

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50332638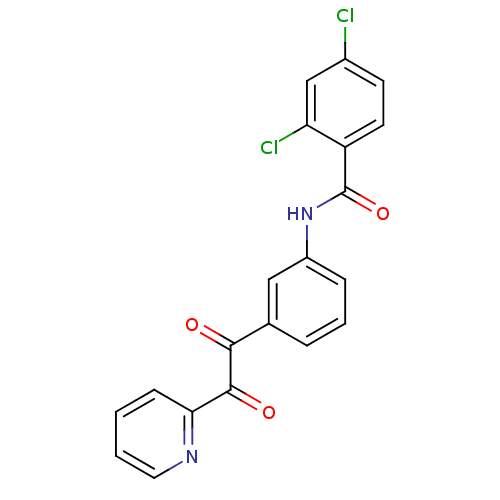 (2,4-dichloro-N-(3-(2-oxo-2-(pyridin-2-yl)acetyl)ph...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 279 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human intestinal CE1 using O-nitrophenyl acetate as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50332637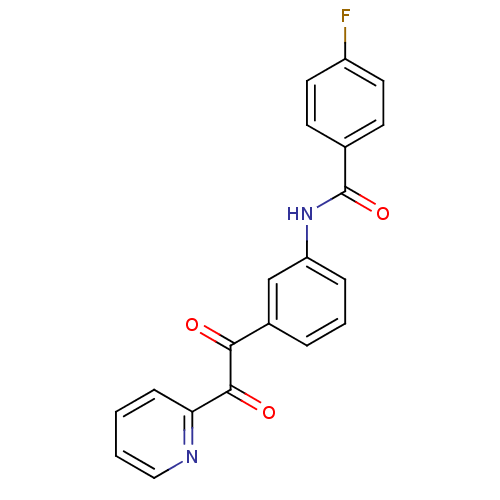 (4-fluoro-N-(3-(2-oxo-2-(pyridin-2-yl)acetyl)phenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 381 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human intestinal CE1 using O-nitrophenyl acetate as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50332640 (CHEMBL1629724 | N-(3-(2-oxo-2-(pyridin-2-yl)acetyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 437 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human intestinal CE1 using O-nitrophenyl acetate as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50332635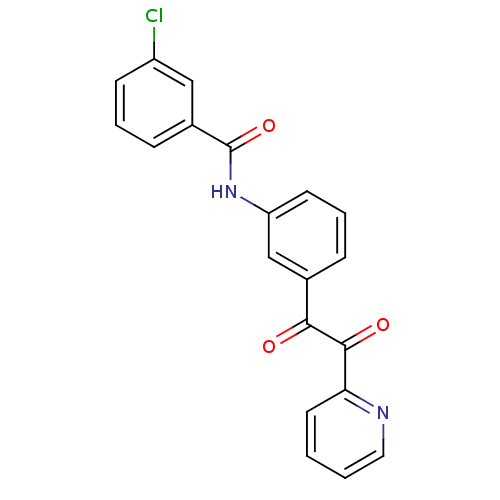 (3-chloro-N-(3-(2-oxo-2-(pyridin-2-yl)acetyl)phenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 756 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human intestinal CE1 using O-nitrophenyl acetate as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50332641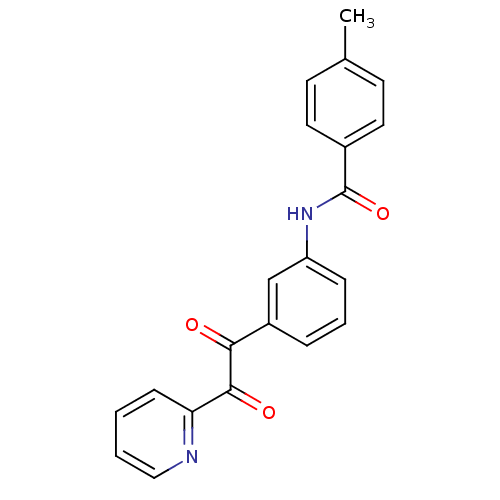 (4-methyl-N-(3-(2-oxo-2-(pyridin-2-yl)acetyl)phenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human intestinal CE1 using O-nitrophenyl acetate as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50332642 (3,4-difluoro-N-(3-(2-oxo-2-(pyridin-2-yl)acetyl)ph...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human intestinal CE1 using O-nitrophenyl acetate as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50332636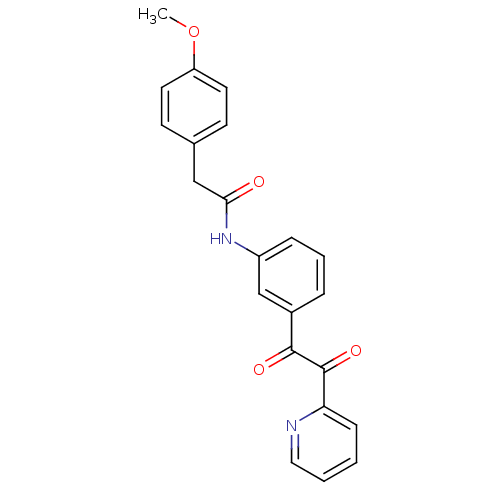 (2-(4-methoxyphenyl)-N-(3-(2-oxo-2-(pyridin-2-yl)ac...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human intestinal CE1 using O-nitrophenyl acetate as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50332638 (2,4-dichloro-N-(3-(2-oxo-2-(pyridin-2-yl)acetyl)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50332639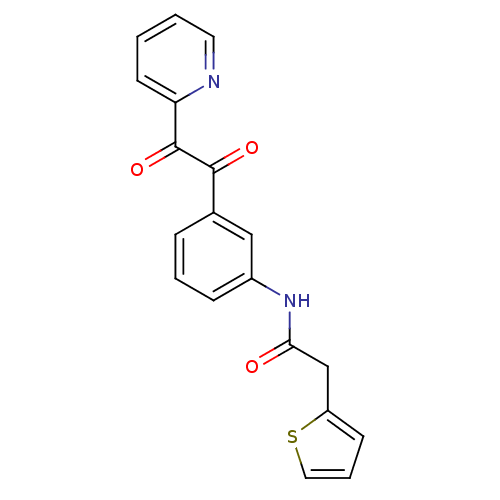 (CHEMBL1630809 | N-(3-(2-oxo-2-(pyridin-2-yl)acetyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50332640 (CHEMBL1629724 | N-(3-(2-oxo-2-(pyridin-2-yl)acetyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50332641 (4-methyl-N-(3-(2-oxo-2-(pyridin-2-yl)acetyl)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50332642 (3,4-difluoro-N-(3-(2-oxo-2-(pyridin-2-yl)acetyl)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50332643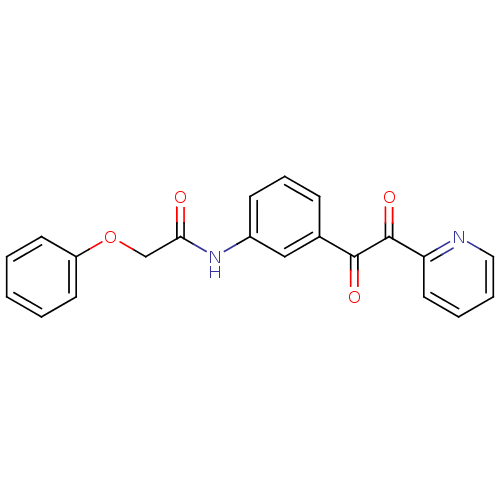 (CHEMBL1630812 | N-(3-(2-oxo-2-(pyridin-2-yl)acetyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50332644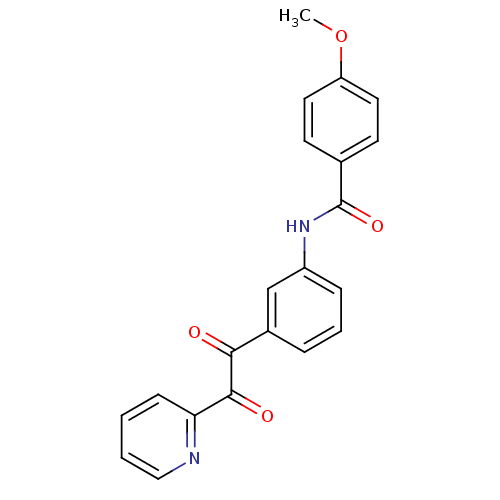 (4-methoxy-N-(3-(2-oxo-2-(pyridin-2-yl)acetyl)pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50332635 (3-chloro-N-(3-(2-oxo-2-(pyridin-2-yl)acetyl)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50332636 (2-(4-methoxyphenyl)-N-(3-(2-oxo-2-(pyridin-2-yl)ac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50332637 (4-fluoro-N-(3-(2-oxo-2-(pyridin-2-yl)acetyl)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50332638 (2,4-dichloro-N-(3-(2-oxo-2-(pyridin-2-yl)acetyl)ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50332639 (CHEMBL1630809 | N-(3-(2-oxo-2-(pyridin-2-yl)acetyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50332640 (CHEMBL1629724 | N-(3-(2-oxo-2-(pyridin-2-yl)acetyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50332641 (4-methyl-N-(3-(2-oxo-2-(pyridin-2-yl)acetyl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50332642 (3,4-difluoro-N-(3-(2-oxo-2-(pyridin-2-yl)acetyl)ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50332643 (CHEMBL1630812 | N-(3-(2-oxo-2-(pyridin-2-yl)acetyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50332644 (4-methoxy-N-(3-(2-oxo-2-(pyridin-2-yl)acetyl)pheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human intestinal CE1 using O-nitrophenyl acetate as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50332643 (CHEMBL1630812 | N-(3-(2-oxo-2-(pyridin-2-yl)acetyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human intestinal CE1 using O-nitrophenyl acetate as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50332639 (CHEMBL1630809 | N-(3-(2-oxo-2-(pyridin-2-yl)acetyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human intestinal CE1 using O-nitrophenyl acetate as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50332637 (4-fluoro-N-(3-(2-oxo-2-(pyridin-2-yl)acetyl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50332644 (4-methoxy-N-(3-(2-oxo-2-(pyridin-2-yl)acetyl)pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50332635 (3-chloro-N-(3-(2-oxo-2-(pyridin-2-yl)acetyl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50332636 (2-(4-methoxyphenyl)-N-(3-(2-oxo-2-(pyridin-2-yl)ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine as substrate by spectrophotometry | J Med Chem 53: 8709-15 (2010) Article DOI: 10.1021/jm101101q BindingDB Entry DOI: 10.7270/Q2RN3847 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50145037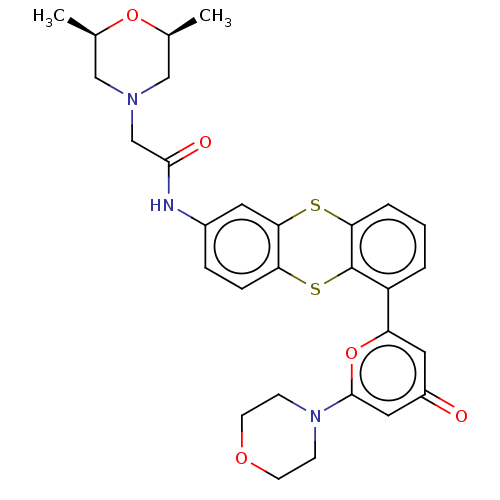 (CHEMBL3765783) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ATM (unknown origin) | J Med Chem 59: 559-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01092 BindingDB Entry DOI: 10.7270/Q2TM7D0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM92862 (US9284315, BEZ-235 | mTOR Inhibitor, BEZ235) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ATM (unknown origin) | J Med Chem 59: 559-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01092 BindingDB Entry DOI: 10.7270/Q2TM7D0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50208517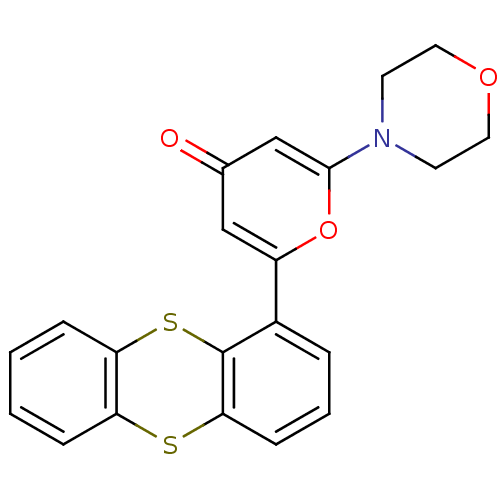 (2-morpholin-4-yl-6-thianthren-1-yl-pyran-4-one | C...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human ATM using p53 as substrate preincubated for 10 mins by ELISA | J Med Chem 59: 559-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01092 BindingDB Entry DOI: 10.7270/Q2TM7D0W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50145038 (CHEMBL2143829) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University School of Medicine Curated by ChEMBL | Assay Description Inhibition of flag-tagged ATM (unknown origin) using p53 as substrate by ELISA | J Med Chem 59: 559-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01092 BindingDB Entry DOI: 10.7270/Q2TM7D0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM50028142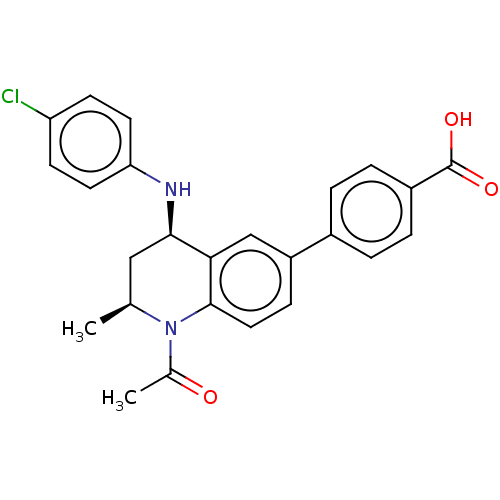 (CHEMBL2177300) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of recombinant human BRD2 bromodomain 2 (342 to 460 residues) using biotin labeled peptide substrate preincubated for 15 mins followed by ... | Bioorg Med Chem 26: 25-36 (2018) Article DOI: 10.1016/j.bmc.2017.10.042 BindingDB Entry DOI: 10.7270/Q2B56NB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM50365262 ((+)-JQ1 | (S)-JQ1 (1) | CHEMBL1957266 | JQ1 | US10...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of recombinant human BRD2 bromodomain 2 (342 to 460 residues) using biotin labeled peptide substrate preincubated for 15 mins followed by ... | Bioorg Med Chem 26: 25-36 (2018) Article DOI: 10.1016/j.bmc.2017.10.042 BindingDB Entry DOI: 10.7270/Q2B56NB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50208517 (2-morpholin-4-yl-6-thianthren-1-yl-pyran-4-one | C...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ATM in human U2OS cells assessed as inhibition of p53 phosphorylation at Ser15 residue | J Med Chem 59: 559-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01092 BindingDB Entry DOI: 10.7270/Q2TM7D0W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50208517 (2-morpholin-4-yl-6-thianthren-1-yl-pyran-4-one | C...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ATM kinase in human MCF7 cells after 1 hr by immunofluorescence assay | J Med Chem 59: 559-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01092 BindingDB Entry DOI: 10.7270/Q2TM7D0W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM50365262 ((+)-JQ1 | (S)-JQ1 (1) | CHEMBL1957266 | JQ1 | US10...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of recombinant human BRD2 bromodomain 1 (49 to 170 residues) using biotin labeled peptide substrate preincubated for 15 mins followed by s... | Bioorg Med Chem 26: 25-36 (2018) Article DOI: 10.1016/j.bmc.2017.10.042 BindingDB Entry DOI: 10.7270/Q2B56NB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50145038 (CHEMBL2143829) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ATM kinase in human MCF7 cells after 1 hr by immunofluorescence assay | J Med Chem 59: 559-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01092 BindingDB Entry DOI: 10.7270/Q2TM7D0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM50452557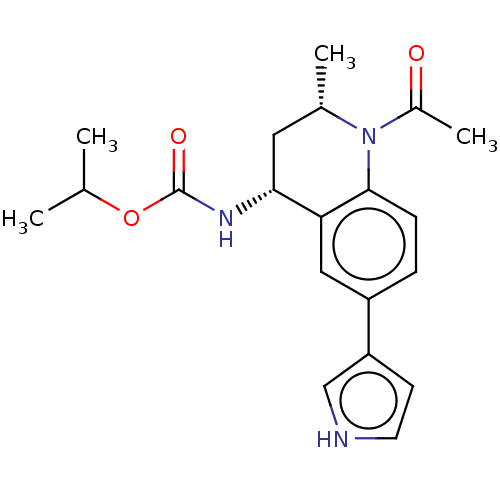 (CHEMBL4206677 | US11028051, Cmpd 55) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of recombinant human BRD2 bromodomain 2 (342 to 460 residues) using biotin labeled peptide substrate preincubated for 15 mins followed by ... | Bioorg Med Chem 26: 25-36 (2018) Article DOI: 10.1016/j.bmc.2017.10.042 BindingDB Entry DOI: 10.7270/Q2B56NB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM50452551 (CHEMBL4209110 | US11028051, Cmpd 50) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of recombinant human BRD2 bromodomain 2 (342 to 460 residues) using biotin labeled peptide substrate preincubated for 15 mins followed by ... | Bioorg Med Chem 26: 25-36 (2018) Article DOI: 10.1016/j.bmc.2017.10.042 BindingDB Entry DOI: 10.7270/Q2B56NB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM50028142 (CHEMBL2177300) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of recombinant human BRD2 bromodomain 1 (49 to 170 residues) using biotin labeled peptide substrate preincubated for 15 mins followed by s... | Bioorg Med Chem 26: 25-36 (2018) Article DOI: 10.1016/j.bmc.2017.10.042 BindingDB Entry DOI: 10.7270/Q2B56NB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50144958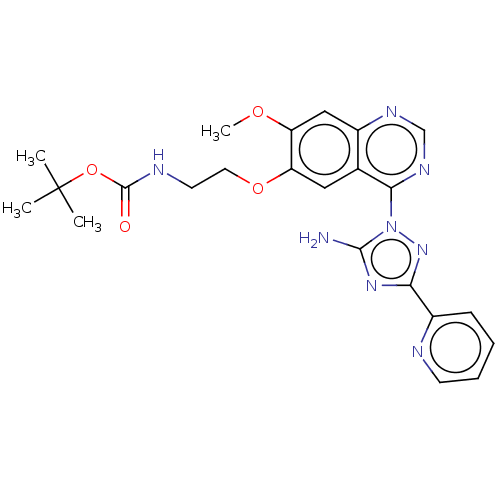 (CHEMBL3763939) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ATM kinase in human MCF7 cells after 1 hr by immunofluorescence assay | J Med Chem 59: 559-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01092 BindingDB Entry DOI: 10.7270/Q2TM7D0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50144957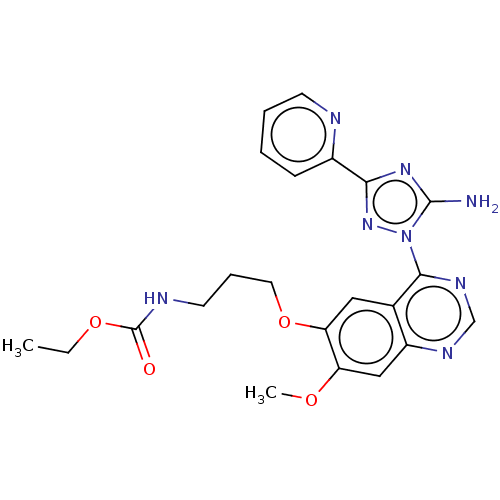 (CHEMBL3765373) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ATM kinase in human MCF7 cells after 1 hr by immunofluorescence assay | J Med Chem 59: 559-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01092 BindingDB Entry DOI: 10.7270/Q2TM7D0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50144959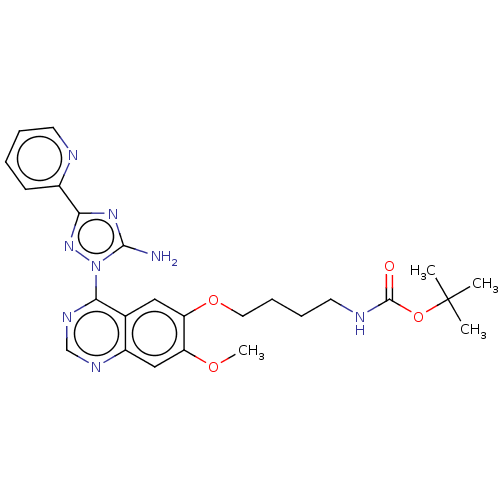 (CHEMBL3764231) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ATM kinase in human MCF7 cells after 1 hr by immunofluorescence assay | J Med Chem 59: 559-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01092 BindingDB Entry DOI: 10.7270/Q2TM7D0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM50452552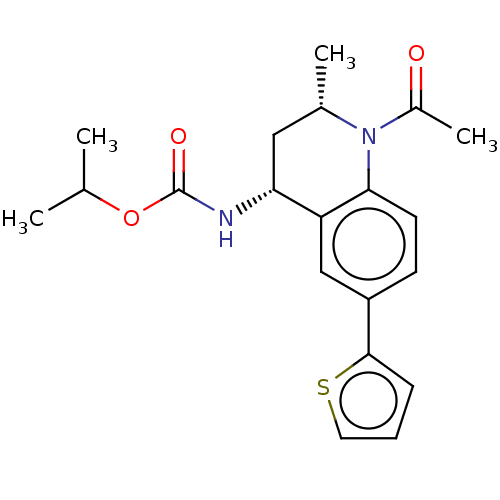 (CHEMBL4205876 | US11028051, Cmpd 52) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of recombinant human BRD2 bromodomain 2 (342 to 460 residues) using biotin labeled peptide substrate preincubated for 15 mins followed by ... | Bioorg Med Chem 26: 25-36 (2018) Article DOI: 10.1016/j.bmc.2017.10.042 BindingDB Entry DOI: 10.7270/Q2B56NB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50144960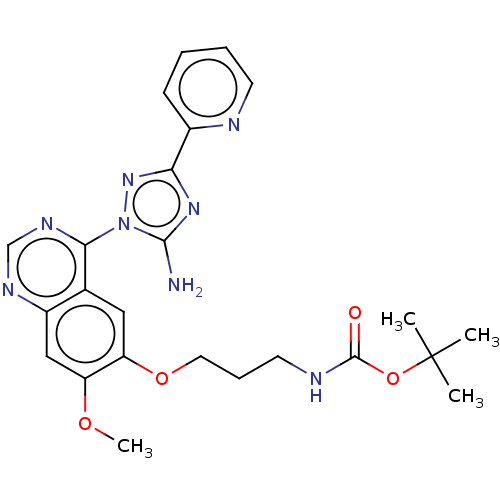 (CHEMBL3763565) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ATM kinase in human MCF7 cells after 1 hr by immunofluorescence assay | J Med Chem 59: 559-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01092 BindingDB Entry DOI: 10.7270/Q2TM7D0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50144956 (CHEMBL3764768) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ATM kinase in human MCF7 cells after 1 hr by immunofluorescence assay | J Med Chem 59: 559-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01092 BindingDB Entry DOI: 10.7270/Q2TM7D0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM50452556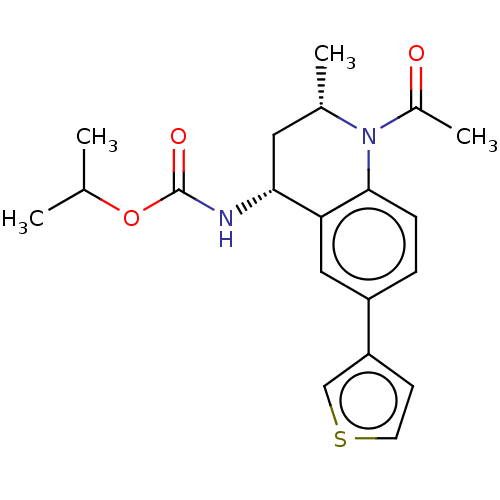 (CHEMBL4210405 | US11028051, Cmpd 53) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of recombinant human BRD2 bromodomain 2 (342 to 460 residues) using biotin labeled peptide substrate preincubated for 15 mins followed by ... | Bioorg Med Chem 26: 25-36 (2018) Article DOI: 10.1016/j.bmc.2017.10.042 BindingDB Entry DOI: 10.7270/Q2B56NB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 169 total ) | Next | Last >> |