

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]R-alpha-methylistamine from human histamine H3 receptor by cell-based assay | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274200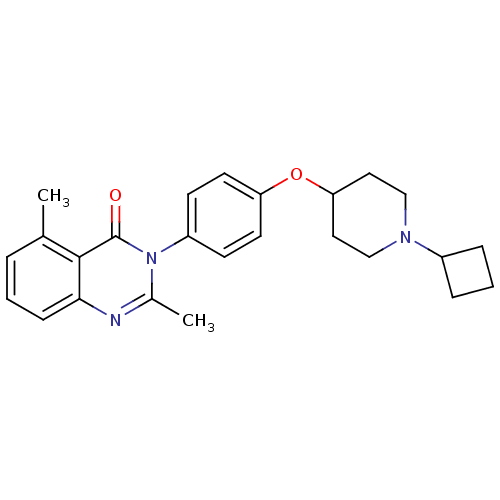 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50274200 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in HEK293 cells | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50274235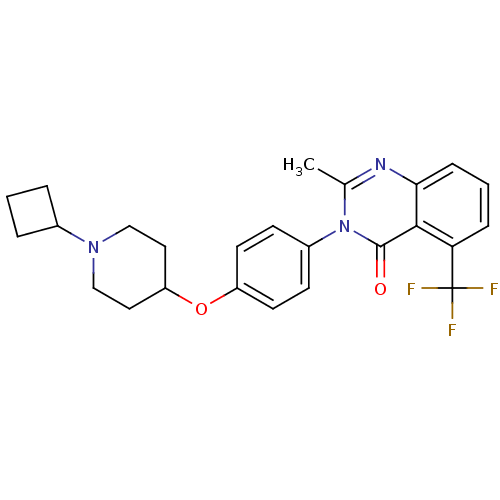 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in HEK293 cells | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]R-alpha-methylistamine from rat histamine H3 receptor by cell-based assay | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127501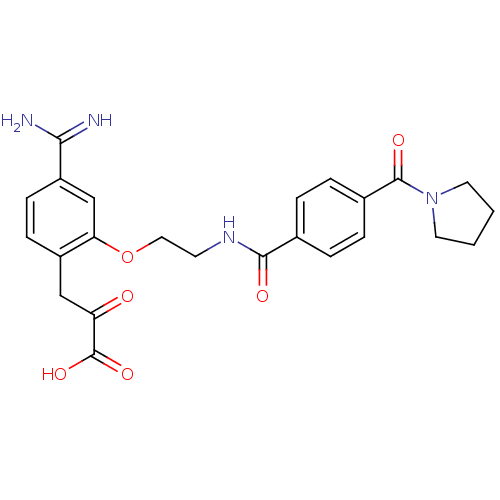 (3-(4-Carbamimidoyl-2-{2-[4-(pyrrolidine-1-carbonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274235 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50274235 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse recombinant histamine H3 receptor | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50274200 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse recombinant histamine H3 receptor | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50262939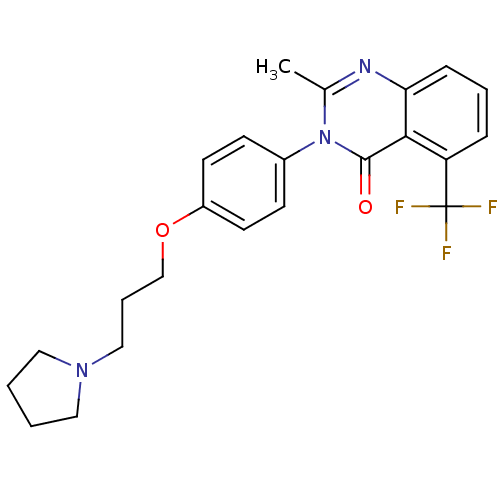 (2-Methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rhesus monkey histamine H3 receptor expressed in HEK293T cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]R-alpha-methylistamine from mouse histamine H3 receptor by cell-based assay | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50274692 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-5-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in HEK293 cells | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274692 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-5-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262939 (2-Methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50274692 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-5-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse recombinant histamine H3 receptor | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127492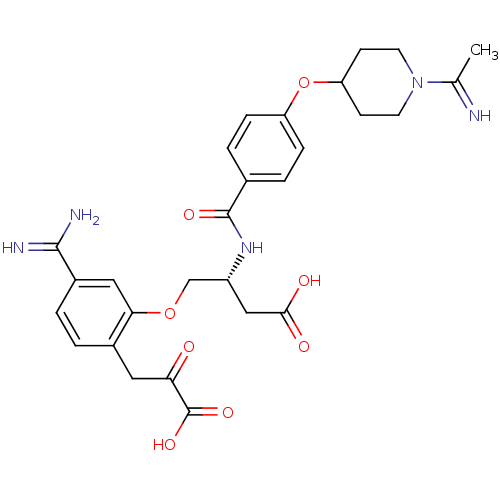 (4-[5-Carbamimidoyl-2-(2-carboxy-2-oxo-ethyl)-pheno...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127502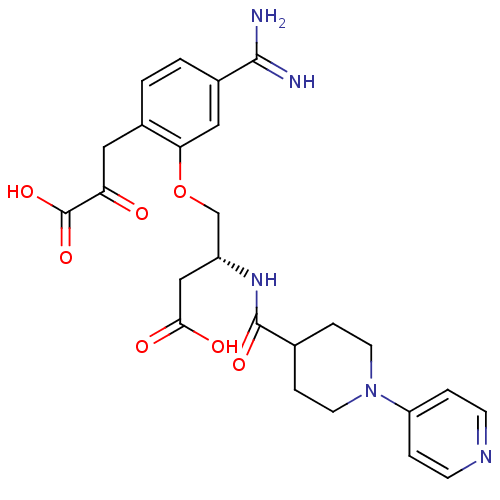 (4-[5-Carbamimidoyl-2-(2-carboxy-2-oxo-ethyl)-pheno...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127504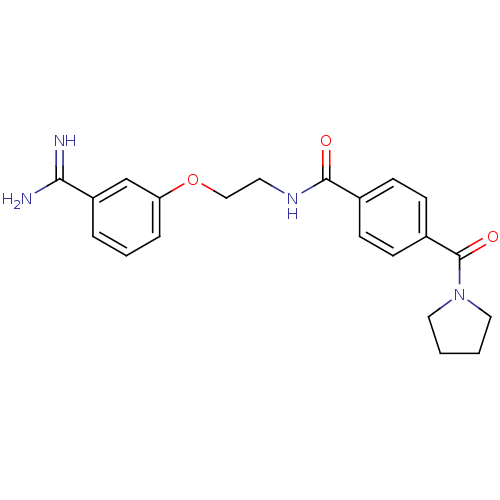 (CHEMBL55770 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127495 (3-[4-Carbamimidoyl-2-(2-{4-[1-(1-imino-ethyl)-pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127494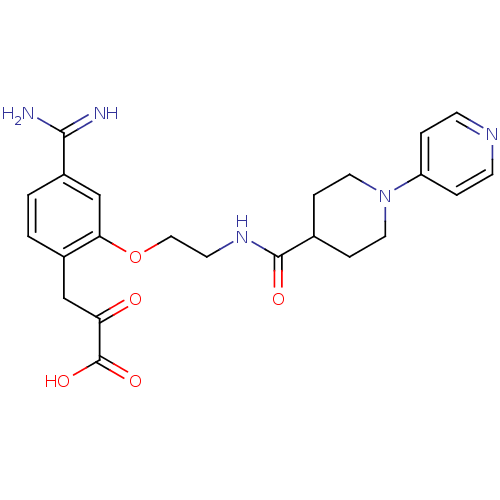 (3-(4-Carbamimidoyl-2-{2-[(3,4,5,6-tetrahydro-2H-[1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127498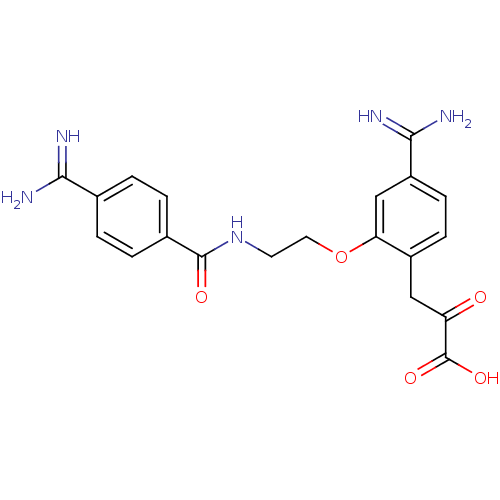 (3-{4-Carbamimidoyl-2-[2-(4-carbamimidoyl-benzoylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127503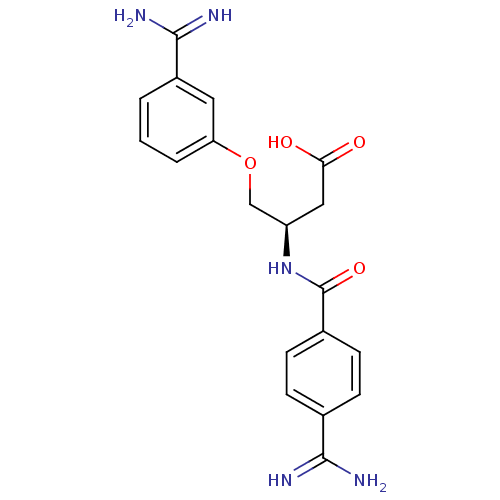 (3-(4-Carbamimidoyl-benzoylamino)-4-(3-carbamimidoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50262939 (2-Methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rat histamine H3 receptor expressed in HEK293 cells coexpressed with CRE-beta-lactamase | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127493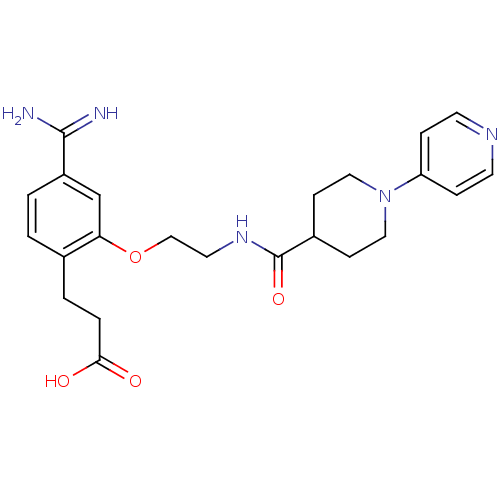 (3-(4-Carbamimidoyl-2-{2-[(3,4,5,6-tetrahydro-2H-[1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127496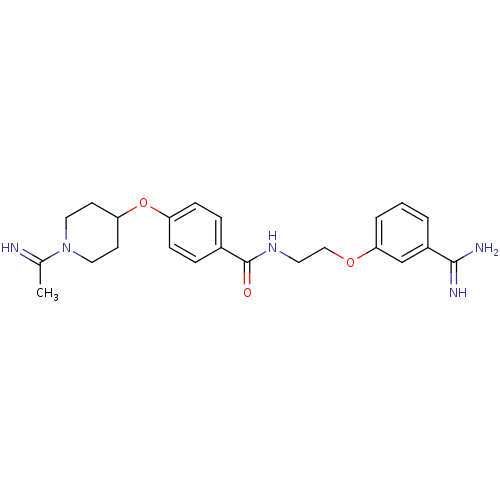 (CHEMBL51796 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127506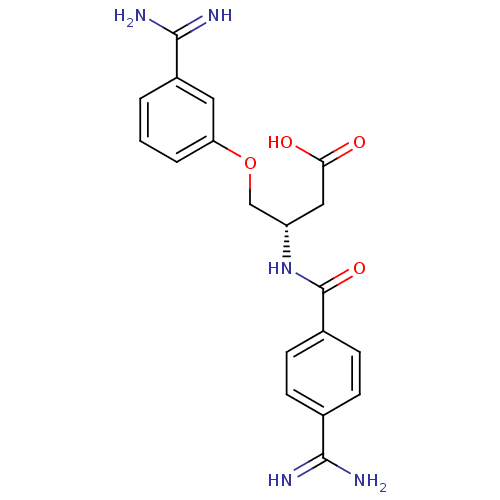 (3-(4-Carbamimidoyl-benzoylamino)-4-(3-carbamimidoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127497 (4-Carbamimidoyl-N-[2-(3-carbamimidoyl-phenoxy)-eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127500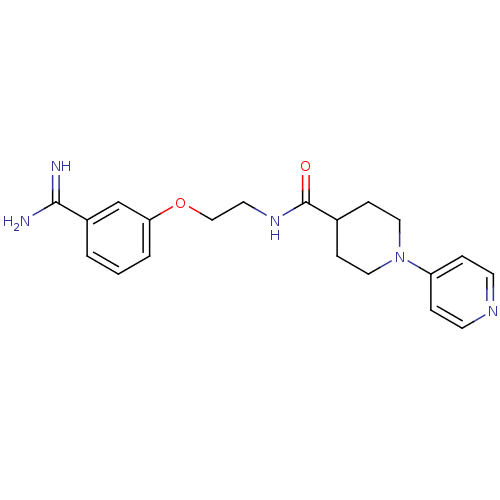 (3,4,5,6-Tetrahydro-2H-[1,4']bipyridinyl-4-carboxyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127499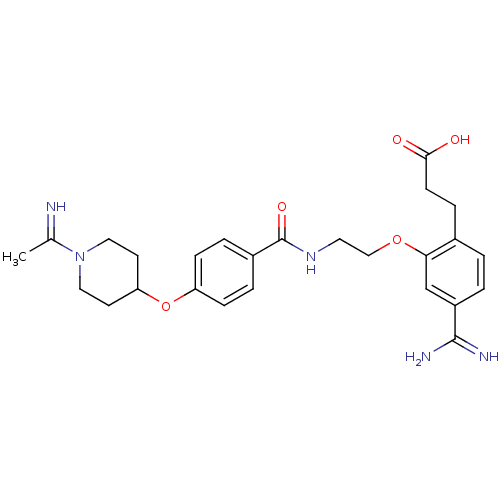 (3-[4-Carbamimidoyl-2-(2-{4-[1-(1-imino-ethyl)-pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127505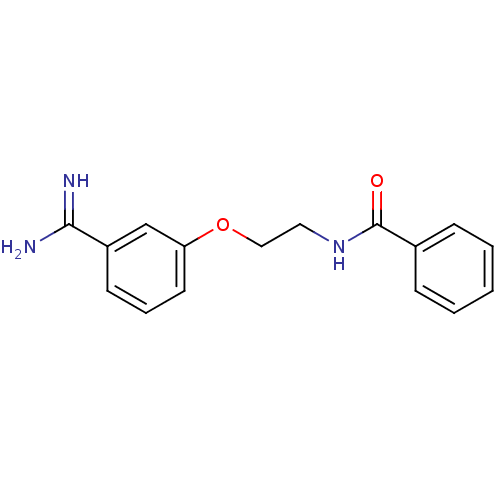 (CHEMBL55364 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127501 (3-(4-Carbamimidoyl-2-{2-[4-(pyrrolidine-1-carbonyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127498 (3-{4-Carbamimidoyl-2-[2-(4-carbamimidoyl-benzoylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127495 (3-[4-Carbamimidoyl-2-(2-{4-[1-(1-imino-ethyl)-pipe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127504 (CHEMBL55770 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127494 (3-(4-Carbamimidoyl-2-{2-[(3,4,5,6-tetrahydro-2H-[1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127496 (CHEMBL51796 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 4.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127492 (4-[5-Carbamimidoyl-2-(2-carboxy-2-oxo-ethyl)-pheno...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 5.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127502 (4-[5-Carbamimidoyl-2-(2-carboxy-2-oxo-ethyl)-pheno...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127497 (4-Carbamimidoyl-N-[2-(3-carbamimidoyl-phenoxy)-eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127505 (CHEMBL55364 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127500 (3,4,5,6-Tetrahydro-2H-[1,4']bipyridinyl-4-carboxyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127503 (3-(4-Carbamimidoyl-benzoylamino)-4-(3-carbamimidoy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127506 (3-(4-Carbamimidoyl-benzoylamino)-4-(3-carbamimidoy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127499 (3-[4-Carbamimidoyl-2-(2-{4-[1-(1-imino-ethyl)-pipe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127493 (3-(4-Carbamimidoyl-2-{2-[(3,4,5,6-tetrahydro-2H-[1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296179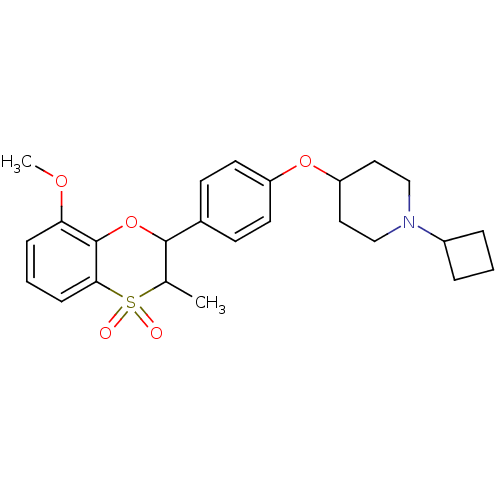 ((+/-)-1-Cyclobutyl-4-[4-(8-methoxy-3-methyl-4,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296178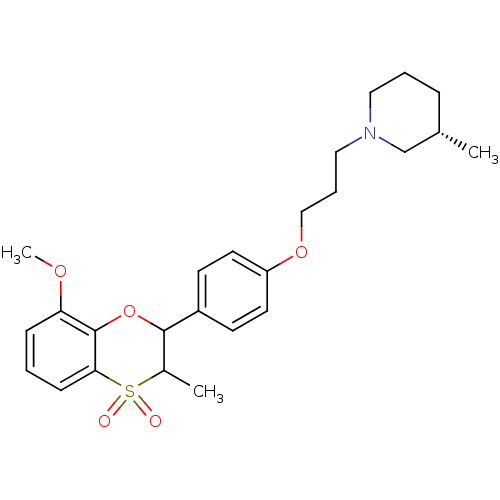 ((+/-)-(S)-1-{3-[4-(8-Methoxy-3-methyl-4,4-dioxo-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296176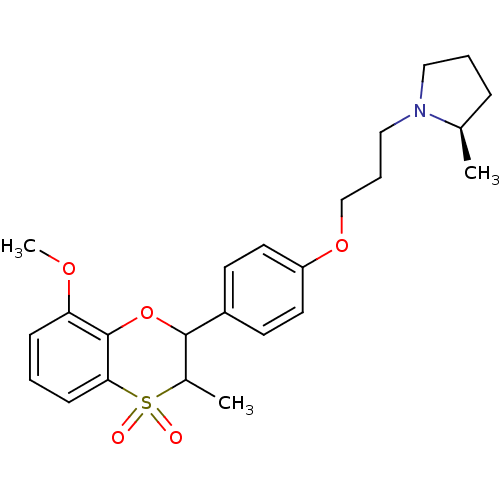 ((+/-)-(R)-1-{3-[4-(8-Methoxy-3-methyl-4,4-dioxo-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274200 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296174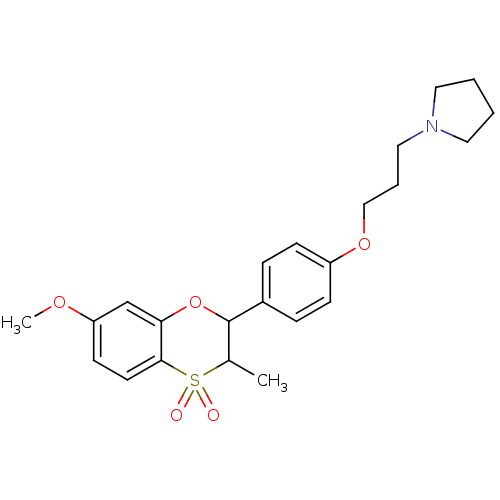 ((+/-)-1-{3-[4-(7-Methoxy-3-methyl-4,4-dioxo-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 682 total ) | Next | Last >> |