

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50444605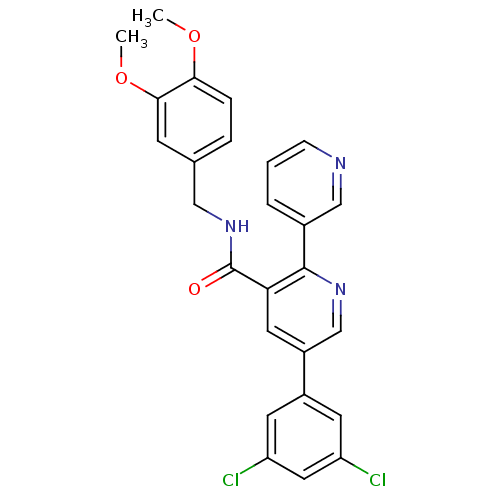 (CHEMBL3099899) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SB-674042 from human orexin-2 receptor after 60 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6620-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.045 BindingDB Entry DOI: 10.7270/Q2WH2RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50318697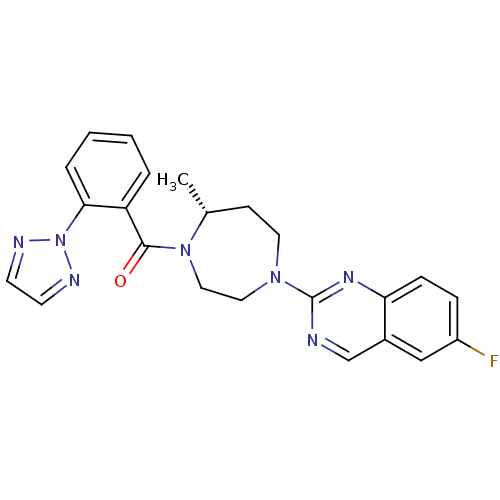 (6-Fluoro-2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000485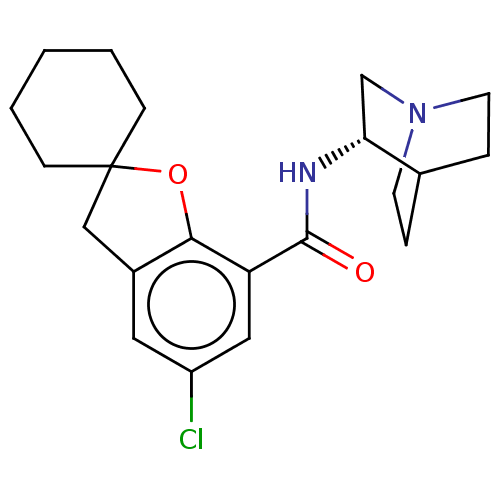 ((S)-2-(5-chlorospiro[2,3-dihydrobenzo[b]furan-2,1'...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50318698 (6,7-Fluoro-2-{(5R)-5-Methyl-4-[2-(2H-1,2,3-triazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50318699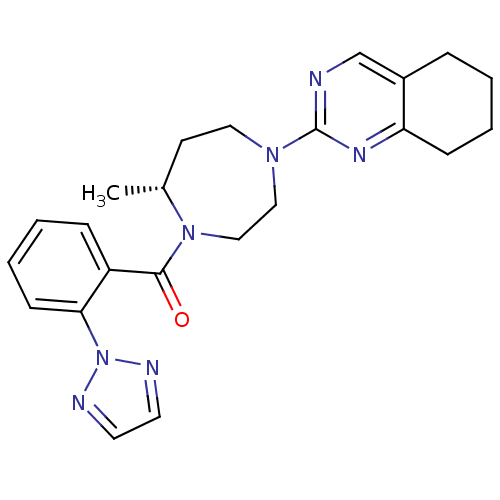 (2-{(5R)-5-Methyl-4-[2-(2H-1,2,3-triazol-2-yl)benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50028040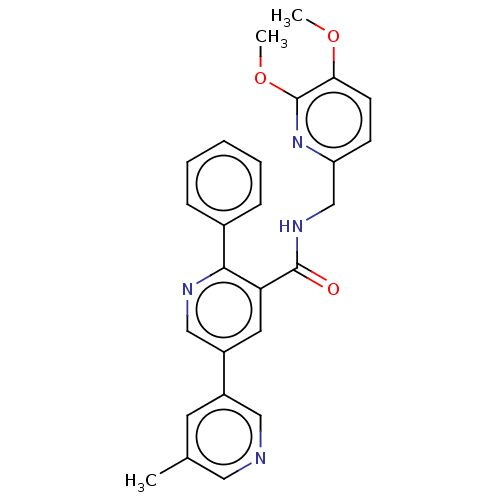 (CHEMBL3338846) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-([3H]-1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... | Bioorg Med Chem Lett 24: 4884-90 (2014) Article DOI: 10.1016/j.bmcl.2014.08.041 BindingDB Entry DOI: 10.7270/Q2GX4D4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50028045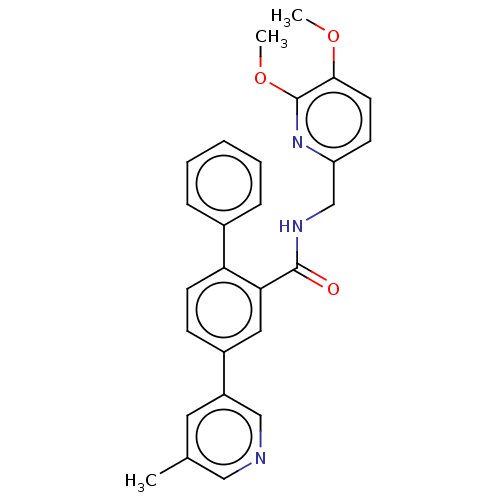 (CHEMBL3338852) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-([3H]-1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... | Bioorg Med Chem Lett 24: 4884-90 (2014) Article DOI: 10.1016/j.bmcl.2014.08.041 BindingDB Entry DOI: 10.7270/Q2GX4D4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17759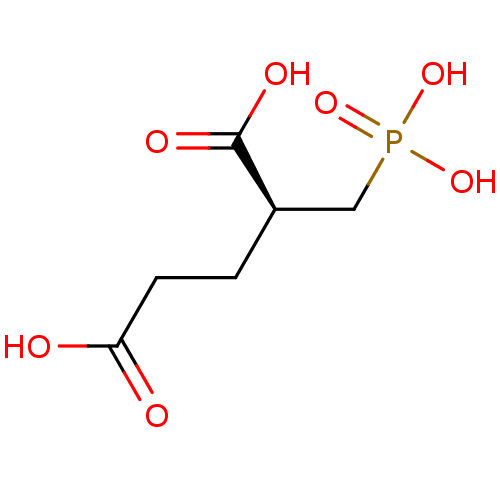 ((2S)-2-(phosphonomethyl)pentanedioic acid | (S)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of GCPII (unknown origin) NAALADase activity | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128044 BindingDB Entry DOI: 10.7270/Q2SQ9459 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50318701 (CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50318695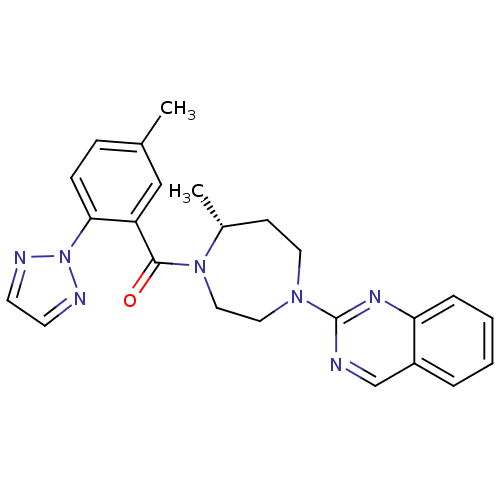 (2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-triazol-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50318695 (2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-triazol-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(biphenyl-2-yl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX1R expressed in C... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50318695 (2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-triazol-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-cyclobutyl-5-methyl-N-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)ethyl)-2-(2H-1,2,3-triazol-2-yl)benzamide from human OX1R expre... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50318696 (2-{(5R)-5-Methyl-4-[2-(2H-1,2,3-triazol-2-yl)benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM106968 (US8592457, 1-5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-([3H]-1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... | Bioorg Med Chem Lett 24: 4884-90 (2014) Article DOI: 10.1016/j.bmcl.2014.08.041 BindingDB Entry DOI: 10.7270/Q2GX4D4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50318701 (CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-cyclobutyl-5-methyl-N-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)ethyl)-2-(2H-1,2,3-triazol-2-yl)benzamide from human OX1R expre... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50318701 (CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(biphenyl-2-yl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX1R expressed in C... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50258741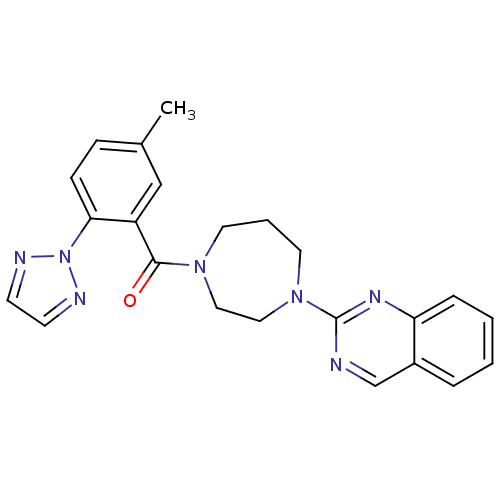 ((5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)(4-(quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50444606 (CHEMBL3099898) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SB-674042 from human orexin-2 receptor after 60 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6620-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.045 BindingDB Entry DOI: 10.7270/Q2WH2RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50444611 (CHEMBL3099893) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SB-674042 from human orexin-2 receptor after 60 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6620-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.045 BindingDB Entry DOI: 10.7270/Q2WH2RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50053947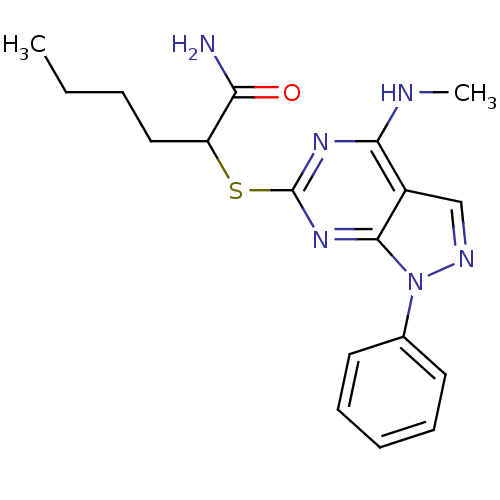 (2-(4-Methylamino-1-phenyl-1H-pyrazolo[3,4-d]pyrimi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.745 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Griffith University Curated by ChEMBL | Assay Description Displacement of [3H]-N6-PIA binding from A1 receptor in whole rat brain membranes | Bioorg Med Chem Lett 11: 191-3 (2001) BindingDB Entry DOI: 10.7270/Q23F4NW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50318699 (2-{(5R)-5-Methyl-4-[2-(2H-1,2,3-triazol-2-yl)benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(biphenyl-2-yl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX1R expressed in C... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50318699 (2-{(5R)-5-Methyl-4-[2-(2H-1,2,3-triazol-2-yl)benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-cyclobutyl-5-methyl-N-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)ethyl)-2-(2H-1,2,3-triazol-2-yl)benzamide from human OX1R expre... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50444610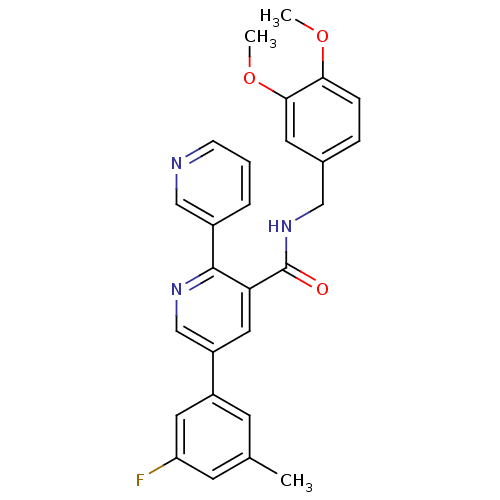 (CHEMBL3099894) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SB-674042 from human orexin-2 receptor after 60 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6620-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.045 BindingDB Entry DOI: 10.7270/Q2WH2RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000482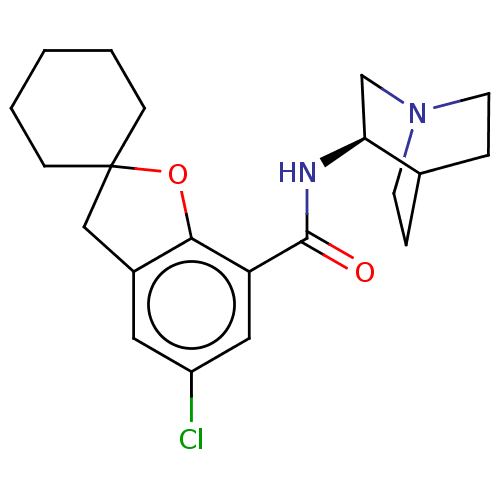 ((R)-2-(5-chlorospiro[2,3-dihydrobenzo[b]furan-2,1'...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM106970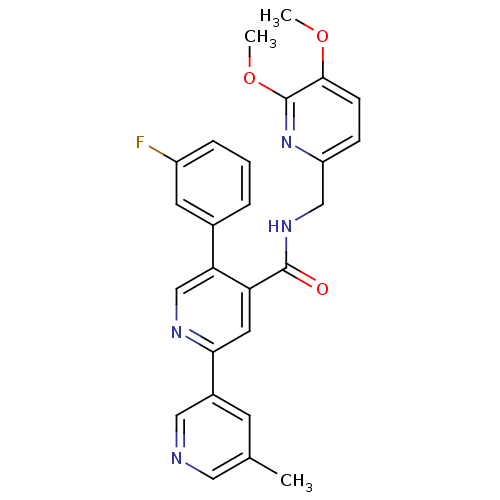 (US8592457, 1-16) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-([3H]-1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... | Bioorg Med Chem Lett 24: 4884-90 (2014) Article DOI: 10.1016/j.bmcl.2014.08.041 BindingDB Entry DOI: 10.7270/Q2GX4D4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase 2A (Homo sapiens (Human)) | BDBM200712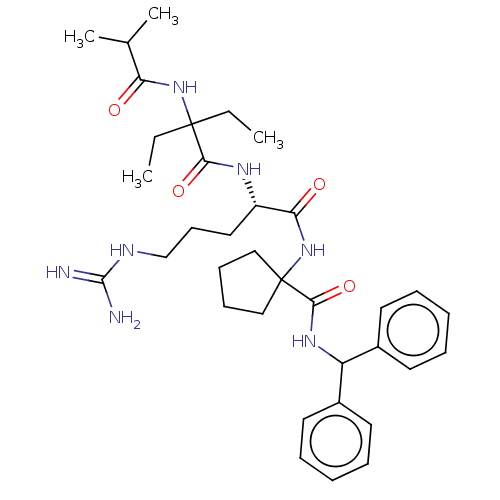 (US9233086, 10A) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of MLL1 binding to N-terminal His-tagged WRD5 23 deletion mutant (24 to 334 residues) (unknown origin) expressed in Escherichia coli Roset... | Bioorg Med Chem 26: 356-365 (2018) Article DOI: 10.1016/j.bmc.2017.11.045 BindingDB Entry DOI: 10.7270/Q2SQ92ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM333146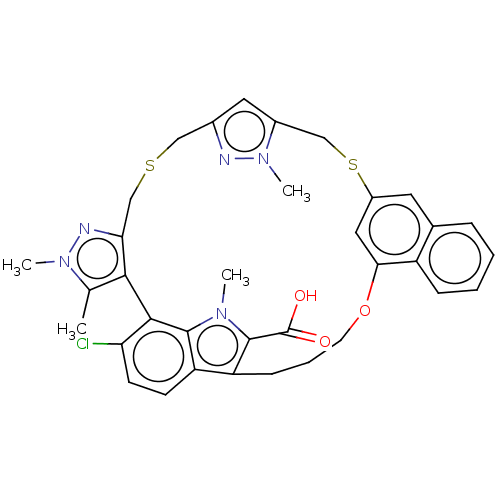 (Compound I | US10196404, Example 1 | US10196404, E...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FITC-labeled Bak BH3 peptide binding to GST-tagged MCL1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00682 BindingDB Entry DOI: 10.7270/Q2MC93VV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase 2A (Homo sapiens (Human)) | BDBM200723 (US9233086, 10L) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of MLL1 binding to N-terminal His-tagged WRD5 23 deletion mutant (24 to 334 residues) (unknown origin) expressed in Escherichia coli Roset... | Bioorg Med Chem 26: 356-365 (2018) Article DOI: 10.1016/j.bmc.2017.11.045 BindingDB Entry DOI: 10.7270/Q2SQ92ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase 2A (Homo sapiens (Human)) | BDBM200722 (US9233086, 10K) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of MLL1 binding to N-terminal His-tagged WRD5 23 deletion mutant (24 to 334 residues) (unknown origin) expressed in Escherichia coli Roset... | Bioorg Med Chem 26: 356-365 (2018) Article DOI: 10.1016/j.bmc.2017.11.045 BindingDB Entry DOI: 10.7270/Q2SQ92ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM106971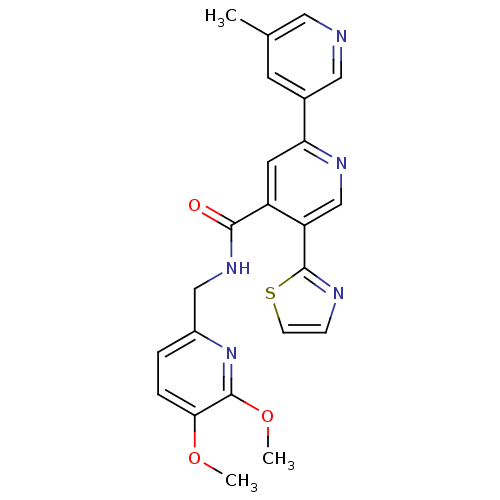 (US8592457, 7-5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-([3H]-1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... | Bioorg Med Chem Lett 24: 4884-90 (2014) Article DOI: 10.1016/j.bmcl.2014.08.041 BindingDB Entry DOI: 10.7270/Q2GX4D4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000480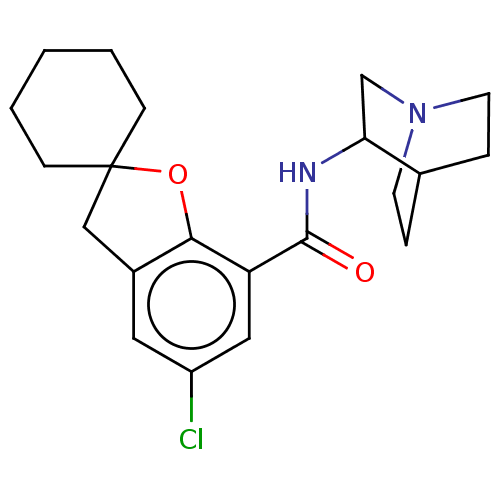 (2-(5-chlorospiro[2,3-dihydrobenzo[b]furan-2,1'-cyc...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000479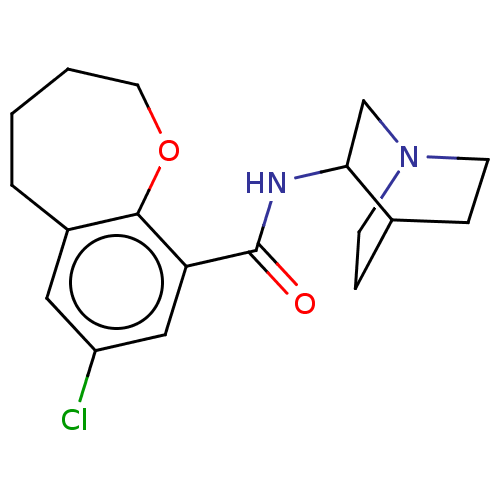 (7-Chloro-2,3,4,5-tetrahydro-benzo[b]oxepine-9-carb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50519614 (CHEMBL4439276) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | <1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to C-terminal MBP-fused human MCL1 (173 to 321 residues) expressed in Escherichia coli BL21(DE3)pLysS incubated for 2 hrs by fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00682 BindingDB Entry DOI: 10.7270/Q2MC93VV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50258741 ((5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)(4-(quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-cyclobutyl-5-methyl-N-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)ethyl)-2-(2H-1,2,3-triazol-2-yl)benzamide from human OX1R expre... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50258741 ((5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)(4-(quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(biphenyl-2-yl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX1R expressed in C... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50444607 (CHEMBL3099897) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SB-674042 from human orexin-2 receptor after 60 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6620-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.045 BindingDB Entry DOI: 10.7270/Q2WH2RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50028049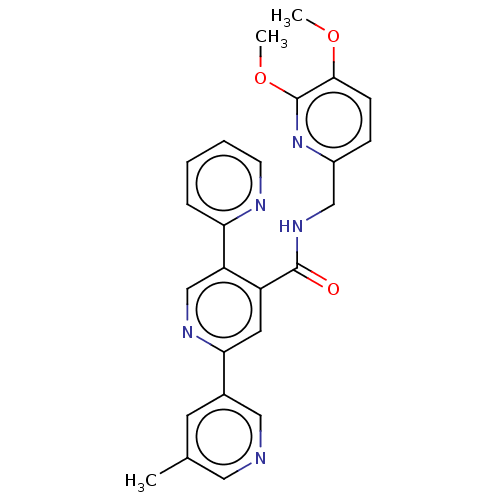 (CHEMBL3338857) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-([3H]-1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... | Bioorg Med Chem Lett 24: 4884-90 (2014) Article DOI: 10.1016/j.bmcl.2014.08.041 BindingDB Entry DOI: 10.7270/Q2GX4D4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50028042 (CHEMBL3338849) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-([3H]-1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... | Bioorg Med Chem Lett 24: 4884-90 (2014) Article DOI: 10.1016/j.bmcl.2014.08.041 BindingDB Entry DOI: 10.7270/Q2GX4D4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50028048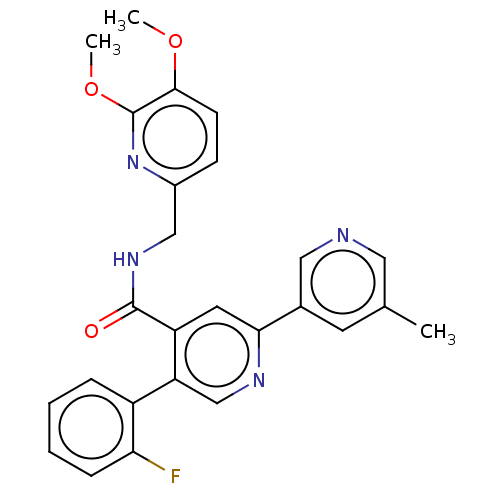 (CHEMBL3338855) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-([3H]-1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... | Bioorg Med Chem Lett 24: 4884-90 (2014) Article DOI: 10.1016/j.bmcl.2014.08.041 BindingDB Entry DOI: 10.7270/Q2GX4D4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000492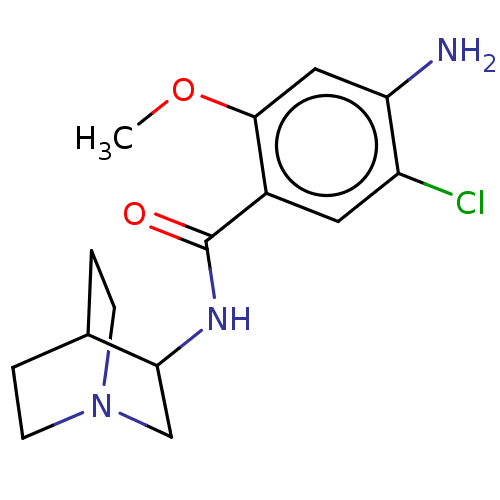 ((zacopride)4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000495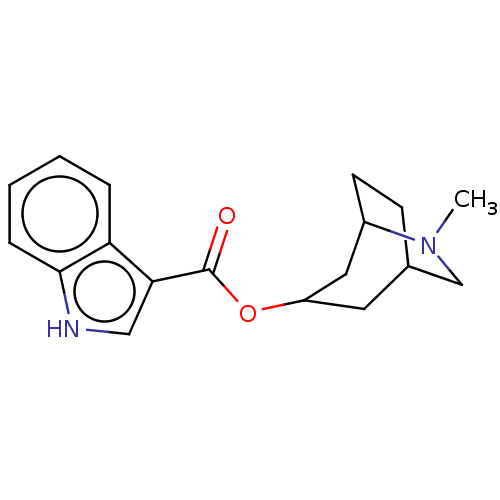 ((ICS 205-930)1H-Indole-3-carboxylic acid 6-methyl-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000483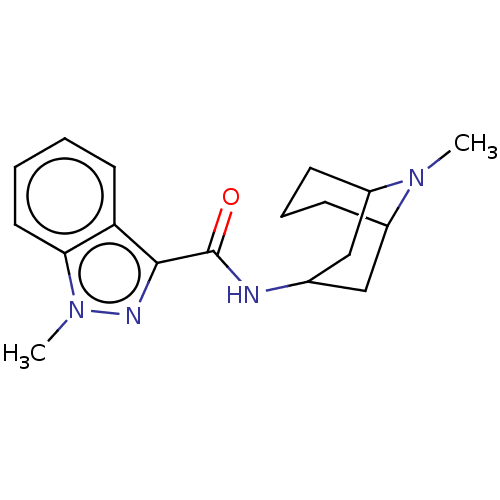 ((BRL 43694)1-Methyl-1H-indazole-3-carboxylic acid ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50318697 (6-Fluoro-2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(biphenyl-2-yl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX1R expressed in C... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50318698 (6,7-Fluoro-2-{(5R)-5-Methyl-4-[2-(2H-1,2,3-triazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-cyclobutyl-5-methyl-N-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)ethyl)-2-(2H-1,2,3-triazol-2-yl)benzamide from human OX1R expre... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50318697 (6-Fluoro-2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-cyclobutyl-5-methyl-N-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)ethyl)-2-(2H-1,2,3-triazol-2-yl)benzamide from human OX1R expre... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50318698 (6,7-Fluoro-2-{(5R)-5-Methyl-4-[2-(2H-1,2,3-triazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(biphenyl-2-yl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX1R expressed in C... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50106836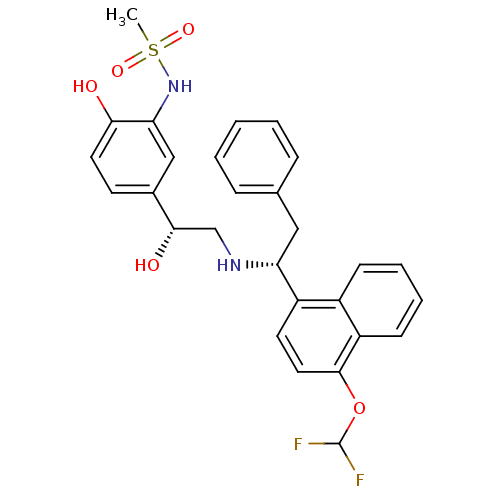 (CHEMBL105758 | N-(5-{(R)-2-[(R)-1-(4-Difluorometho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to CHO cells expressing the cloned human beta-3 adrenergic receptor in the presence of [125I]-iodocyanopindolol | Bioorg Med Chem Lett 11: 3041-4 (2001) BindingDB Entry DOI: 10.7270/Q2794402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000494 (7-Bromo-2,3,4,5-tetrahydro-benzo[b]oxepine-9-carbo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50300041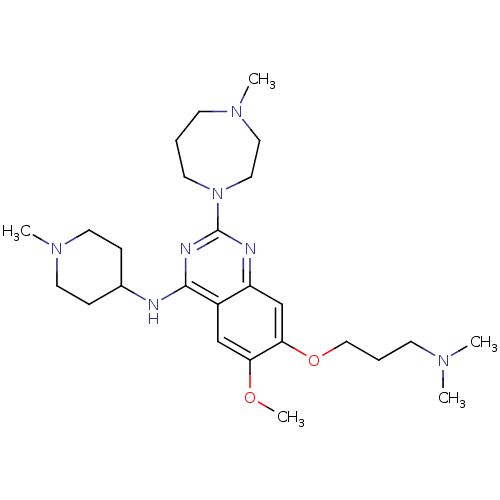 (7-(3-(dimethylamino)propoxy)-6-methoxy-2-(4-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of G9a (unknown origin) using histone H3 (1 to 25 residues) as substrate preincubated for 2 mins followed by substrate addition measured f... | Bioorg Med Chem 24: 6102-6108 (2016) Article DOI: 10.1016/j.bmc.2016.09.071 BindingDB Entry DOI: 10.7270/Q29C70D7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50444618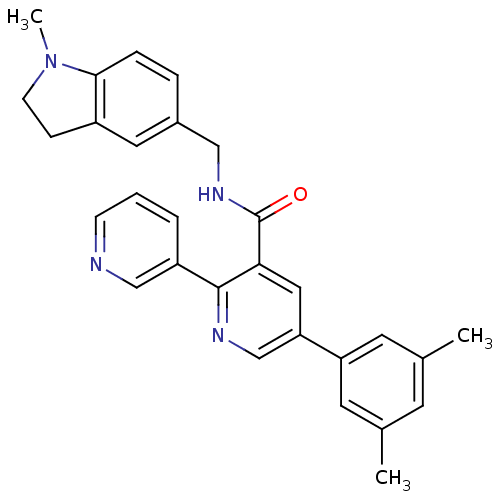 (CHEMBL3099886) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SB-674042 from human orexin-2 receptor after 60 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6620-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.045 BindingDB Entry DOI: 10.7270/Q2WH2RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3406 total ) | Next | Last >> |