

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330795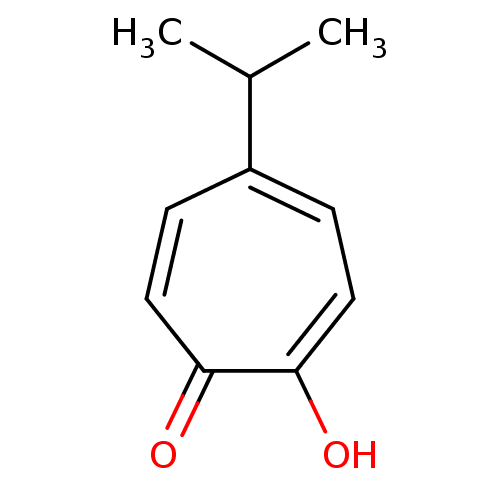 (2-hydroxy-5-isopropyl-2,4,6-cycloheptatrien-1-one ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Theoretical Medicine, Inc. Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase after 15 mins by Lineweaver-Bulk plot analysis | Bioorg Med Chem 18: 8112-8 (2010) Article DOI: 10.1016/j.bmc.2010.08.056 BindingDB Entry DOI: 10.7270/Q2JH3MF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330794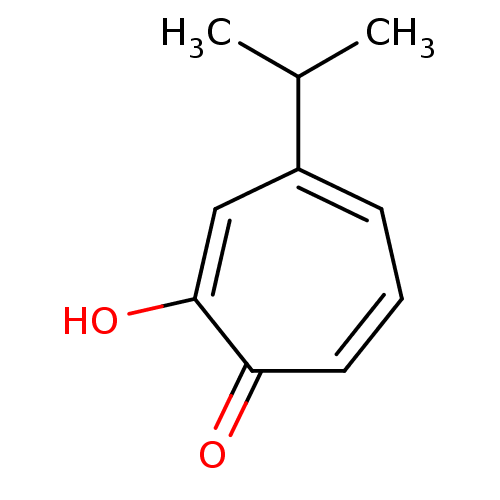 (2-Hydroxy-4-isopropyl-cyclohepta-2,4,6-trienone | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Theoretical Medicine, Inc. Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase after 15 mins by Lineweaver-Bulk plot analysis | Bioorg Med Chem 18: 8112-8 (2010) Article DOI: 10.1016/j.bmc.2010.08.056 BindingDB Entry DOI: 10.7270/Q2JH3MF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330793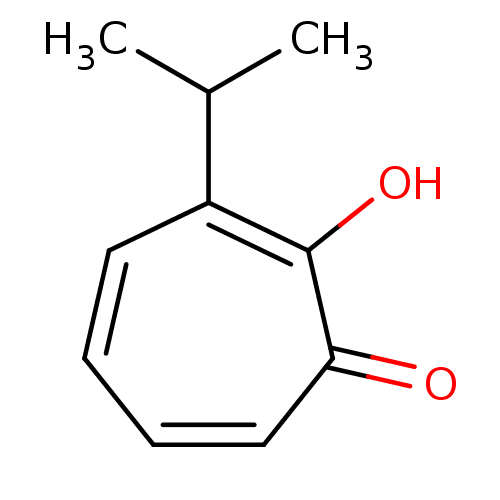 (2-hydroxy-3-isopropyl-2,4,6-cycloheptatrien-1-one ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Theoretical Medicine, Inc. Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase after 15 mins by Lineweaver-Bulk plot analysis | Bioorg Med Chem 18: 8112-8 (2010) Article DOI: 10.1016/j.bmc.2010.08.056 BindingDB Entry DOI: 10.7270/Q2JH3MF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50354041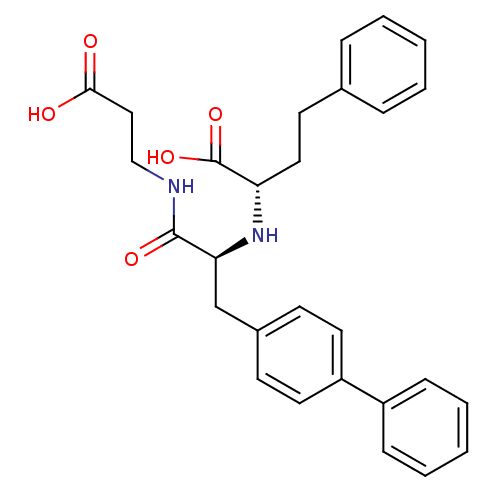 (CHEMBL1829584) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Kao Corporation Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human fibroblasts homogenates using glutaryl-Ala-Ala-Phe-4-methoxy-2-naphtylamide as substrate after 1 hrs by ... | Bioorg Med Chem 19: 5935-47 (2011) Article DOI: 10.1016/j.bmc.2011.08.064 BindingDB Entry DOI: 10.7270/Q2154HF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50354042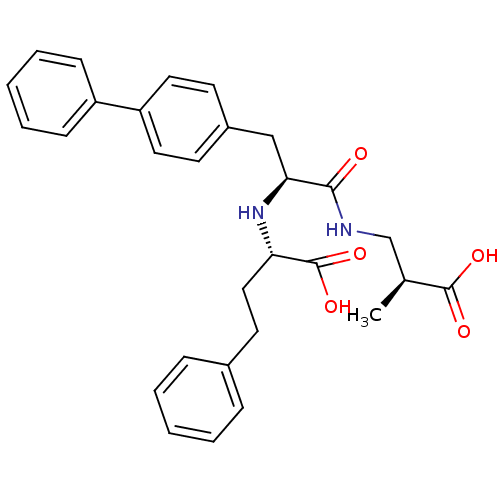 (CHEMBL1829585) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Kao Corporation Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human fibroblasts homogenates using glutaryl-Ala-Ala-Phe-4-methoxy-2-naphtylamide as substrate after 1 hrs by ... | Bioorg Med Chem 19: 5935-47 (2011) Article DOI: 10.1016/j.bmc.2011.08.064 BindingDB Entry DOI: 10.7270/Q2154HF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50251742 ((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Kao Corporation Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human fibroblasts homogenates using glutaryl-Ala-Ala-Phe-4-methoxy-2-naphtylamide as substrate after 1 hrs by ... | Bioorg Med Chem 19: 5935-47 (2011) Article DOI: 10.1016/j.bmc.2011.08.064 BindingDB Entry DOI: 10.7270/Q2154HF6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50354040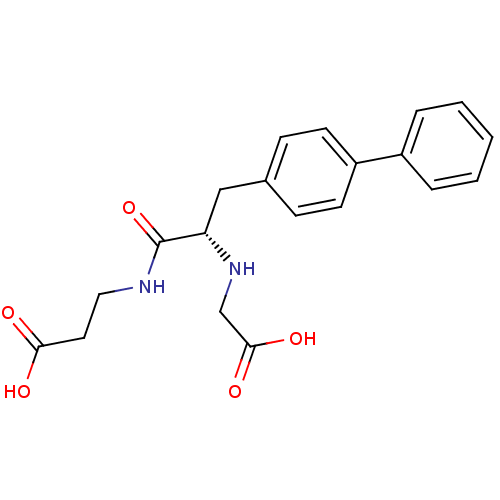 (CHEMBL1829583) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Kao Corporation Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human fibroblasts homogenates using glutaryl-Ala-Ala-Phe-4-methoxy-2-naphtylamide as substrate after 1 hrs by ... | Bioorg Med Chem 19: 5935-47 (2011) Article DOI: 10.1016/j.bmc.2011.08.064 BindingDB Entry DOI: 10.7270/Q2154HF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330795 (2-hydroxy-5-isopropyl-2,4,6-cycloheptatrien-1-one ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Theoretical Medicine, Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of mushroom tyrosinase | Bioorg Med Chem 22: 6193-200 (2014) Article DOI: 10.1016/j.bmc.2014.08.027 BindingDB Entry DOI: 10.7270/Q2DV1MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330795 (2-hydroxy-5-isopropyl-2,4,6-cycloheptatrien-1-one ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Theoretical Medicine, Inc. Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase after 15 mins | Bioorg Med Chem 18: 8112-8 (2010) Article DOI: 10.1016/j.bmc.2010.08.056 BindingDB Entry DOI: 10.7270/Q2JH3MF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50157547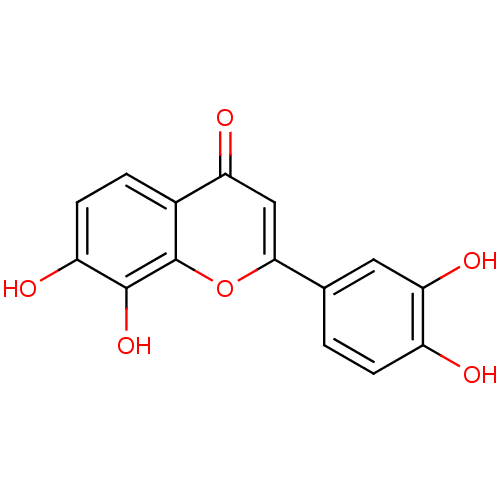 (2-(3,4-dihydroxyphenyl)-7,8-dihydroxy-4H-chromen-4...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Theoretical Medicine, Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of mushroom tyrosinase | Bioorg Med Chem 22: 6193-200 (2014) Article DOI: 10.1016/j.bmc.2014.08.027 BindingDB Entry DOI: 10.7270/Q2DV1MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330794 (2-Hydroxy-4-isopropyl-cyclohepta-2,4,6-trienone | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Theoretical Medicine, Inc. Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase after 15 mins | Bioorg Med Chem 18: 8112-8 (2010) Article DOI: 10.1016/j.bmc.2010.08.056 BindingDB Entry DOI: 10.7270/Q2JH3MF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330794 (2-Hydroxy-4-isopropyl-cyclohepta-2,4,6-trienone | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Theoretical Medicine, Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of mushroom tyrosinase | Bioorg Med Chem 22: 6193-200 (2014) Article DOI: 10.1016/j.bmc.2014.08.027 BindingDB Entry DOI: 10.7270/Q2DV1MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50354044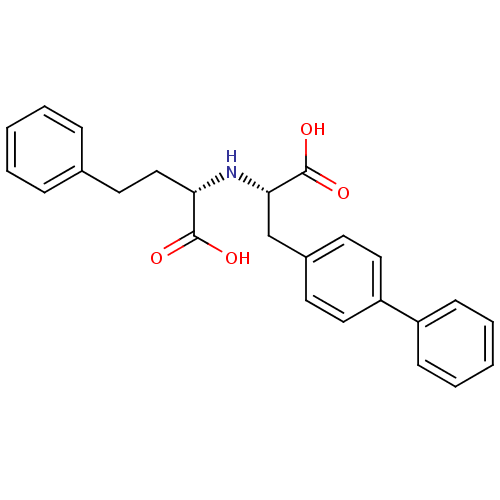 (CHEMBL1829587) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Kao Corporation Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human fibroblasts homogenates using glutaryl-Ala-Ala-Phe-4-methoxy-2-naphtylamide as substrate after 1 hrs by ... | Bioorg Med Chem 19: 5935-47 (2011) Article DOI: 10.1016/j.bmc.2011.08.064 BindingDB Entry DOI: 10.7270/Q2154HF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50354043 (CHEMBL1829586) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Kao Corporation Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human fibroblasts homogenates using glutaryl-Ala-Ala-Phe-4-methoxy-2-naphtylamide as substrate after 1 hrs by ... | Bioorg Med Chem 19: 5935-47 (2011) Article DOI: 10.1016/j.bmc.2011.08.064 BindingDB Entry DOI: 10.7270/Q2154HF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM15236 (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged Glyoxalase 1 expressed in Sf21-Baculovirus system | Bioorg Med Chem 16: 3969-75 (2008) Article DOI: 10.1016/j.bmc.2008.01.031 BindingDB Entry DOI: 10.7270/Q2Z037XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM15236 (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged glyoxalase 1 expressed in Escherichia coli BL21 assessed as formation of S-D-lactoylglutathione after 5 mi... | Bioorg Med Chem Lett 21: 4337-42 (2011) Article DOI: 10.1016/j.bmcl.2011.05.046 BindingDB Entry DOI: 10.7270/Q2WW7J38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50045936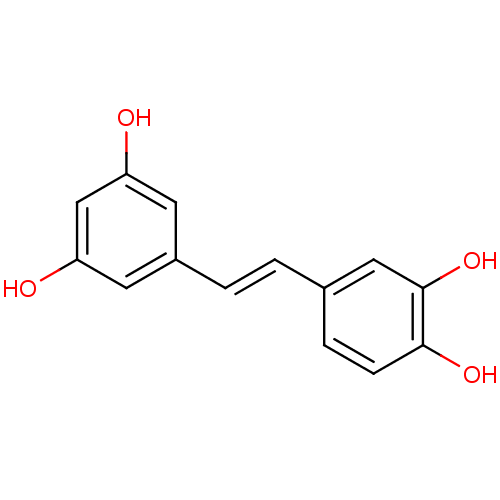 ((E)-4-(3,5-dihydroxystyryl)benzene-1,2-diol | (E)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GLO1 expressed in baculovirus infected sf21 cells assessed as reduction in S-D-lactoylglutathione formatio... | Bioorg Med Chem Lett 27: 1169-1174 (2017) Article DOI: 10.1016/j.bmcl.2017.01.070 BindingDB Entry DOI: 10.7270/Q29W0HQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50330795 (2-hydroxy-5-isopropyl-2,4,6-cycloheptatrien-1-one ...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Theoretical Medicine, Inc. Curated by ChEMBL | Assay Description Competitive inhibition of tyrosinase in human G-361 cells incubated for 10 mins measured for 2 hrs by MBTH-based spectrophotometry | Bioorg Med Chem 22: 6193-200 (2014) Article DOI: 10.1016/j.bmc.2014.08.027 BindingDB Entry DOI: 10.7270/Q2DV1MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50326997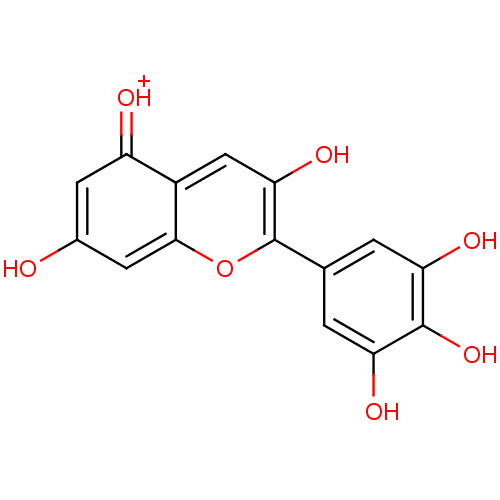 (CHEMBL590878 | Delphinidin) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition of human recombinant glyoxalase 1 assessed as S-D-lactoylglutathione after 15 mins by spectrophotometric analysis | Bioorg Med Chem 18: 7029-33 (2010) Article DOI: 10.1016/j.bmc.2010.08.012 BindingDB Entry DOI: 10.7270/Q2PC32K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50355547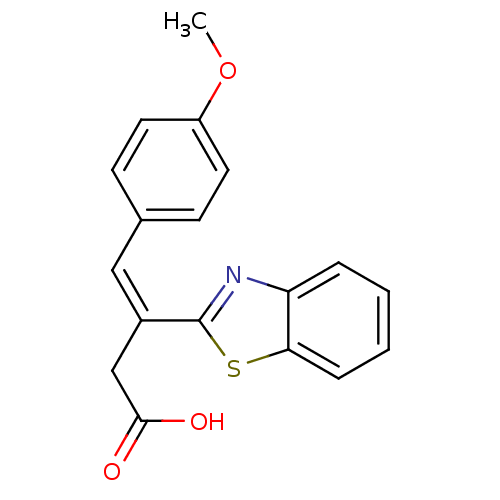 (CHEMBL1910548) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged glyoxalase 1 expressed in Escherichia coli BL21 assessed as formation of S-D-lactoylglutathione after 5 mi... | Bioorg Med Chem Lett 21: 4337-42 (2011) Article DOI: 10.1016/j.bmcl.2011.05.046 BindingDB Entry DOI: 10.7270/Q2WW7J38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569128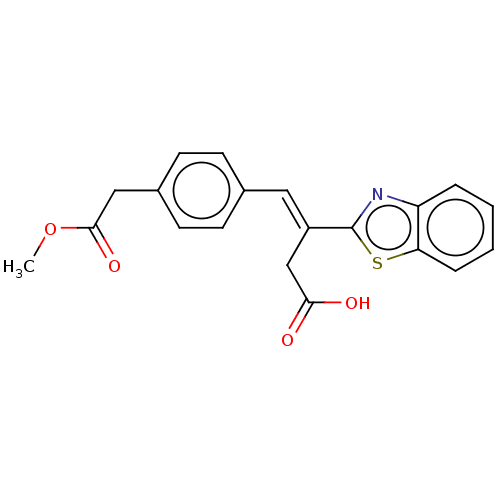 (CHEMBL4853249) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569120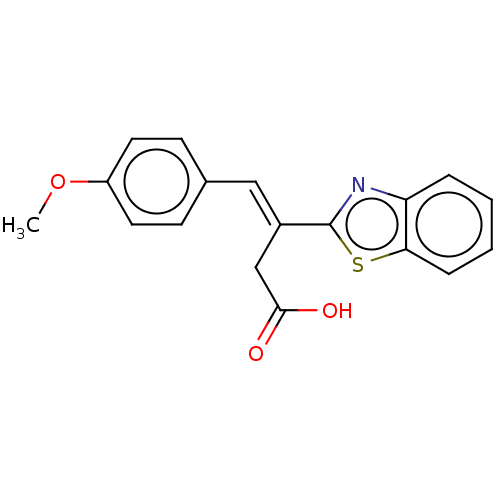 (CHEMBL4861582) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569133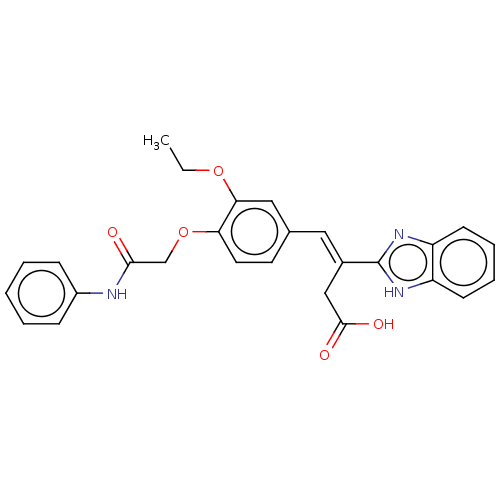 (CHEMBL4875229) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50192743 (3,4-Dihydroxybenzaldehyde | CHEBI:50205 | Protocat...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Theoretical Medicine, Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of mushroom tyrosinase | Bioorg Med Chem 22: 6193-200 (2014) Article DOI: 10.1016/j.bmc.2014.08.027 BindingDB Entry DOI: 10.7270/Q2DV1MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569134 (CHEMBL4863408) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569131 (CHEMBL4852940) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged Glyoxalase 1 expressed in Sf21-Baculovirus system | Bioorg Med Chem 16: 3969-75 (2008) Article DOI: 10.1016/j.bmc.2008.01.031 BindingDB Entry DOI: 10.7270/Q2Z037XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569129 (CHEMBL4851336) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569125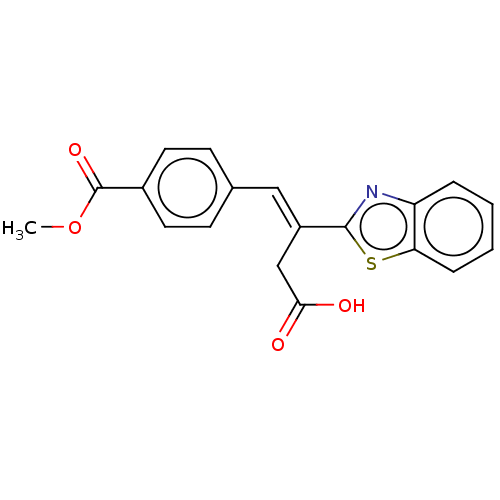 (CHEMBL4871220) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569130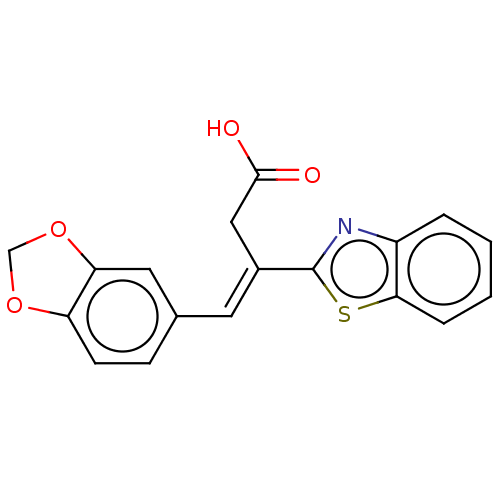 (CHEMBL1303934) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50145829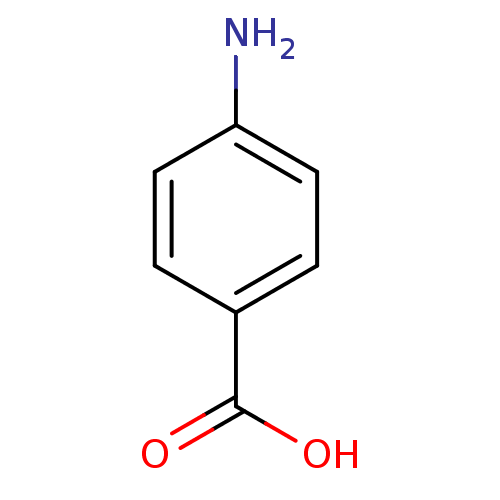 (4-Aminobenzoesaeure | 4-aminobenzoic acid | CHEMBL...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate assessed as dopachrome formation preincubated for 10 mins followed by protein additio... | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 BindingDB Entry DOI: 10.7270/Q27P92WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50145829 (4-Aminobenzoesaeure | 4-aminobenzoic acid | CHEMBL...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate assessed as dopachrome formation preincubated for 10 mins followed by protein additio... | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 BindingDB Entry DOI: 10.7270/Q27P92WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569127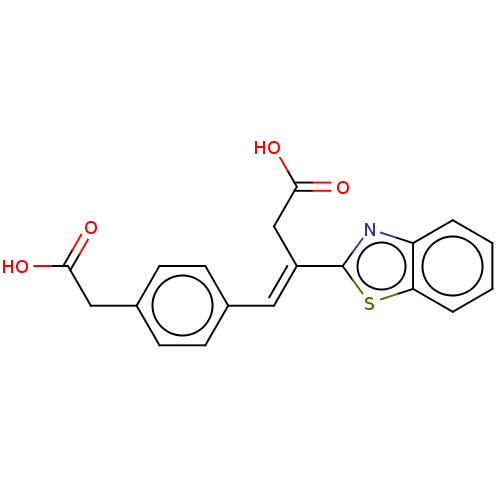 (CHEMBL4877301) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569121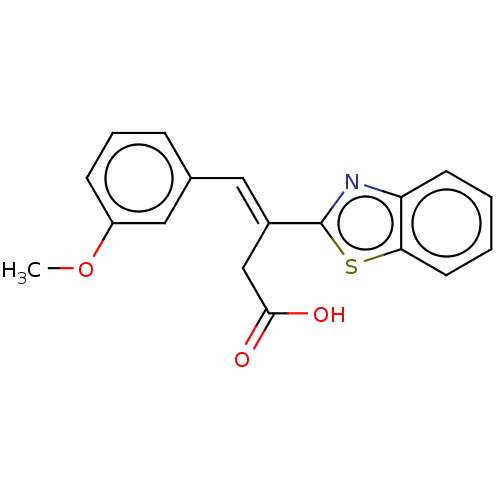 (CHEMBL1162436) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM54054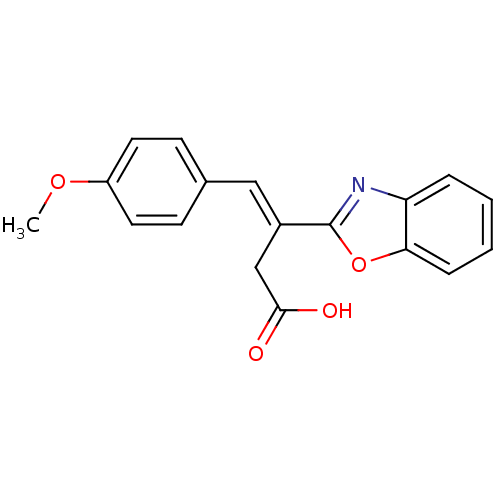 ((E)-3-(1,3-benzoxazol-2-yl)-4-(4-methoxyphenyl)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569132 (CHEMBL4848220) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569124 (CHEMBL4877073) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50195793 (CHEMBL123234) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase monophenolase activity using L-tyrosine as substrate measured every 60 s for 25 times | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 BindingDB Entry DOI: 10.7270/Q27P92WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50195793 (CHEMBL123234) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hinoki Shinyaku Co., Ltd Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase monophenolase activity using L-tyrosine as substrate measured every 60 s for 25 times | Bioorg Med Chem 24: 4509-4515 (2016) Article DOI: 10.1016/j.bmc.2016.07.060 BindingDB Entry DOI: 10.7270/Q27P92WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM7459 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged Glyoxalase 1 expressed in Sf21-Baculovirus system | Bioorg Med Chem 16: 3969-75 (2008) Article DOI: 10.1016/j.bmc.2008.01.031 BindingDB Entry DOI: 10.7270/Q2Z037XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569123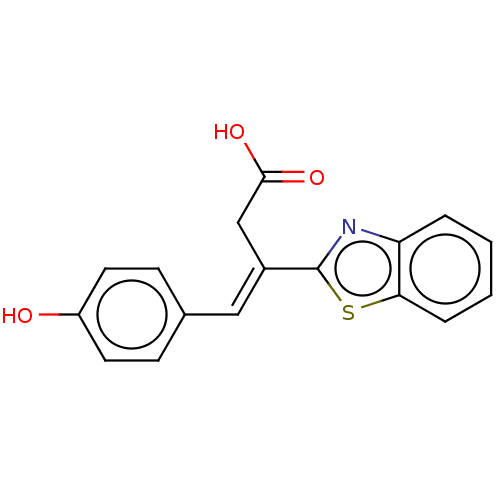 (CHEMBL4874418) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50192745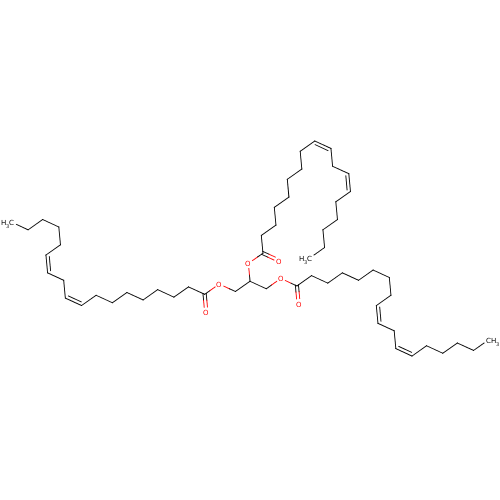 (CHEBI:75844 | CHEMBL3343985) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Theoretical Medicine, Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of mushroom tyrosinase | Bioorg Med Chem 22: 6193-200 (2014) Article DOI: 10.1016/j.bmc.2014.08.027 BindingDB Entry DOI: 10.7270/Q2DV1MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50330794 (2-Hydroxy-4-isopropyl-cyclohepta-2,4,6-trienone | ...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Theoretical Medicine, Inc. Curated by ChEMBL | Assay Description Competitive inhibition of tyrosinase in human G-361 cells incubated for 10 mins measured for 2 hrs by MBTH-based spectrophotometry | Bioorg Med Chem 22: 6193-200 (2014) Article DOI: 10.1016/j.bmc.2014.08.027 BindingDB Entry DOI: 10.7270/Q2DV1MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50192746 (CHEMBL3343986) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Theoretical Medicine, Inc. Curated by ChEMBL | Assay Description Inhibition of tyrosinase in human HMV-2 melanoma cells after 5 mins by spectrophotometry | Bioorg Med Chem 22: 6193-200 (2014) Article DOI: 10.1016/j.bmc.2014.08.027 BindingDB Entry DOI: 10.7270/Q2DV1MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569126 (CHEMBL4849720) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330793 (2-hydroxy-3-isopropyl-2,4,6-cycloheptatrien-1-one ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Theoretical Medicine, Inc. Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase after 15 mins | Bioorg Med Chem 18: 8112-8 (2010) Article DOI: 10.1016/j.bmc.2010.08.056 BindingDB Entry DOI: 10.7270/Q2JH3MF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330793 (2-hydroxy-3-isopropyl-2,4,6-cycloheptatrien-1-one ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Theoretical Medicine, Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of mushroom tyrosinase | Bioorg Med Chem 22: 6193-200 (2014) Article DOI: 10.1016/j.bmc.2014.08.027 BindingDB Entry DOI: 10.7270/Q2DV1MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50009001 (5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged Glyoxalase 1 expressed in Sf21-Baculovirus system | Bioorg Med Chem 16: 3969-75 (2008) Article DOI: 10.1016/j.bmc.2008.01.031 BindingDB Entry DOI: 10.7270/Q2Z037XF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50241503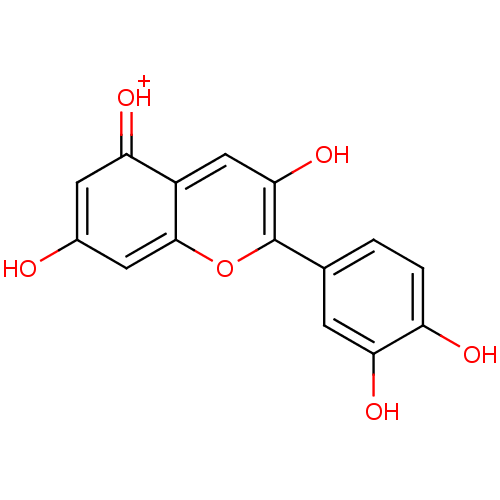 (2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-1-Benzopy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition of human recombinant glyoxalase 1 assessed as S-D-lactoylglutathione after 15 mins by spectrophotometric analysis | Bioorg Med Chem 18: 7029-33 (2010) Article DOI: 10.1016/j.bmc.2010.08.012 BindingDB Entry DOI: 10.7270/Q2PC32K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM50270346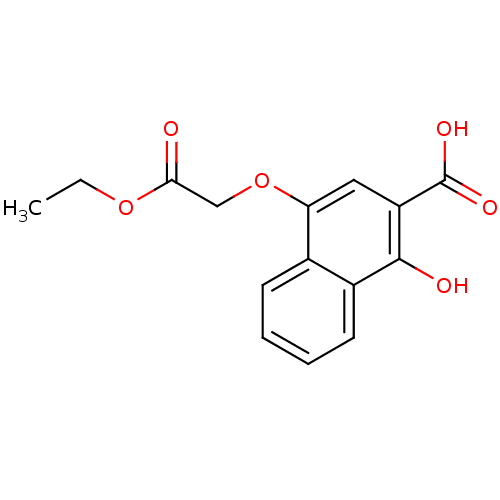 (4-(ethoxycarbonylmethoxy)-1-hydroxy-naphthalene-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition of human recombinant caspase 3 preincubated for 10 mins | Bioorg Med Chem 16: 4854-9 (2008) Article DOI: 10.1016/j.bmc.2008.03.046 BindingDB Entry DOI: 10.7270/Q25B0281 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 97 total ) | Next | Last >> |