 Found 186 hits with Last Name = 'yoo' and Initial = 'm'
Found 186 hits with Last Name = 'yoo' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Procathepsin L
(Homo sapiens (Human)) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602543
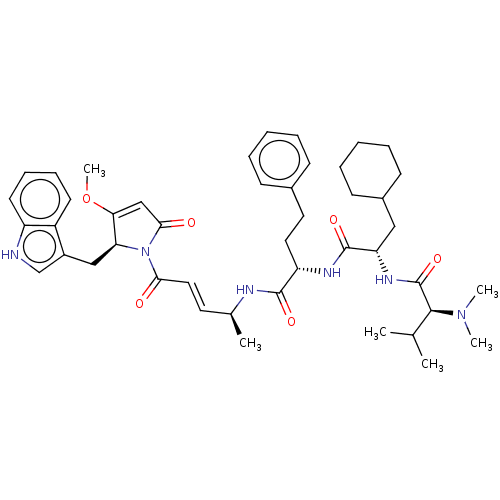
(CHEMBL4598338)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Mus musculus) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Mus musculus) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Mus musculus) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Mus musculus) | BDBM50602543

(CHEMBL4598338)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Mus musculus) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 238 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602543

(CHEMBL4598338)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 853 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 861 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602543

(CHEMBL4598338)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.149 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.224 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.278 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.304 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.367 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.416 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.623 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50344779
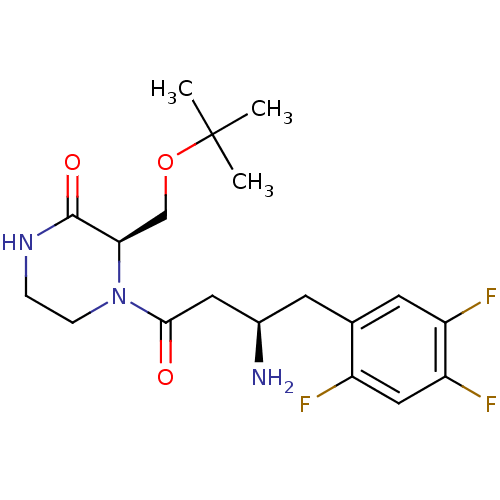
((R)-4-((R)-3-amino-4-(2,4,5-trifluorophenyl)butano...)Show SMILES CC(C)(C)OC[C@H]1N(CCNC1=O)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C19H26F3N3O3/c1-19(2,3)28-10-16-18(27)24-4-5-25(16)17(26)8-12(23)6-11-7-14(21)15(22)9-13(11)20/h7,9,12,16H,4-6,8,10,23H2,1-3H3,(H,24,27)/t12-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 assessed as H-Gly-Pro-AMC cleavage after 1 hr by fluorescence assay |
Bioorg Med Chem Lett 21: 3809-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.029
BindingDB Entry DOI: 10.7270/Q2XS5VQK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50344783
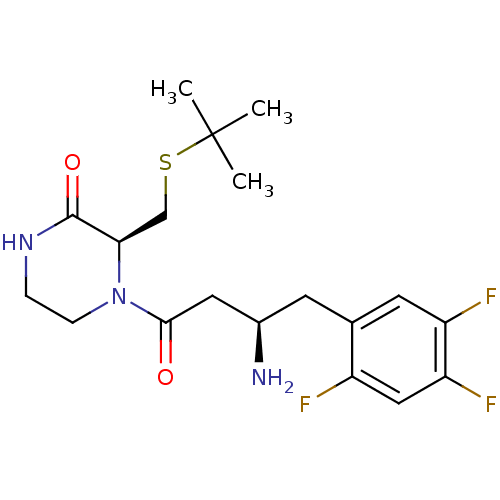
((S)-4-((R)-3-amino-4-(2,4,5-trifluorophenyl)butano...)Show SMILES CC(C)(C)SC[C@H]1N(CCNC1=O)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C19H26F3N3O2S/c1-19(2,3)28-10-16-18(27)24-4-5-25(16)17(26)8-12(23)6-11-7-14(21)15(22)9-13(11)20/h7,9,12,16H,4-6,8,10,23H2,1-3H3,(H,24,27)/t12-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 assessed as H-Gly-Pro-AMC cleavage after 1 hr by fluorescence assay |
Bioorg Med Chem Lett 21: 3809-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.029
BindingDB Entry DOI: 10.7270/Q2XS5VQK |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
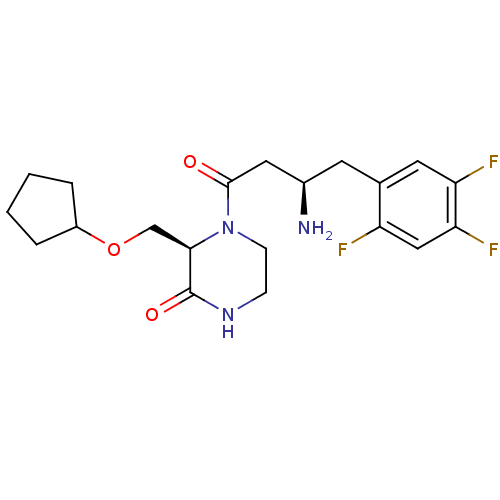
(Homo sapiens (Human)) | BDBM50344777
((R)-4-((R)-3-amino-4-(2,4,5-trifluorophenyl)butano...)Show SMILES N[C@@H](CC(=O)N1CCNC(=O)[C@H]1COC1CCCC1)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C20H26F3N3O3/c21-15-10-17(23)16(22)8-12(15)7-13(24)9-19(27)26-6-5-25-20(28)18(26)11-29-14-3-1-2-4-14/h8,10,13-14,18H,1-7,9,11,24H2,(H,25,28)/t13-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 assessed as H-Gly-Pro-AMC cleavage after 1 hr by fluorescence assay |
Bioorg Med Chem Lett 21: 3809-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.029
BindingDB Entry DOI: 10.7270/Q2XS5VQK |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM14361

((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to rolipram binding site in isolated rat brain by rolipram binding assay |
Bioorg Med Chem Lett 11: 611-4 (2001)
BindingDB Entry DOI: 10.7270/Q2PK0JBS |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50261163

(CHEMBL4081756)Show SMILES Cc1cccc(n1)C(=O)N[C@H]1C[C@H](C1)n1cnc2c(NCc3cccc(Br)c3)ncnc12 |r,wU:12.15,10.10,(36.41,-23.55,;35.32,-24.63,;33.83,-24.23,;32.74,-25.33,;33.14,-26.81,;34.63,-27.21,;35.72,-26.12,;35.02,-28.69,;36.54,-29.01,;33.99,-29.84,;32.49,-29.52,;31.2,-30.36,;30.36,-29.07,;31.65,-28.23,;28.86,-28.74,;28.23,-27.34,;26.7,-27.5,;26.37,-29.01,;25.04,-29.78,;23.71,-29.01,;22.38,-29.78,;21.04,-29.01,;21.04,-27.47,;19.7,-26.7,;18.37,-27.47,;18.37,-29.01,;17.04,-29.78,;19.7,-29.78,;25.04,-31.32,;26.37,-32.09,;27.71,-31.32,;27.71,-29.78,)| Show InChI InChI=1S/C23H22BrN7O/c1-14-4-2-7-19(29-14)23(32)30-17-9-18(10-17)31-13-28-20-21(26-12-27-22(20)31)25-11-15-5-3-6-16(24)8-15/h2-8,12-13,17-18H,9-11H2,1H3,(H,30,32)(H,25,26,27)/t17-,18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/CyclinA (unknown origin) using ULingt-4E-BP as substrate after 1 hr in presence of ATP by fluorescence assay |
Bioorg Med Chem Lett 27: 4399-4404 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.018
BindingDB Entry DOI: 10.7270/Q2W66P60 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50344778
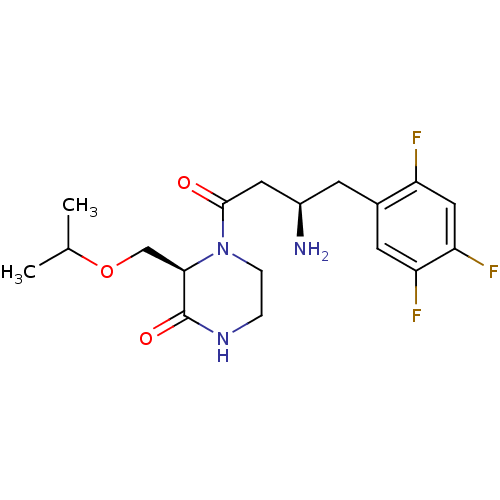
((R)-4-((R)-3-amino-4-(2,4,5-trifluorophenyl)butano...)Show SMILES CC(C)OC[C@H]1N(CCNC1=O)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C18H24F3N3O3/c1-10(2)27-9-16-18(26)23-3-4-24(16)17(25)7-12(22)5-11-6-14(20)15(21)8-13(11)19/h6,8,10,12,16H,3-5,7,9,22H2,1-2H3,(H,23,26)/t12-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 assessed as H-Gly-Pro-AMC cleavage after 1 hr by fluorescence assay |
Bioorg Med Chem Lett 21: 3809-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.029
BindingDB Entry DOI: 10.7270/Q2XS5VQK |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50261164

(CHEMBL4103221)Show SMILES Cc1cccc(n1)C(=O)N[C@H]1C[C@H](C1)n1cnc2c(NCc3cccc(Cl)c3)ncnc12 |r,wU:12.15,10.10,(26.01,-22.02,;24.92,-23.11,;23.43,-22.71,;22.35,-23.8,;22.75,-25.29,;24.23,-25.68,;25.32,-24.6,;24.63,-27.17,;26.14,-27.49,;23.6,-28.32,;22.09,-28,;20.8,-28.83,;19.97,-27.54,;21.26,-26.71,;18.46,-27.22,;17.83,-25.81,;16.3,-25.98,;15.98,-27.48,;14.65,-28.26,;13.31,-27.48,;11.98,-28.26,;10.65,-27.48,;10.65,-25.94,;9.31,-25.18,;7.98,-25.94,;7.98,-27.48,;6.64,-28.26,;9.31,-28.26,;14.65,-29.8,;15.98,-30.56,;17.31,-29.8,;17.31,-28.26,)| Show InChI InChI=1S/C23H22ClN7O/c1-14-4-2-7-19(29-14)23(32)30-17-9-18(10-17)31-13-28-20-21(26-12-27-22(20)31)25-11-15-5-3-6-16(24)8-15/h2-8,12-13,17-18H,9-11H2,1H3,(H,30,32)(H,25,26,27)/t17-,18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p35 (unknown origin) using ULingt-4E-BP as substrate after 1 hr in presence of ATP by fluorescence assay |
Bioorg Med Chem Lett 27: 4399-4404 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.018
BindingDB Entry DOI: 10.7270/Q2W66P60 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50344776
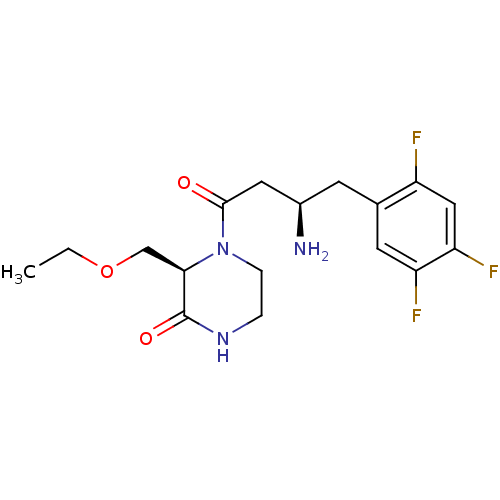
((R)-4-((R)-3-amino-4-(2,4,5-trifluorophenyl)butano...)Show SMILES CCOC[C@H]1N(CCNC1=O)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C17H22F3N3O3/c1-2-26-9-15-17(25)22-3-4-23(15)16(24)7-11(21)5-10-6-13(19)14(20)8-12(10)18/h6,8,11,15H,2-5,7,9,21H2,1H3,(H,22,25)/t11-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 assessed as H-Gly-Pro-AMC cleavage after 1 hr by fluorescence assay |
Bioorg Med Chem Lett 21: 3809-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.029
BindingDB Entry DOI: 10.7270/Q2XS5VQK |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50261163

(CHEMBL4081756)Show SMILES Cc1cccc(n1)C(=O)N[C@H]1C[C@H](C1)n1cnc2c(NCc3cccc(Br)c3)ncnc12 |r,wU:12.15,10.10,(36.41,-23.55,;35.32,-24.63,;33.83,-24.23,;32.74,-25.33,;33.14,-26.81,;34.63,-27.21,;35.72,-26.12,;35.02,-28.69,;36.54,-29.01,;33.99,-29.84,;32.49,-29.52,;31.2,-30.36,;30.36,-29.07,;31.65,-28.23,;28.86,-28.74,;28.23,-27.34,;26.7,-27.5,;26.37,-29.01,;25.04,-29.78,;23.71,-29.01,;22.38,-29.78,;21.04,-29.01,;21.04,-27.47,;19.7,-26.7,;18.37,-27.47,;18.37,-29.01,;17.04,-29.78,;19.7,-29.78,;25.04,-31.32,;26.37,-32.09,;27.71,-31.32,;27.71,-29.78,)| Show InChI InChI=1S/C23H22BrN7O/c1-14-4-2-7-19(29-14)23(32)30-17-9-18(10-17)31-13-28-20-21(26-12-27-22(20)31)25-11-15-5-3-6-16(24)8-15/h2-8,12-13,17-18H,9-11H2,1H3,(H,30,32)(H,25,26,27)/t17-,18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p35 (unknown origin) using ULingt-4E-BP as substrate after 1 hr in presence of ATP by fluorescence assay |
Bioorg Med Chem Lett 27: 4399-4404 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.018
BindingDB Entry DOI: 10.7270/Q2W66P60 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50344782
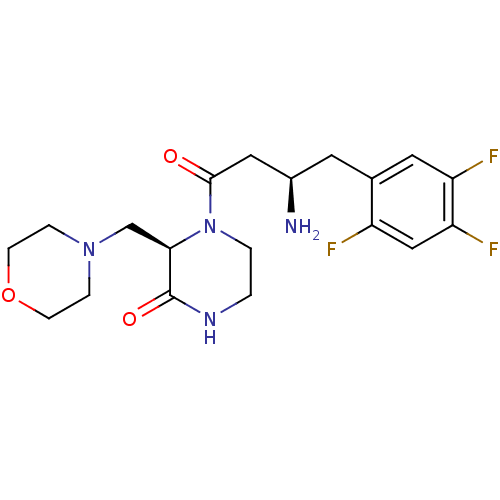
((R)-4-((R)-3-amino-4-(2,4,5-trifluorophenyl)butano...)Show SMILES N[C@@H](CC(=O)N1CCNC(=O)[C@H]1CN1CCOCC1)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C19H25F3N4O3/c20-14-10-16(22)15(21)8-12(14)7-13(23)9-18(27)26-2-1-24-19(28)17(26)11-25-3-5-29-6-4-25/h8,10,13,17H,1-7,9,11,23H2,(H,24,28)/t13-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 assessed as H-Gly-Pro-AMC cleavage after 1 hr by fluorescence assay |
Bioorg Med Chem Lett 21: 3809-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.029
BindingDB Entry DOI: 10.7270/Q2XS5VQK |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50261205

(CHEMBL4091107)Show SMILES Cc1cccc(n1)C(=O)N[C@H]1C[C@H](C1)n1cnc2c(NCc3ccc(F)cc3)ncnc12 |r,wU:12.15,10.10,(29.02,-22.38,;27.93,-23.46,;26.44,-23.06,;25.35,-24.16,;25.75,-25.64,;27.24,-26.04,;28.33,-24.95,;27.64,-27.52,;29.15,-27.85,;26.61,-28.67,;25.1,-28.35,;23.81,-29.19,;22.97,-27.9,;24.26,-27.06,;21.47,-27.58,;20.84,-26.17,;19.31,-26.33,;18.99,-27.84,;17.65,-28.61,;16.32,-27.84,;14.99,-28.61,;13.66,-27.84,;12.32,-28.61,;10.99,-27.84,;10.99,-26.3,;9.65,-25.53,;12.32,-25.53,;13.66,-26.3,;17.65,-30.15,;18.99,-30.92,;20.32,-30.15,;20.32,-28.61,)| Show InChI InChI=1S/C23H22FN7O/c1-14-3-2-4-19(29-14)23(32)30-17-9-18(10-17)31-13-28-20-21(26-12-27-22(20)31)25-11-15-5-7-16(24)8-6-15/h2-8,12-13,17-18H,9-11H2,1H3,(H,30,32)(H,25,26,27)/t17-,18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/CyclinA (unknown origin) using ULingt-4E-BP as substrate after 1 hr in presence of ATP by fluorescence assay |
Bioorg Med Chem Lett 27: 4399-4404 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.018
BindingDB Entry DOI: 10.7270/Q2W66P60 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50261165

(CHEMBL4070146)Show SMILES Cc1cccc(n1)C(=O)N[C@H]1C[C@H](C1)n1cnc2c(NCc3cccc(F)c3)ncnc12 |r,wU:12.15,10.10,(31.27,-11.82,;30.17,-12.91,;28.69,-12.51,;27.6,-13.6,;28,-15.09,;29.49,-15.48,;30.57,-14.39,;29.89,-16.97,;31.39,-17.29,;28.85,-18.12,;27.35,-17.8,;26.06,-18.63,;25.22,-17.34,;26.51,-16.51,;23.71,-17.02,;23.08,-15.61,;21.55,-15.77,;21.23,-17.28,;19.9,-18.05,;18.57,-17.28,;17.23,-18.05,;15.9,-17.28,;15.9,-15.74,;14.57,-14.97,;13.24,-15.74,;13.24,-17.28,;11.9,-18.05,;14.57,-18.05,;19.9,-19.59,;21.23,-20.36,;22.56,-19.59,;22.56,-18.05,)| Show InChI InChI=1S/C23H22FN7O/c1-14-4-2-7-19(29-14)23(32)30-17-9-18(10-17)31-13-28-20-21(26-12-27-22(20)31)25-11-15-5-3-6-16(24)8-15/h2-8,12-13,17-18H,9-11H2,1H3,(H,30,32)(H,25,26,27)/t17-,18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p35 (unknown origin) using ULingt-4E-BP as substrate after 1 hr in presence of ATP by fluorescence assay |
Bioorg Med Chem Lett 27: 4399-4404 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.018
BindingDB Entry DOI: 10.7270/Q2W66P60 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50261164

(CHEMBL4103221)Show SMILES Cc1cccc(n1)C(=O)N[C@H]1C[C@H](C1)n1cnc2c(NCc3cccc(Cl)c3)ncnc12 |r,wU:12.15,10.10,(26.01,-22.02,;24.92,-23.11,;23.43,-22.71,;22.35,-23.8,;22.75,-25.29,;24.23,-25.68,;25.32,-24.6,;24.63,-27.17,;26.14,-27.49,;23.6,-28.32,;22.09,-28,;20.8,-28.83,;19.97,-27.54,;21.26,-26.71,;18.46,-27.22,;17.83,-25.81,;16.3,-25.98,;15.98,-27.48,;14.65,-28.26,;13.31,-27.48,;11.98,-28.26,;10.65,-27.48,;10.65,-25.94,;9.31,-25.18,;7.98,-25.94,;7.98,-27.48,;6.64,-28.26,;9.31,-28.26,;14.65,-29.8,;15.98,-30.56,;17.31,-29.8,;17.31,-28.26,)| Show InChI InChI=1S/C23H22ClN7O/c1-14-4-2-7-19(29-14)23(32)30-17-9-18(10-17)31-13-28-20-21(26-12-27-22(20)31)25-11-15-5-3-6-16(24)8-15/h2-8,12-13,17-18H,9-11H2,1H3,(H,30,32)(H,25,26,27)/t17-,18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/CyclinA (unknown origin) using ULingt-4E-BP as substrate after 1 hr in presence of ATP by fluorescence assay |
Bioorg Med Chem Lett 27: 4399-4404 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.018
BindingDB Entry DOI: 10.7270/Q2W66P60 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50344775
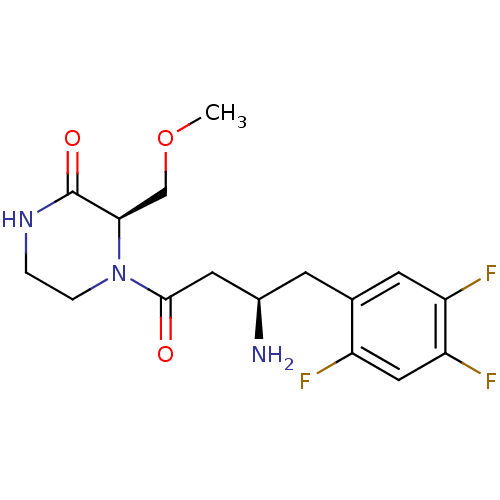
((R)-4-((R)-3-amino-4-(2,4,5-trifluorophenyl)butano...)Show SMILES COC[C@H]1N(CCNC1=O)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H20F3N3O3/c1-25-8-14-16(24)21-2-3-22(14)15(23)6-10(20)4-9-5-12(18)13(19)7-11(9)17/h5,7,10,14H,2-4,6,8,20H2,1H3,(H,21,24)/t10-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 assessed as H-Gly-Pro-AMC cleavage after 1 hr by fluorescence assay |
Bioorg Med Chem Lett 21: 3809-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.029
BindingDB Entry DOI: 10.7270/Q2XS5VQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50344788
((R)-4-(3-amino-4-(2,4,5-trifluorophenyl)butanoyl)-...)Show SMILES COc1cc(CN2CCN(CC2=O)C(=O)C[C@H](N)Cc2cc(F)c(F)cc2F)c(cc1OC)[N+]([O-])=O |r| Show InChI InChI=1S/C23H25F3N4O6/c1-35-20-7-14(19(30(33)34)10-21(20)36-2)11-28-3-4-29(12-23(28)32)22(31)8-15(27)5-13-6-17(25)18(26)9-16(13)24/h6-7,9-10,15H,3-5,8,11-12,27H2,1-2H3/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 assessed as H-Gly-Pro-AMC cleavage after 1 hr by fluorescence assay |
Bioorg Med Chem Lett 21: 3809-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.029
BindingDB Entry DOI: 10.7270/Q2XS5VQK |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50261168

(CHEMBL4067424)Show SMILES Cc1cccc(n1)C(=O)N[C@H]1C[C@H](C1)n1cnc2c(NCc3cccnc3)ncnc12 |r,wU:12.15,10.10,(29.63,-23.6,;28.54,-24.68,;27.05,-24.28,;25.97,-25.38,;26.36,-26.86,;27.85,-27.26,;28.94,-26.17,;28.25,-28.74,;29.75,-29.07,;27.22,-29.89,;25.71,-29.57,;24.42,-30.41,;23.58,-29.12,;24.87,-28.28,;22.07,-28.8,;21.45,-27.39,;19.92,-27.55,;19.6,-29.06,;18.27,-29.83,;16.93,-29.06,;15.59,-29.83,;14.26,-29.06,;12.93,-29.83,;11.6,-29.06,;11.6,-27.52,;12.93,-26.75,;14.26,-27.52,;18.27,-31.37,;19.6,-32.14,;20.93,-31.37,;20.93,-29.83,)| Show InChI InChI=1S/C22H22N8O/c1-14-4-2-6-18(28-14)22(31)29-16-8-17(9-16)30-13-27-19-20(25-12-26-21(19)30)24-11-15-5-3-7-23-10-15/h2-7,10,12-13,16-17H,8-9,11H2,1H3,(H,29,31)(H,24,25,26)/t16-,17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p35 (unknown origin) using ULingt-4E-BP as substrate after 1 hr in presence of ATP by fluorescence assay |
Bioorg Med Chem Lett 27: 4399-4404 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.018
BindingDB Entry DOI: 10.7270/Q2W66P60 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50344781
((R)-4-((R)-3-amino-4-(2,4,5-trifluorophenyl)butano...)Show SMILES CCN(CC)C[C@H]1N(CCNC1=O)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C19H27F3N4O2/c1-3-25(4-2)11-17-19(28)24-5-6-26(17)18(27)9-13(23)7-12-8-15(21)16(22)10-14(12)20/h8,10,13,17H,3-7,9,11,23H2,1-2H3,(H,24,28)/t13-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 assessed as H-Gly-Pro-AMC cleavage after 1 hr by fluorescence assay |
Bioorg Med Chem Lett 21: 3809-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.029
BindingDB Entry DOI: 10.7270/Q2XS5VQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50344774
((R)-4-((R)-3-amino-4-(2,4,5-trifluorophenyl)butano...)Show SMILES N[C@@H](CC(=O)N1CCNC(=O)[C@H]1CO)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C15H18F3N3O3/c16-10-6-12(18)11(17)4-8(10)3-9(19)5-14(23)21-2-1-20-15(24)13(21)7-22/h4,6,9,13,22H,1-3,5,7,19H2,(H,20,24)/t9-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 assessed as H-Gly-Pro-AMC cleavage after 1 hr by fluorescence assay |
Bioorg Med Chem Lett 21: 3809-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.029
BindingDB Entry DOI: 10.7270/Q2XS5VQK |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50261204
(CHEMBL4098255)Show SMILES Cc1cccc(n1)C(=O)N[C@H]1C[C@H](C1)n1cnc2c(NCc3ccc(F)c(F)c3)ncnc12 |r,wU:12.15,10.10,(29.23,-17.32,;28.14,-18.41,;26.65,-18.01,;25.57,-19.1,;25.97,-20.59,;27.45,-20.98,;28.54,-19.89,;27.85,-22.47,;29.36,-22.79,;26.82,-23.62,;25.31,-23.3,;24.02,-24.13,;23.19,-22.84,;24.48,-22.01,;21.68,-22.52,;21.05,-21.11,;19.52,-21.28,;19.2,-22.78,;17.87,-23.56,;16.53,-22.78,;15.2,-23.56,;13.87,-22.78,;13.87,-21.24,;12.54,-20.48,;11.2,-21.24,;9.86,-20.48,;11.2,-22.78,;9.86,-23.56,;12.54,-23.56,;17.87,-25.09,;19.2,-25.86,;20.53,-25.09,;20.53,-23.56,)| Show InChI InChI=1S/C23H21F2N7O/c1-13-3-2-4-19(30-13)23(33)31-15-8-16(9-15)32-12-29-20-21(27-11-28-22(20)32)26-10-14-5-6-17(24)18(25)7-14/h2-7,11-12,15-16H,8-10H2,1H3,(H,31,33)(H,26,27,28)/t15-,16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p35 (unknown origin) using ULingt-4E-BP as substrate after 1 hr in presence of ATP by fluorescence assay |
Bioorg Med Chem Lett 27: 4399-4404 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.018
BindingDB Entry DOI: 10.7270/Q2W66P60 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50344769
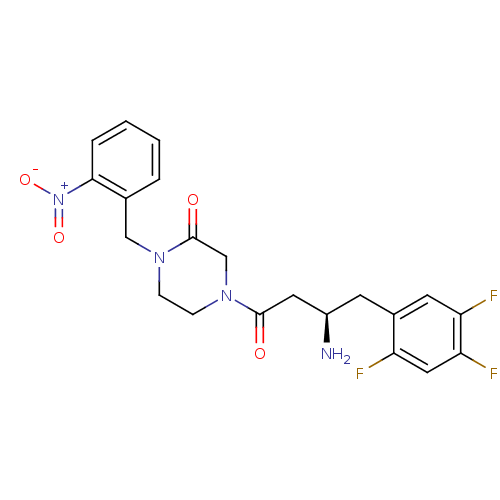
((R)-4-(3-amino-4-(2,4,5-trifluorophenyl)butanoyl)-...)Show SMILES N[C@@H](CC(=O)N1CCN(Cc2ccccc2[N+]([O-])=O)C(=O)C1)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C21H21F3N4O4/c22-16-10-18(24)17(23)8-14(16)7-15(25)9-20(29)27-6-5-26(21(30)12-27)11-13-3-1-2-4-19(13)28(31)32/h1-4,8,10,15H,5-7,9,11-12,25H2/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 assessed as H-Gly-Pro-AMC cleavage after 1 hr by fluorescence assay |
Bioorg Med Chem Lett 21: 3809-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.029
BindingDB Entry DOI: 10.7270/Q2XS5VQK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data