

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM11002 ((1R)-N-methyl-N-(prop-2-yn-1-yl)-2,3-dihydro-1H-in...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 600 | -35.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically at 316 nm and 250 nm using kynuramine and benzylamine as substrates, respectively. ... | J Med Chem 47: 1760-6 (2004) Article DOI: 10.1021/jm0310885 BindingDB Entry DOI: 10.7270/Q2P8494M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM10989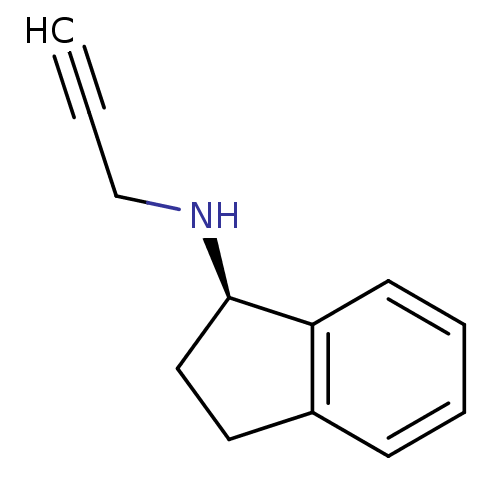 ((1R)-N-(prop-2-yn-1-yl)-2,3-dihydro-1H-inden-1-ami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 700 | -35.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically at 316 nm and 250 nm using kynuramine and benzylamine as substrates, respectively. ... | J Med Chem 47: 1760-6 (2004) Article DOI: 10.1021/jm0310885 BindingDB Entry DOI: 10.7270/Q2P8494M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50018671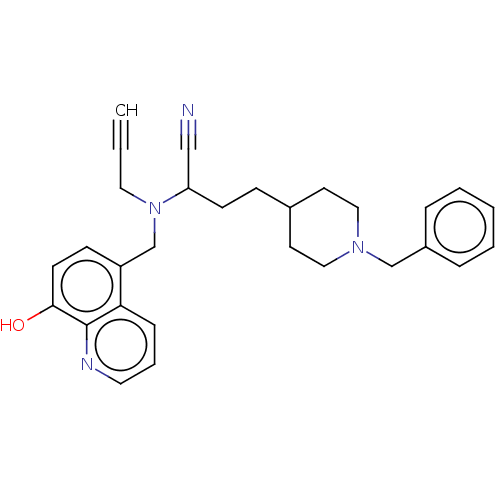 (CHEMBL3291019) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Reversible inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM10799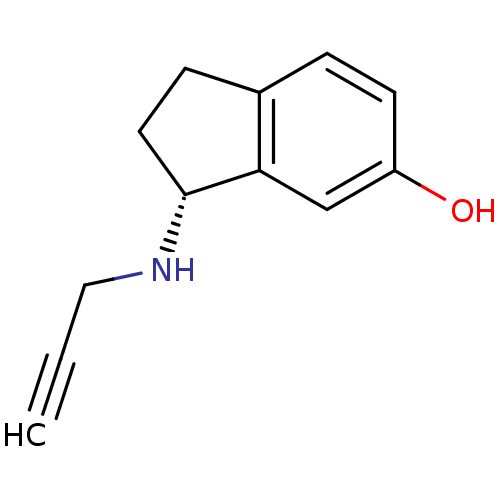 ((3R)-3-(prop-2-yn-1-ylamino)-2,3-dihydro-1H-inden-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.90E+3 | -32.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically at 316 nm and 250 nm using kynuramine and benzylamine as substrates, respectively. ... | J Med Chem 47: 1760-6 (2004) Article DOI: 10.1021/jm0310885 BindingDB Entry DOI: 10.7270/Q2P8494M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM11002 ((1R)-N-methyl-N-(prop-2-yn-1-yl)-2,3-dihydro-1H-in...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.90E+3 | -30.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically at 316 nm and 250 nm using kynuramine and benzylamine as substrates, respectively. ... | J Med Chem 47: 1760-6 (2004) Article DOI: 10.1021/jm0310885 BindingDB Entry DOI: 10.7270/Q2P8494M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM10989 ((1R)-N-(prop-2-yn-1-yl)-2,3-dihydro-1H-inden-1-ami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 9.70E+3 | -28.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically at 316 nm and 250 nm using kynuramine and benzylamine as substrates, respectively. ... | J Med Chem 47: 1760-6 (2004) Article DOI: 10.1021/jm0310885 BindingDB Entry DOI: 10.7270/Q2P8494M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM10799 ((3R)-3-(prop-2-yn-1-ylamino)-2,3-dihydro-1H-inden-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.70E+4 | -27.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically at 316 nm and 250 nm using kynuramine and benzylamine as substrates, respectively. ... | J Med Chem 47: 1760-6 (2004) Article DOI: 10.1021/jm0310885 BindingDB Entry DOI: 10.7270/Q2P8494M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM11000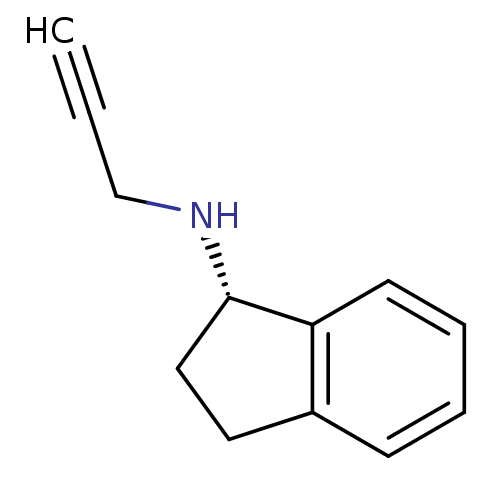 ((1S)-N-(prop-2-yn-1-yl)-2,3-dihydro-1H-inden-1-ami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.12E+5 | -22.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically at 316 nm and 250 nm using kynuramine and benzylamine as substrates, respectively. ... | J Med Chem 47: 1760-6 (2004) Article DOI: 10.1021/jm0310885 BindingDB Entry DOI: 10.7270/Q2P8494M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM11000 ((1S)-N-(prop-2-yn-1-yl)-2,3-dihydro-1H-inden-1-ami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.27E+5 | -22.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically at 316 nm and 250 nm using kynuramine and benzylamine as substrates, respectively. ... | J Med Chem 47: 1760-6 (2004) Article DOI: 10.1021/jm0310885 BindingDB Entry DOI: 10.7270/Q2P8494M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM10750 ((3R)-3-(prop-2-yn-1-ylamino)-2,3-dihydro-1H-inden-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 2.13E+5 | -21.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically at 316 nm and 250 nm using kynuramine and benzylamine as substrates, respectively. ... | J Med Chem 47: 1760-6 (2004) Article DOI: 10.1021/jm0310885 BindingDB Entry DOI: 10.7270/Q2P8494M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM10750 ((3R)-3-(prop-2-yn-1-ylamino)-2,3-dihydro-1H-inden-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 2.27E+5 | -20.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically at 316 nm and 250 nm using kynuramine and benzylamine as substrates, respectively. ... | J Med Chem 47: 1760-6 (2004) Article DOI: 10.1021/jm0310885 BindingDB Entry DOI: 10.7270/Q2P8494M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM10989 ((1R)-N-(prop-2-yn-1-yl)-2,3-dihydro-1H-inden-1-ami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description MAO-A and MAO-B activity was measured using radioactive substrates. The substrate for MAO-A was 5 HT and for MAO-B was PEA. When measuring the activi... | US Patent US9034303 (2015) BindingDB Entry DOI: 10.7270/Q20Z722N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-B in rat liver homogenate using [14C]-phenylethylamine as substrate preincubated for 30 mins followed by substrate addition measure... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM10804 ((3R)-3-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM36552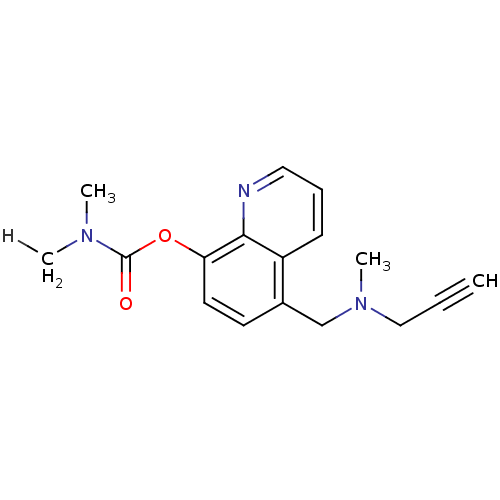 (Prochelators, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Weizmann Institute of Science | Assay Description Inhibition of MAO activity in rat brain homogenates. | ACS Chem Biol 5: 603-10 (2010) Article DOI: 10.1021/cb900264w BindingDB Entry DOI: 10.7270/Q2959FX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM10807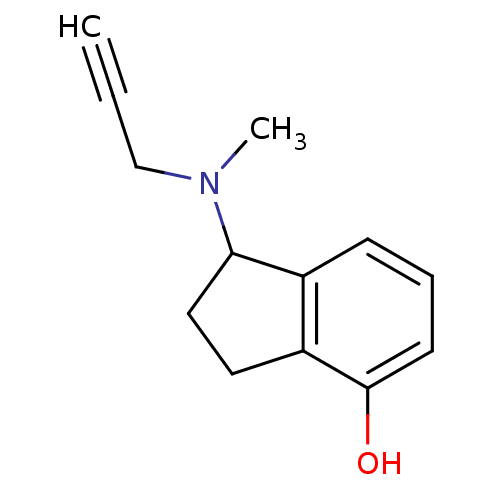 (1-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1H-ind...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10742 (1-amino-2,3-dihydro-1H-inden-4-yl N,N-dimethylcarb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM36553 (Prochelators, 3) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Weizmann Institute of Science | Assay Description Inhibition of MAO activity in rat brain homogenates. | ACS Chem Biol 5: 603-10 (2010) Article DOI: 10.1021/cb900264w BindingDB Entry DOI: 10.7270/Q2959FX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10796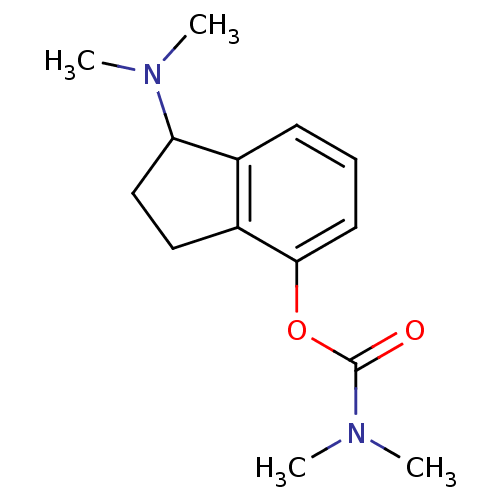 (1-(dimethylamino)-2,3-dihydro-1H-inden-4-yl N,N-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10742 (1-amino-2,3-dihydro-1H-inden-4-yl N,N-dimethylcarb...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM10803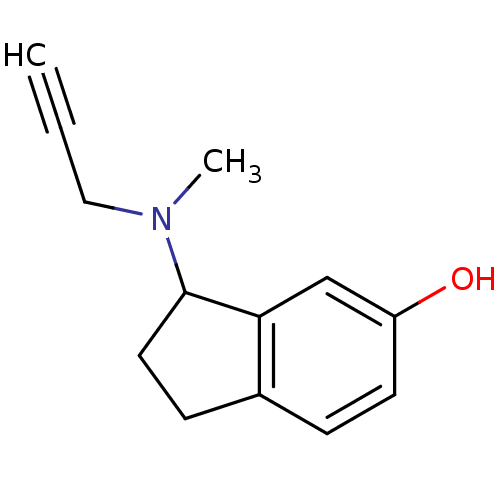 (3-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1H-ind...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10784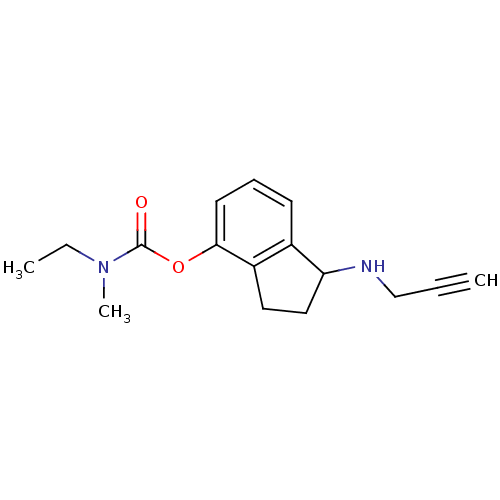 (1-(prop-2-yn-1-ylamino)-2,3-dihydro-1H-inden-4-yl ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver homogenate using [14C]-5HT as substrate preincubated for 30 mins followed by substrate addition measured after 20 mi... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM158502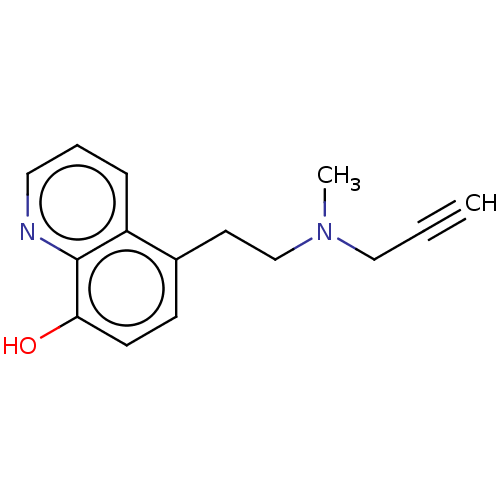 (US9034303, Compound 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description MAO-A and MAO-B activity was measured using radioactive substrates. The substrate for MAO-A was 5 HT and for MAO-B was PEA. When measuring the activi... | US Patent US9034303 (2015) BindingDB Entry DOI: 10.7270/Q20Z722N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM10804 ((3R)-3-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10743 (1-amino-2,3-dihydro-1H-inden-4-yl N-ethyl-N-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM10794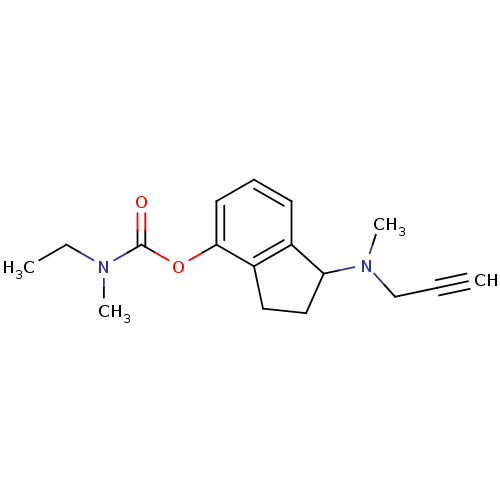 (1-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1H-ind...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM158501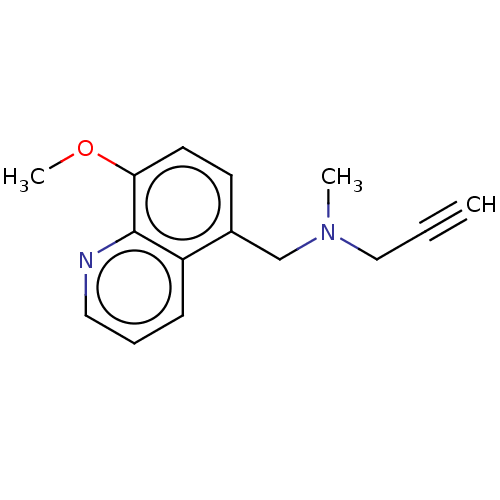 (US9034303, O-Methyl-M30) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description MAO-A and MAO-B activity was measured using radioactive substrates. The substrate for MAO-A was 5 HT and for MAO-B was PEA. When measuring the activi... | US Patent US9034303 (2015) BindingDB Entry DOI: 10.7270/Q20Z722N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver homogenate using [14C]-5HT as substrate preincubated for 30 mins followed by substrate addition measured after 20 mi... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10726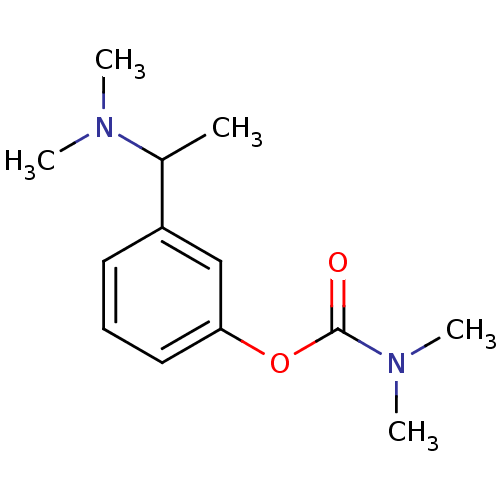 (3-[1-(dimethylamino)ethyl]phenyl N,N-dimethylcarba...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM10803 (3-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1H-ind...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM36551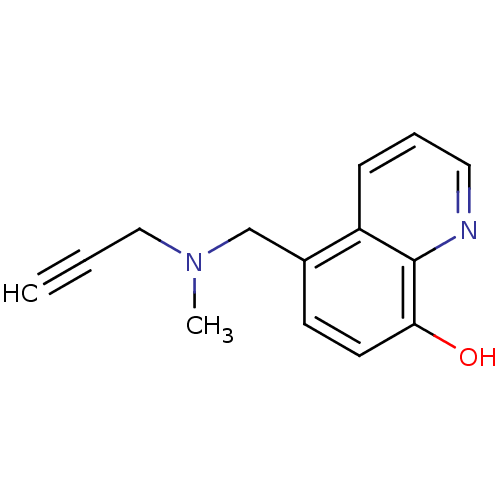 (Chelator, M30 | US9034303, M30) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Weizmann Institute of Science | Assay Description Inhibition of MAO activity in rat brain homogenates. | ACS Chem Biol 5: 603-10 (2010) Article DOI: 10.1021/cb900264w BindingDB Entry DOI: 10.7270/Q2959FX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM36551 (Chelator, M30 | US9034303, M30) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description MAO-A and MAO-B activity was measured using radioactive substrates. The substrate for MAO-A was 5 HT and for MAO-B was PEA. When measuring the activi... | US Patent US9034303 (2015) BindingDB Entry DOI: 10.7270/Q20Z722N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM10806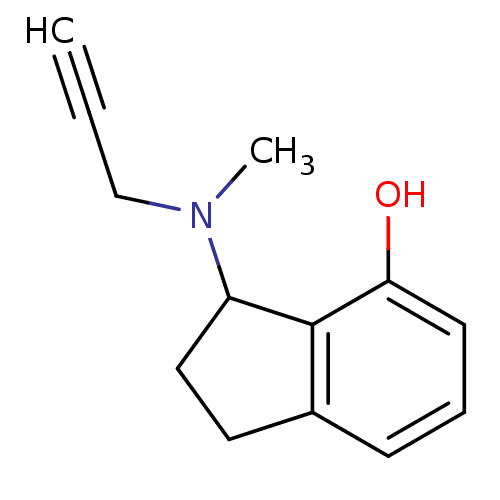 (3-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1H-ind...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10783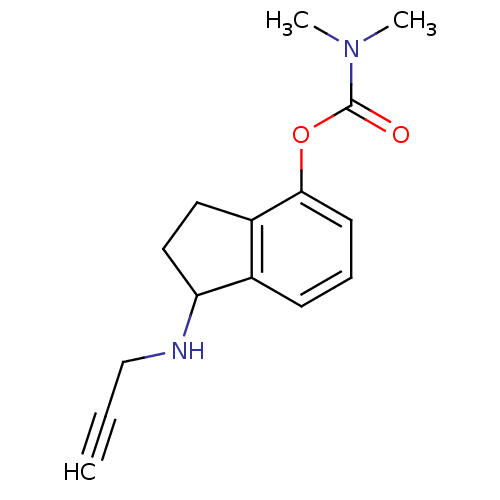 (1-(prop-2-yn-1-ylamino)-2,3-dihydro-1H-inden-4-yl ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10743 (1-amino-2,3-dihydro-1H-inden-4-yl N-ethyl-N-methyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM36551 (Chelator, M30 | US9034303, M30) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver homogenate using [14C]-5HT as substrate preincubated for 30 mins followed by substrate addition measured after 20 mi... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM36551 (Chelator, M30 | US9034303, M30) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description MAO-A and MAO-B activity was measured using radioactive substrates. The substrate for MAO-A was 5 HT and for MAO-B was PEA. When measuring the activi... | US Patent US9034303 (2015) BindingDB Entry DOI: 10.7270/Q20Z722N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM10806 (3-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1H-ind...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM10807 (1-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1H-ind...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM158502 (US9034303, Compound 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description MAO-A and MAO-B activity was measured using radioactive substrates. The substrate for MAO-A was 5 HT and for MAO-B was PEA. When measuring the activi... | US Patent US9034303 (2015) BindingDB Entry DOI: 10.7270/Q20Z722N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM10824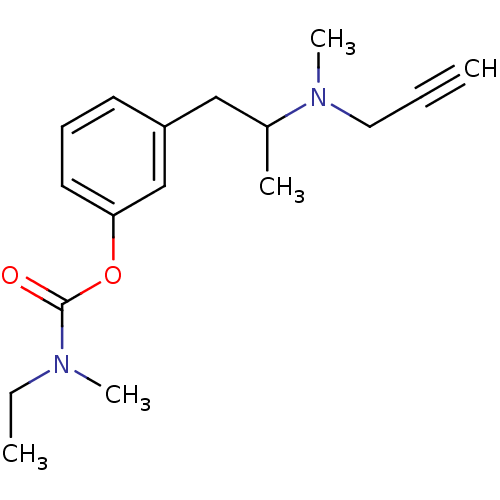 (3-{2-[methyl(prop-2-yn-1-yl)amino]propyl}phenyl N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM158503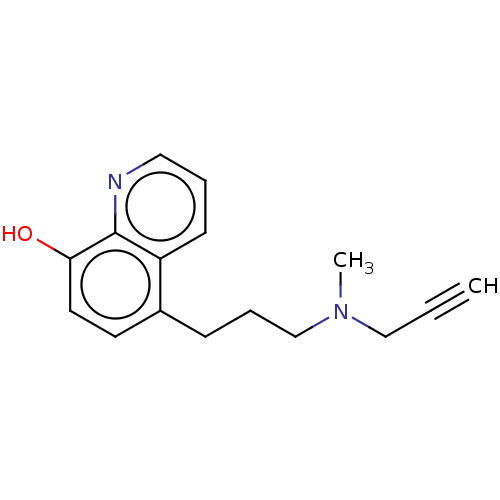 (US9034303, Compound 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description MAO-A and MAO-B activity was measured using radioactive substrates. The substrate for MAO-A was 5 HT and for MAO-B was PEA. When measuring the activi... | US Patent US9034303 (2015) BindingDB Entry DOI: 10.7270/Q20Z722N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM10824 (3-{2-[methyl(prop-2-yn-1-yl)amino]propyl}phenyl N-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM10823 (3-{2-[methyl(prop-2-yn-1-yl)amino]propyl}phenyl N,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10812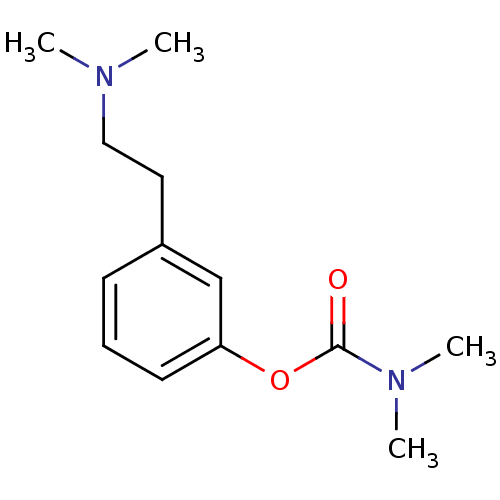 (3-[2-(dimethylamino)ethyl]phenyl N,N-dimethylcarba...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10832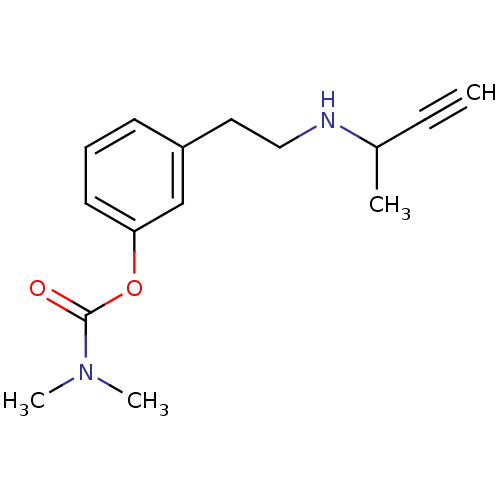 (3-[2-(but-3-yn-2-ylamino)ethyl]phenyl N,N-dimethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM10820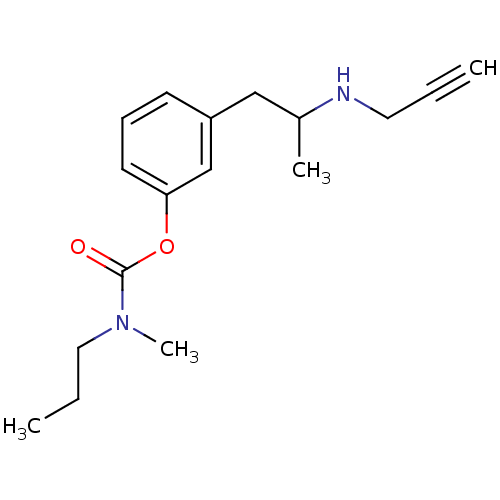 (3-[2-(prop-2-yn-1-ylamino)propyl]phenyl N-methyl-N...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM158503 (US9034303, Compound 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description MAO-A and MAO-B activity was measured using radioactive substrates. The substrate for MAO-A was 5 HT and for MAO-B was PEA. When measuring the activi... | US Patent US9034303 (2015) BindingDB Entry DOI: 10.7270/Q20Z722N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 365 total ) | Next | Last >> |