

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50111101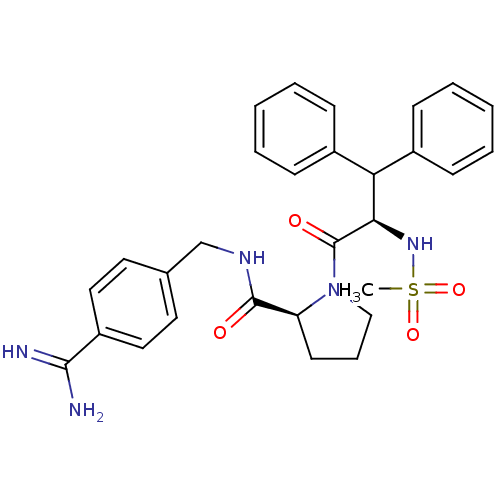 ((S)-1-((R)-2-Methanesulfonylamino-3,3-diphenyl-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111110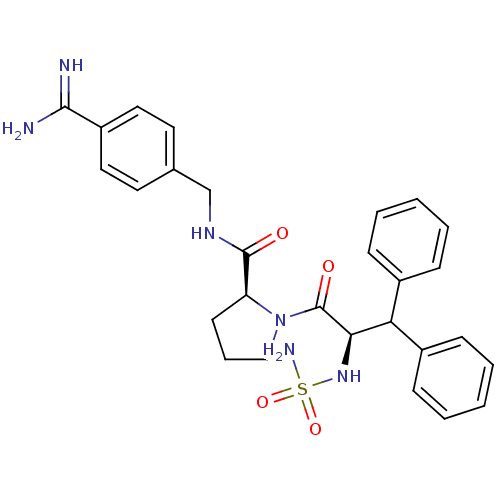 (2N-(4-Benzamidinemethyl)-1-[2-aminosulfonamido-3,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131789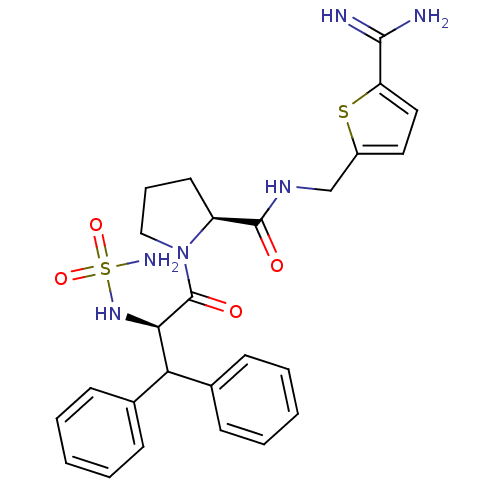 (1-(2-sulfonamide-amino-3,3-diphenyl-propionyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111105 ((S)-1-((R)-2-Methanesulfonylamino-3,3-diphenyl-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131790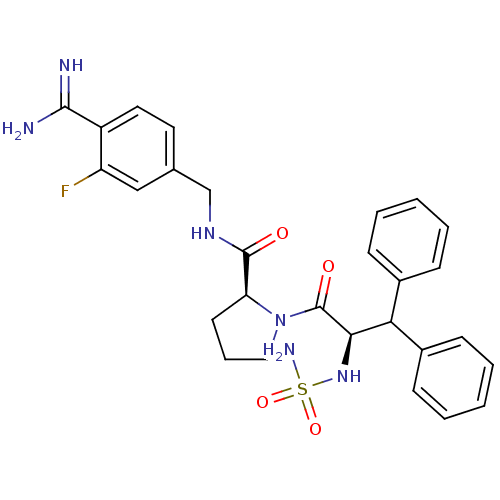 (1-(2-sulfonamideamino-3,3-diphenyl-propionyl)-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131795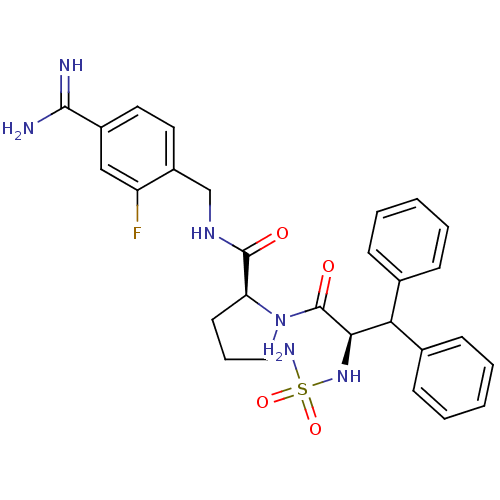 (1-(2-sulfonamideamino-3,3-diphenyl-propionyl)-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131778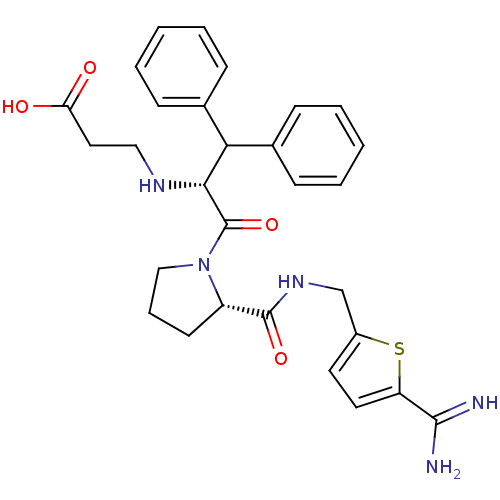 (3-(1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111120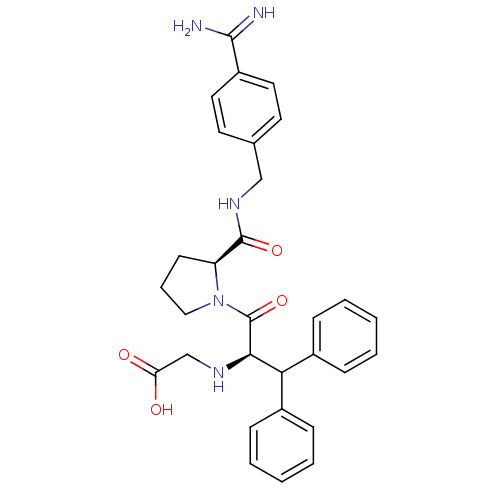 (2N-(4-Benzamidinemethyl)-1-[2-Aminoaceticacid-3,3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131780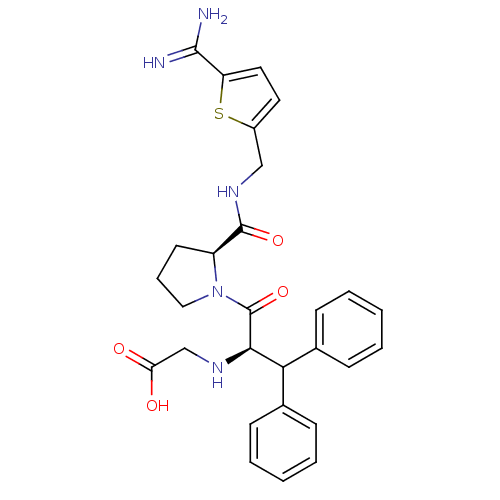 ((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131796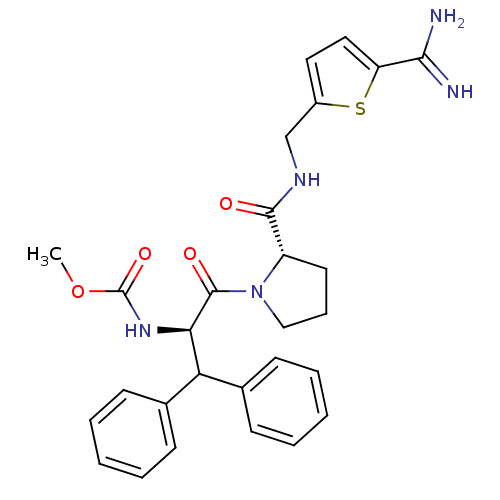 ((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131791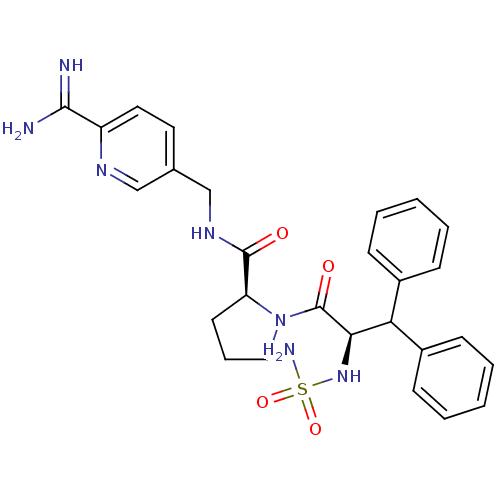 (1-(2-sulfonamideamino-3,3-diphenyl-propionyl)-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131792 ((1-Benzhydryl-2-{2-[(6-carbamimidoyl-pyridin-3-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131798 (1-(2-Methylamino-3,3-diphenyl-propionyl)-pyrrolidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131782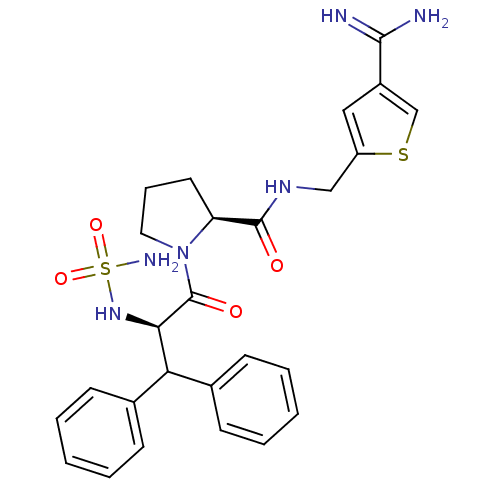 (1-(2-sulfonamide-amino-3,3-diphenyl-propionyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111122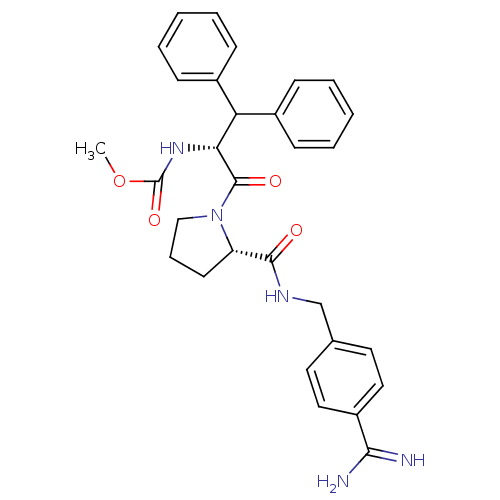 (2N-(4-Benzamidinemethyl)-1-[2-Carbamicacidmethyles...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131787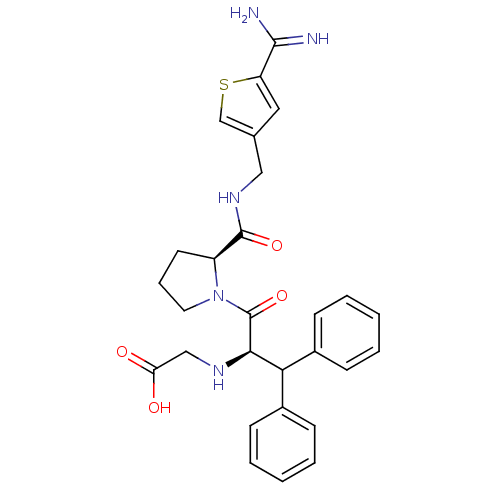 ((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131781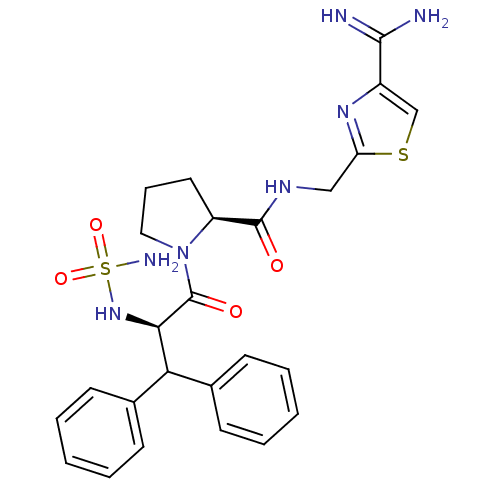 (1-(2-sulfonamide-amino-3,3-diphenyl-propionyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131797 (1-(2-sulfonamide-amino-3,3-diphenyl-propionyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131779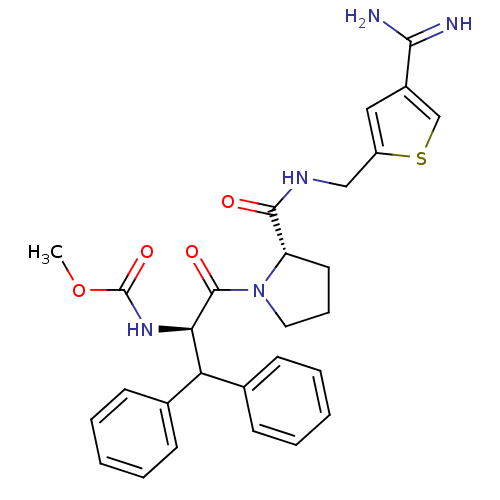 ((1-Benzhydryl-2-{2-[(4-carbamimidoyl-thiophen-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1/Trypsin-2 (Homo sapiens (Human)) | BDBM50131780 ((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-2-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human human trypsin was determined | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131784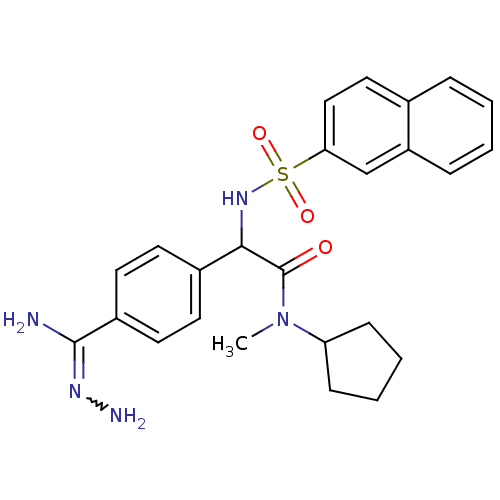 (2-{4-[(Z)-amino(hydrazono)methyl]phenyl}-N-cyclope...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131783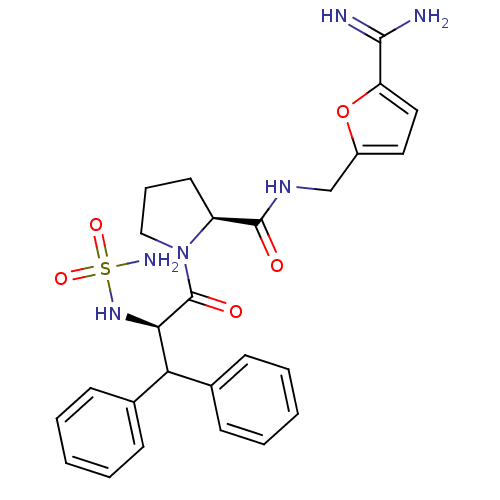 (1-(2-sulfonamide-amino-3,3-diphenyl-propionyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131788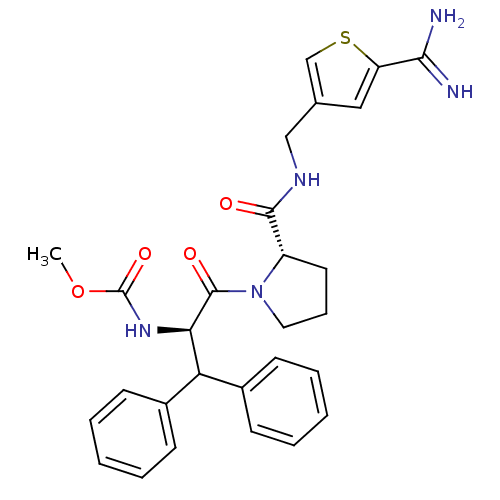 ((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131793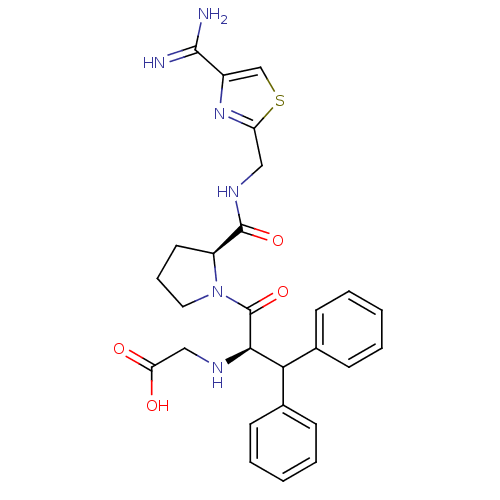 ((1-Benzhydryl-2-{2-[(4-carbamimidoyl-thiazol-2-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131777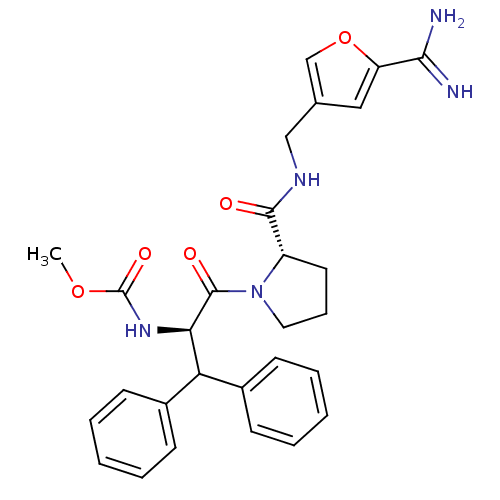 ((1-Benzhydryl-2-{2-[(5-carbamimidoyl-furan-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50131780 ((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-2-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human bovine trypsin was determined | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50038001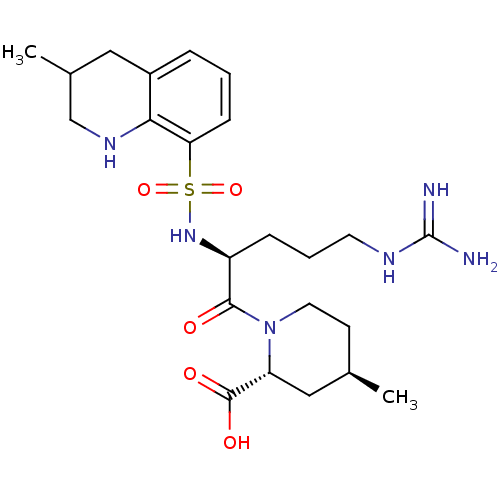 ((2R,4R)-1-((S)-5-(diaminomethyleneamino)-2-(3-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50131780 ((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 22.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human Coagulation factor X was determined | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50131780 ((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 433 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human Tissue type plasminogen activator was determined | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50131780 ((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human human plasmin was determined | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen-related receptor gamma (Homo sapiens (Human)) | BDBM50193253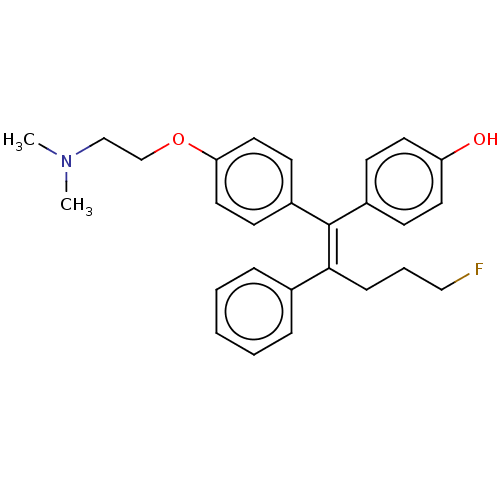 (CHEMBL3961676) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation Curated by ChEMBL | Assay Description Inhibition of fluorescien-conjugated coactivator PGC1a binding to GST-tagged human ERRgamma LBD after 1 hr by TR-FRET assay | Eur J Med Chem 120: 338-52 (2016) Article DOI: 10.1016/j.ejmech.2016.04.076 BindingDB Entry DOI: 10.7270/Q2TH8PN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50193253 (CHEMBL3961676) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation Curated by ChEMBL | Assay Description Inhibition of fluorescien-conjugated coactivator PGC1a binding to ERalpha (unknown origin) after 1 hr by TR-FRET assay | Eur J Med Chem 120: 338-52 (2016) Article DOI: 10.1016/j.ejmech.2016.04.076 BindingDB Entry DOI: 10.7270/Q2TH8PN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen-related receptor gamma (Homo sapiens (Human)) | BDBM50193253 (CHEMBL3961676) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation Curated by ChEMBL | Assay Description Inverse agonist activity at ERRgamma (unknown origin) expressed in human AD293 cells after 24 hrs by beta-galactosidase/luciferase reporter gene assa... | Eur J Med Chem 120: 338-52 (2016) Article DOI: 10.1016/j.ejmech.2016.04.076 BindingDB Entry DOI: 10.7270/Q2TH8PN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen-related receptor gamma (Homo sapiens (Human)) | BDBM50193250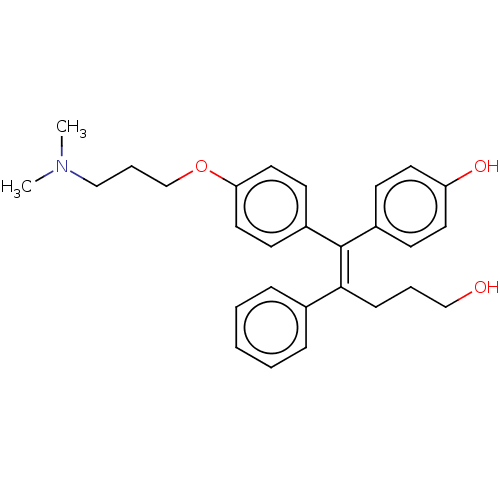 (CHEMBL3897416) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation Curated by ChEMBL | Assay Description Inverse agonist activity at ERRgamma (unknown origin) expressed in human AD293 cells after 24 hrs by beta-galactosidase/luciferase reporter gene assa... | Eur J Med Chem 120: 338-52 (2016) Article DOI: 10.1016/j.ejmech.2016.04.076 BindingDB Entry DOI: 10.7270/Q2TH8PN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen-related receptor gamma (Homo sapiens (Human)) | BDBM22435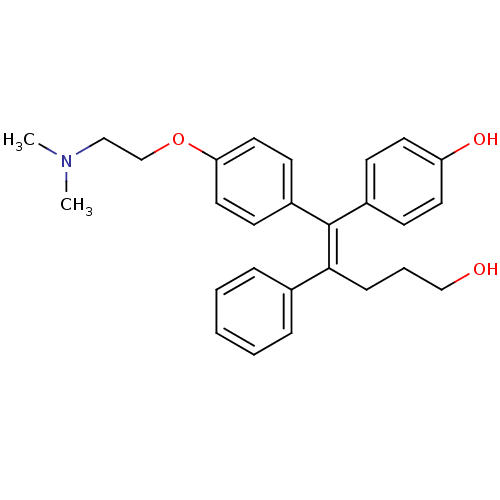 (4-[(1Z)-1-{4-[2-(dimethylamino)ethoxy]phenyl}-5-hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation Curated by ChEMBL | Assay Description Inverse agonist activity at ERRgamma (unknown origin) expressed in human AD293 cells after 24 hrs by beta-galactosidase/luciferase reporter gene assa... | Eur J Med Chem 120: 338-52 (2016) Article DOI: 10.1016/j.ejmech.2016.04.076 BindingDB Entry DOI: 10.7270/Q2TH8PN2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen-related receptor gamma (Homo sapiens (Human)) | BDBM50193252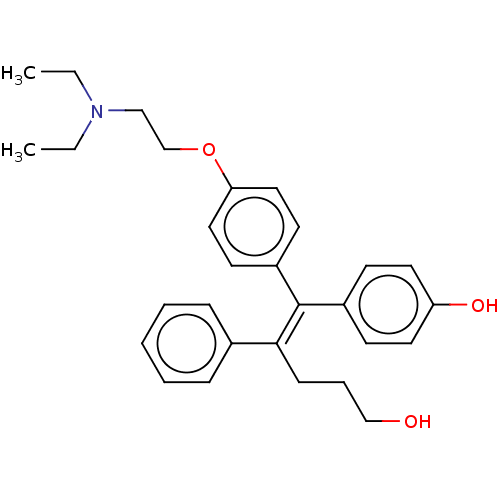 (CHEMBL3957546) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation Curated by ChEMBL | Assay Description Inverse agonist activity at ERRgamma (unknown origin) expressed in human AD293 cells after 24 hrs by beta-galactosidase/luciferase reporter gene assa... | Eur J Med Chem 120: 338-52 (2016) Article DOI: 10.1016/j.ejmech.2016.04.076 BindingDB Entry DOI: 10.7270/Q2TH8PN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen-related receptor gamma (Homo sapiens (Human)) | BDBM50193250 (CHEMBL3897416) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation Curated by ChEMBL | Assay Description Inhibition of fluorescien-conjugated coactivator PGC1a binding to GST-tagged human ERRgamma LBD after 1 hr by TR-FRET assay | Eur J Med Chem 120: 338-52 (2016) Article DOI: 10.1016/j.ejmech.2016.04.076 BindingDB Entry DOI: 10.7270/Q2TH8PN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen-related receptor gamma (Homo sapiens (Human)) | BDBM22435 (4-[(1Z)-1-{4-[2-(dimethylamino)ethoxy]phenyl}-5-hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation Curated by ChEMBL | Assay Description Inhibition of fluorescien-conjugated coactivator PGC1a binding to GST-tagged human ERRgamma LBD after 1 hr by TR-FRET assay | Eur J Med Chem 120: 338-52 (2016) Article DOI: 10.1016/j.ejmech.2016.04.076 BindingDB Entry DOI: 10.7270/Q2TH8PN2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen-related receptor gamma (Homo sapiens (Human)) | BDBM50193262 (CHEMBL3956562) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation Curated by ChEMBL | Assay Description Inverse agonist activity at ERRgamma (unknown origin) expressed in human AD293 cells after 24 hrs by beta-galactosidase/luciferase reporter gene assa... | Eur J Med Chem 120: 338-52 (2016) Article DOI: 10.1016/j.ejmech.2016.04.076 BindingDB Entry DOI: 10.7270/Q2TH8PN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen-related receptor gamma (Homo sapiens (Human)) | BDBM50193251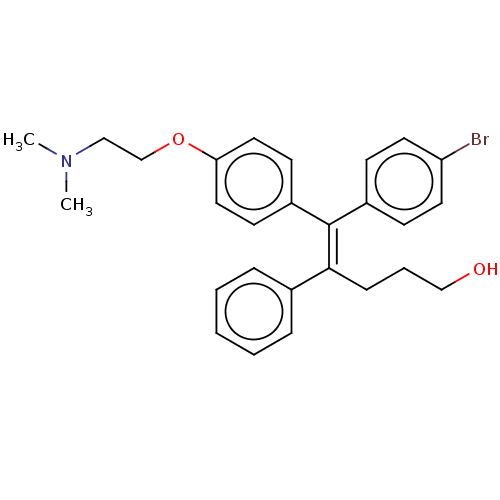 (CHEMBL3978054) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation Curated by ChEMBL | Assay Description Inhibition of fluorescien-conjugated coactivator PGC1a binding to GST-tagged human ERRgamma LBD after 1 hr by TR-FRET assay | Eur J Med Chem 120: 338-52 (2016) Article DOI: 10.1016/j.ejmech.2016.04.076 BindingDB Entry DOI: 10.7270/Q2TH8PN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen-related receptor gamma (Homo sapiens (Human)) | BDBM50193252 (CHEMBL3957546) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation Curated by ChEMBL | Assay Description Inhibition of fluorescien-conjugated coactivator PGC1a binding to GST-tagged human ERRgamma LBD after 1 hr by TR-FRET assay | Eur J Med Chem 120: 338-52 (2016) Article DOI: 10.1016/j.ejmech.2016.04.076 BindingDB Entry DOI: 10.7270/Q2TH8PN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen-related receptor gamma (Homo sapiens (Human)) | BDBM50193264 (CHEMBL3968559) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 187 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation Curated by ChEMBL | Assay Description Inverse agonist activity at ERRgamma (unknown origin) expressed in human AD293 cells after 24 hrs by beta-galactosidase/luciferase reporter gene assa... | Eur J Med Chem 120: 338-52 (2016) Article DOI: 10.1016/j.ejmech.2016.04.076 BindingDB Entry DOI: 10.7270/Q2TH8PN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen-related receptor gamma (Homo sapiens (Human)) | BDBM50193264 (CHEMBL3968559) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation Curated by ChEMBL | Assay Description Inhibition of fluorescien-conjugated coactivator PGC1a binding to GST-tagged human ERRgamma LBD after 1 hr by TR-FRET assay | Eur J Med Chem 120: 338-52 (2016) Article DOI: 10.1016/j.ejmech.2016.04.076 BindingDB Entry DOI: 10.7270/Q2TH8PN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen-related receptor gamma (Homo sapiens (Human)) | BDBM50193255 (CHEMBL3973526) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation Curated by ChEMBL | Assay Description Inhibition of fluorescien-conjugated coactivator PGC1a binding to GST-tagged human ERRgamma LBD after 1 hr by TR-FRET assay | Eur J Med Chem 120: 338-52 (2016) Article DOI: 10.1016/j.ejmech.2016.04.076 BindingDB Entry DOI: 10.7270/Q2TH8PN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen-related receptor gamma (Homo sapiens (Human)) | BDBM50193263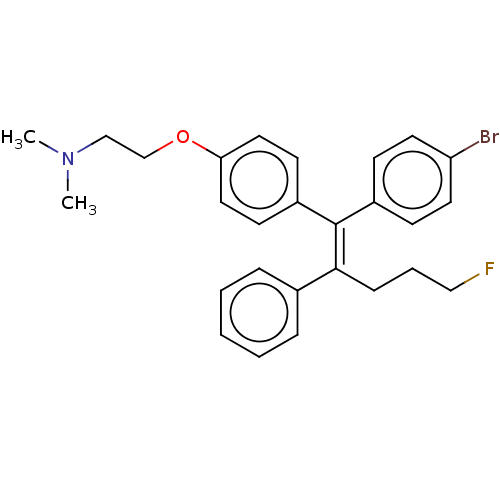 (CHEMBL3891326) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation Curated by ChEMBL | Assay Description Inhibition of fluorescien-conjugated coactivator PGC1a binding to GST-tagged human ERRgamma LBD after 1 hr by TR-FRET assay | Eur J Med Chem 120: 338-52 (2016) Article DOI: 10.1016/j.ejmech.2016.04.076 BindingDB Entry DOI: 10.7270/Q2TH8PN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen-related receptor gamma (Homo sapiens (Human)) | BDBM50193262 (CHEMBL3956562) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation Curated by ChEMBL | Assay Description Inhibition of fluorescien-conjugated coactivator PGC1a binding to GST-tagged human ERRgamma LBD after 1 hr by TR-FRET assay | Eur J Med Chem 120: 338-52 (2016) Article DOI: 10.1016/j.ejmech.2016.04.076 BindingDB Entry DOI: 10.7270/Q2TH8PN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen-related receptor gamma (Homo sapiens (Human)) | BDBM50193273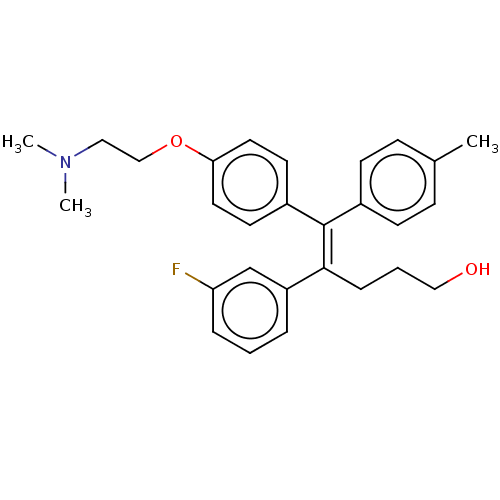 (CHEMBL3982516) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation Curated by ChEMBL | Assay Description Inhibition of fluorescien-conjugated coactivator PGC1a binding to GST-tagged human ERRgamma LBD after 1 hr by TR-FRET assay | Eur J Med Chem 120: 338-52 (2016) Article DOI: 10.1016/j.ejmech.2016.04.076 BindingDB Entry DOI: 10.7270/Q2TH8PN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen-related receptor gamma (Homo sapiens (Human)) | BDBM50193266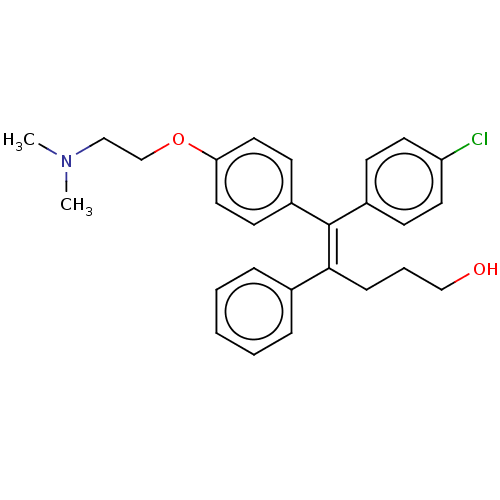 (CHEMBL3940124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation Curated by ChEMBL | Assay Description Inhibition of fluorescien-conjugated coactivator PGC1a binding to GST-tagged human ERRgamma LBD after 1 hr by TR-FRET assay | Eur J Med Chem 120: 338-52 (2016) Article DOI: 10.1016/j.ejmech.2016.04.076 BindingDB Entry DOI: 10.7270/Q2TH8PN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen-related receptor gamma (Homo sapiens (Human)) | BDBM50193255 (CHEMBL3973526) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 669 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation Curated by ChEMBL | Assay Description Inverse agonist activity at ERRgamma (unknown origin) expressed in human AD293 cells after 24 hrs by beta-galactosidase/luciferase reporter gene assa... | Eur J Med Chem 120: 338-52 (2016) Article DOI: 10.1016/j.ejmech.2016.04.076 BindingDB Entry DOI: 10.7270/Q2TH8PN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen-related receptor gamma (Homo sapiens (Human)) | BDBM50193251 (CHEMBL3978054) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 719 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation Curated by ChEMBL | Assay Description Inverse agonist activity at ERRgamma (unknown origin) expressed in human AD293 cells after 24 hrs by beta-galactosidase/luciferase reporter gene assa... | Eur J Med Chem 120: 338-52 (2016) Article DOI: 10.1016/j.ejmech.2016.04.076 BindingDB Entry DOI: 10.7270/Q2TH8PN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 90 total ) | Next | Last >> |