

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122692 (CHEMBL282336 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147818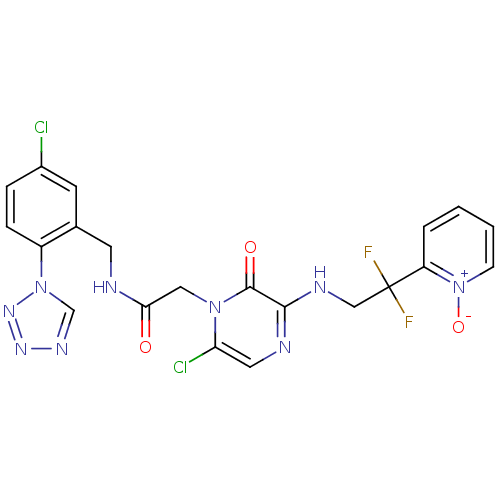 ((2-[6-CHLORO-3-{[2,2-DIFLUORO-2-(1-OXIDOPYRIDIN-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147824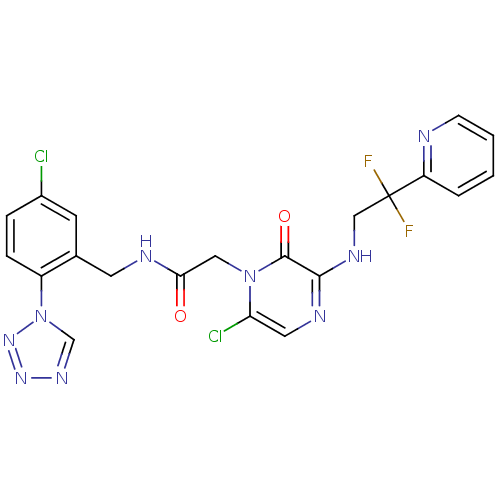 (2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122706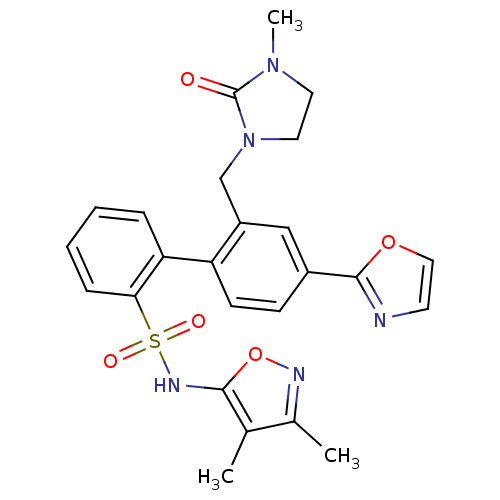 (2'-(3-Methyl-2-oxo-imidazolidin-1-ylmethyl)-4'-oxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122693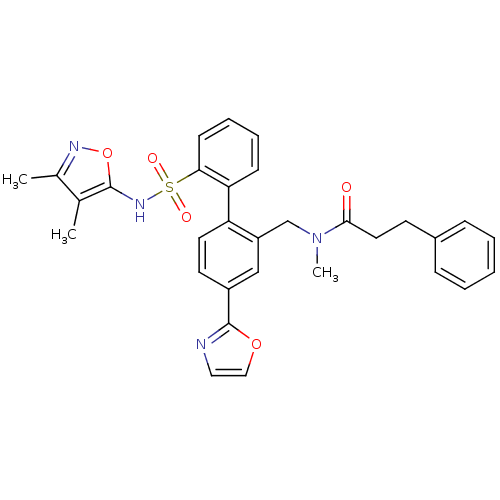 (CHEMBL29346 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122694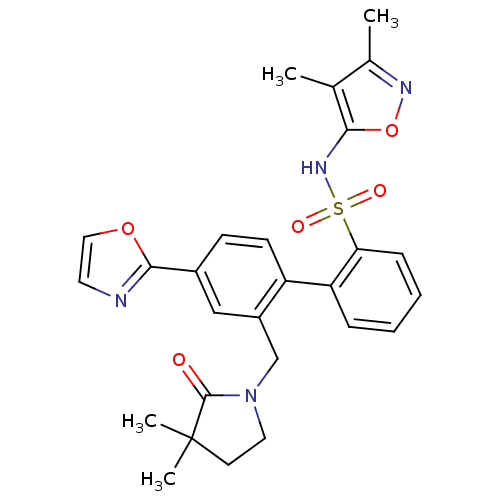 (2'-(3,3-Dimethyl-2-oxo-pyrrolidin-1-ylmethyl)-4'-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122686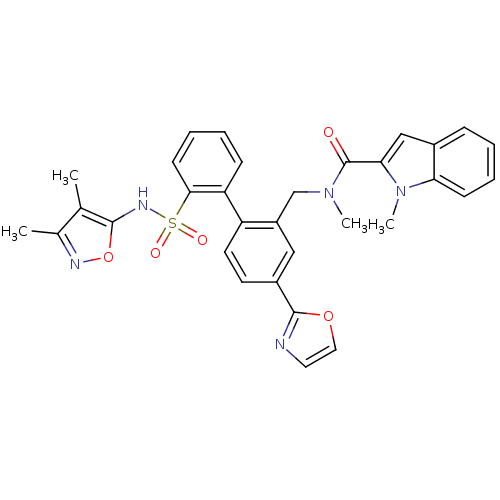 (1-Methyl-1H-indole-2-carboxylic acid [2'-(3,4-dime...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122715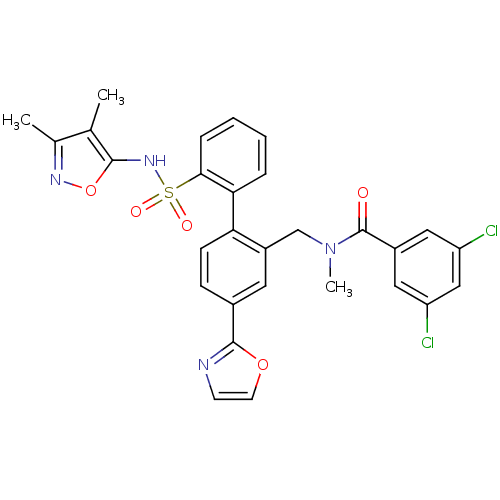 (3,5-Dichloro-N-[2'-(3,4-dimethyl-isoxazol-5-ylsulf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122676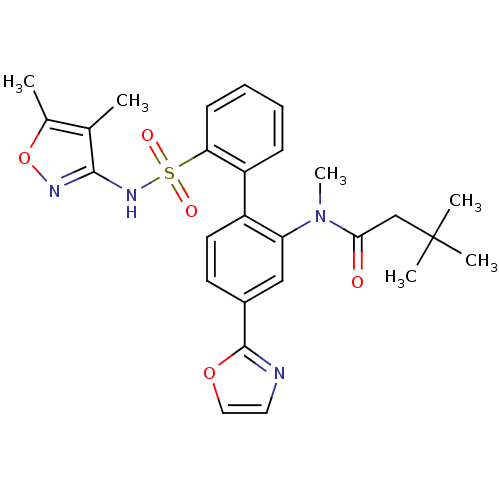 (CHEMBL274489 | N-[2'-(4,5-Dimethyl-isoxazol-3-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122712 (CHEMBL440780 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147793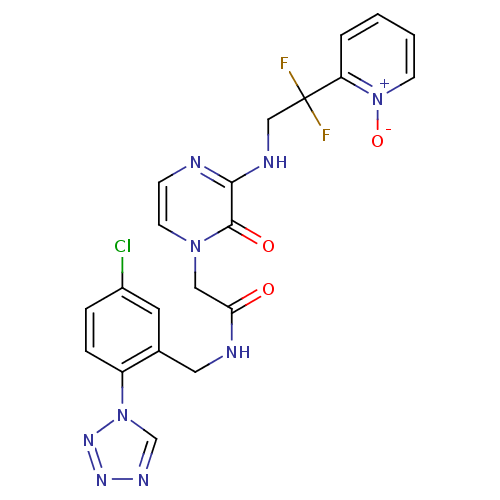 (CHEMBL323583 | N-(5-Chloro-2-tetrazol-1-yl-benzyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147788 (CHEMBL103874 | N-(5-Chloro-2-tetrazol-1-yl-benzyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM364562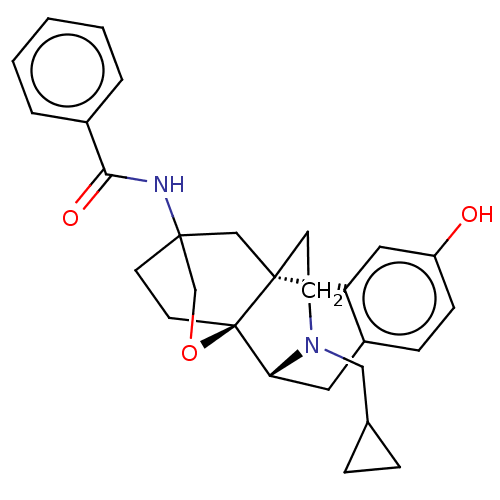 (N-((4bR,6S,8aS,9R)-11- (cyclopropylmethyl)- 3-hydr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg mem... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122700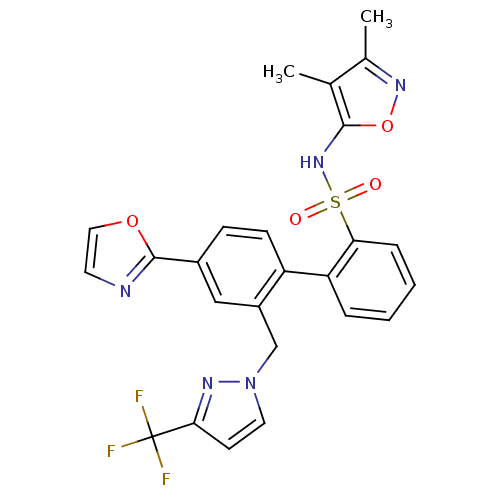 (4'-Oxazol-2-yl-2'-(3-trifluoromethyl-pyrazol-1-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122707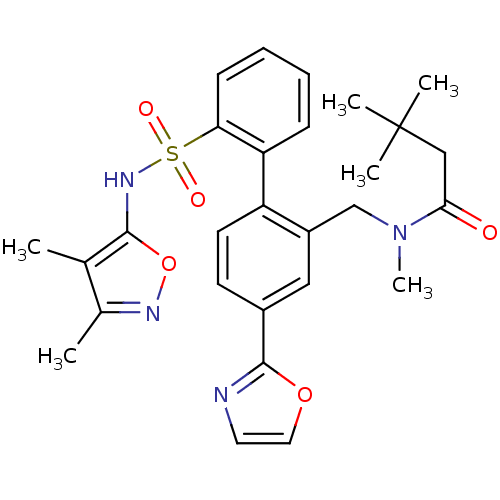 (CHEMBL281659 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122697 (2'-[(Methyl-phenyl-amino)-methyl]-4'-oxazol-2-yl-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131480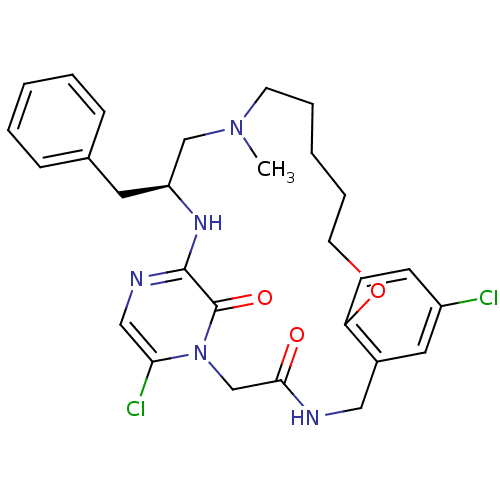 ((S)-20-Benzyl-8,25-dichloro-18-methyl-12-oxa-1,4,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thrombin IIa | Bioorg Med Chem Lett 13: 2781-4 (2003) BindingDB Entry DOI: 10.7270/Q2GF0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM364547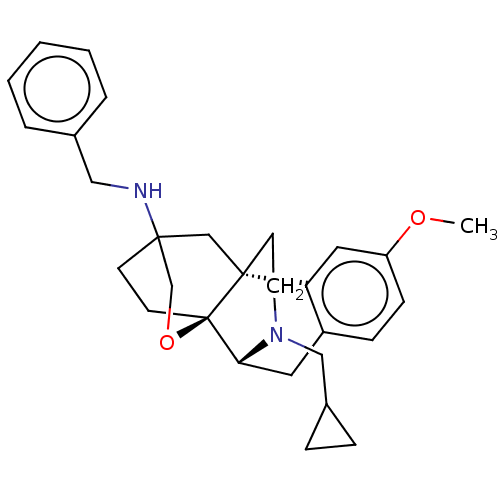 ((4bR,6S,8aS,9R)-N-benzyl-11- (cyclopropylmethyl)-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122681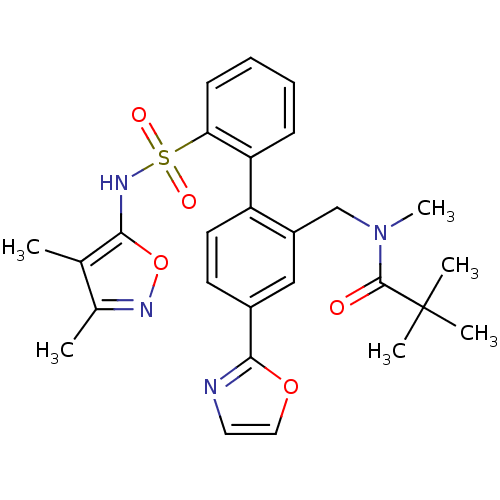 (CHEMBL27855 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM364552 (N-((4bR,6S,8aS,9R)-11- (cyclopropylmethyl)- 3-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147809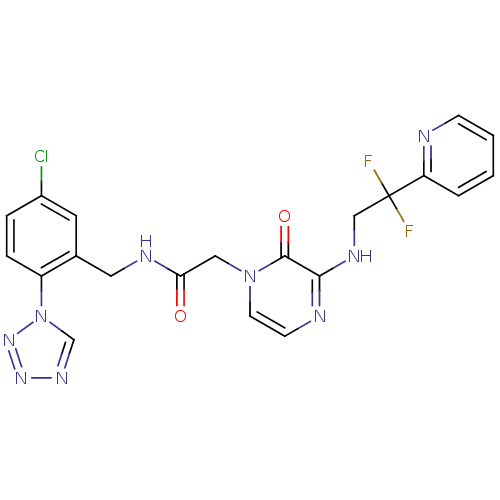 (CHEMBL103342 | N-(5-Chloro-2-tetrazol-1-yl-benzyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122698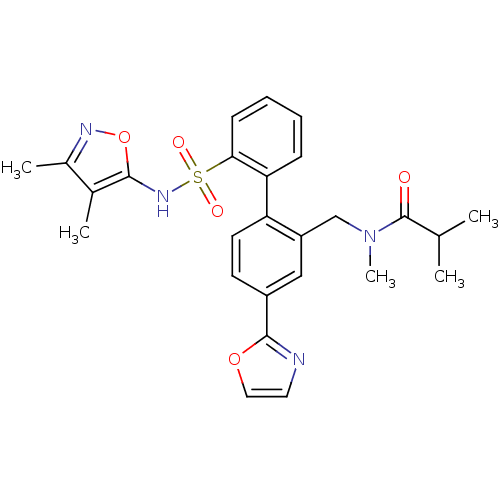 (CHEMBL28863 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122713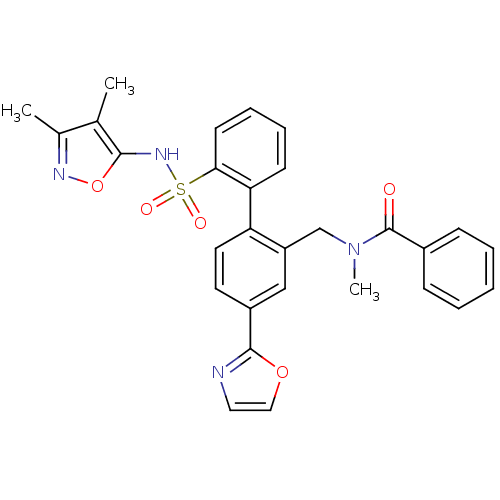 (CHEMBL282359 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122684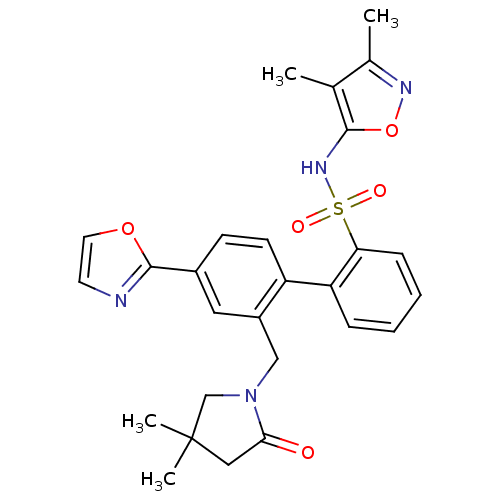 (2'-(4,4-Dimethyl-2-oxo-pyrrolidin-1-ylmethyl)-4'-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122696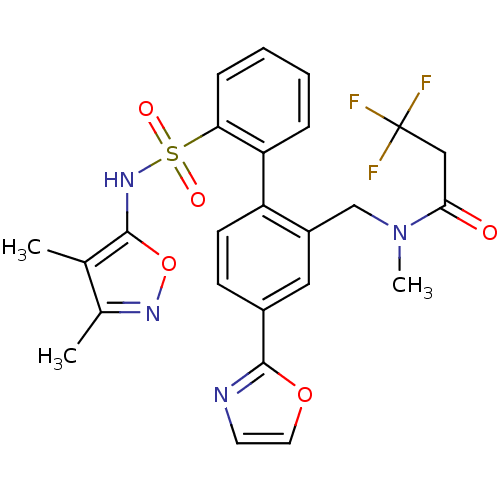 (CHEMBL281549 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131460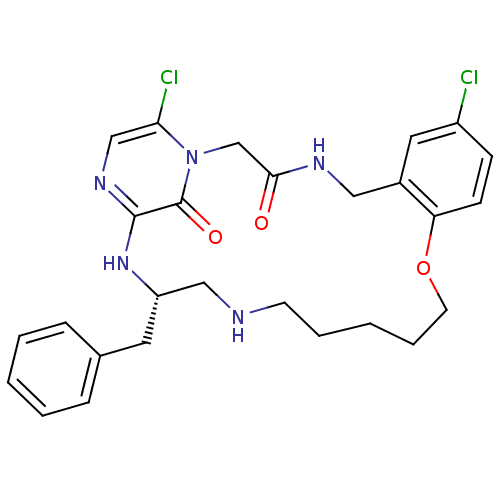 ((S)-20-Benzyl-8,25-dichloro-12-oxa-1,4,18,21,23-pe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thrombin IIa | Bioorg Med Chem Lett 13: 2781-4 (2003) BindingDB Entry DOI: 10.7270/Q2GF0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131464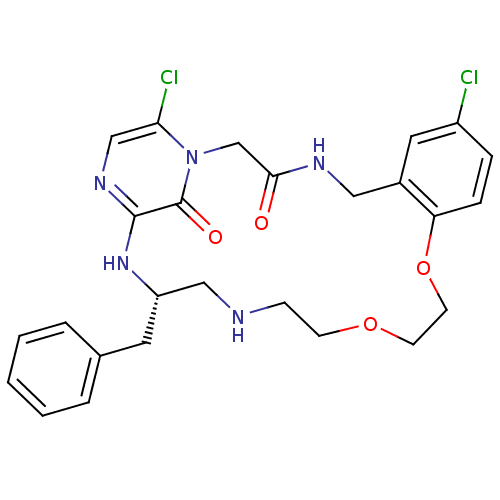 ((S)-20-Benzyl-8,25-dichloro-12,15-dioxa-1,4,18,21,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thrombin IIa | Bioorg Med Chem Lett 13: 2781-4 (2003) BindingDB Entry DOI: 10.7270/Q2GF0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122690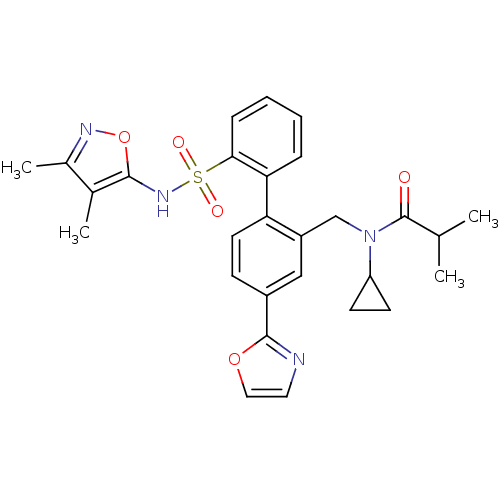 (CHEMBL28963 | N-Cyclopropyl-N-[2'-(3,4-dimethyl-is...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147812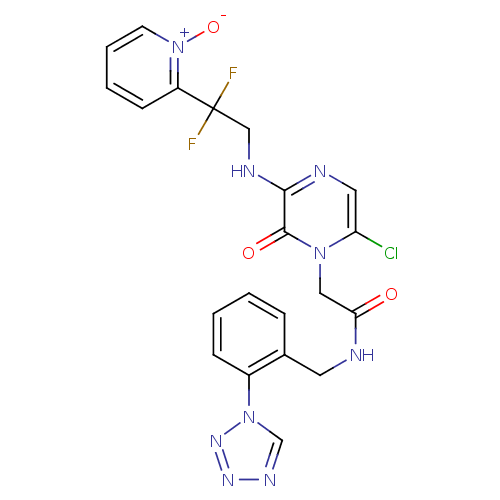 (2-{6-Chloro-3-[2,2-difluoro-2-(1-oxy-pyridin-2-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM364548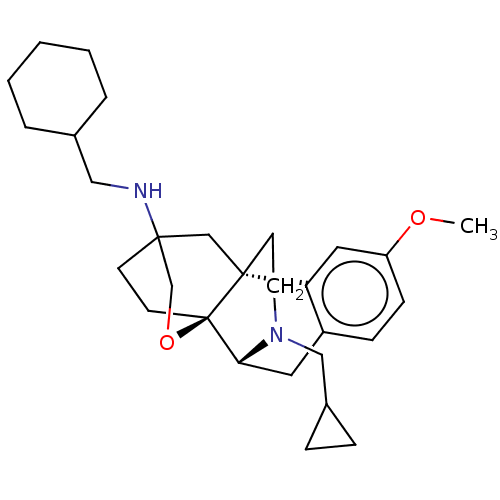 ((4bR,6S,8aS,9R)-N-(cyclohexylmethyl)-11- (cyclopro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM364561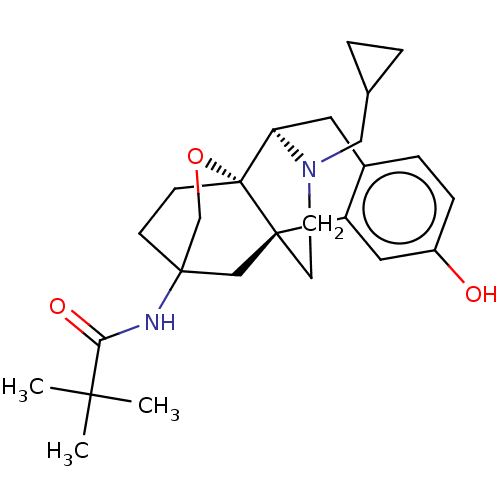 (N-((4bR,6S,8aS,9R)-11- (cyclopropylmethyl)- 3-hydr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg mem... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM364559 (4-Chloro-N-((4bR,6S,8aS,9R)-11- (cyclopropylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM364561 (N-((4bR,6S,8aS,9R)-11- (cyclopropylmethyl)- 3-hydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131471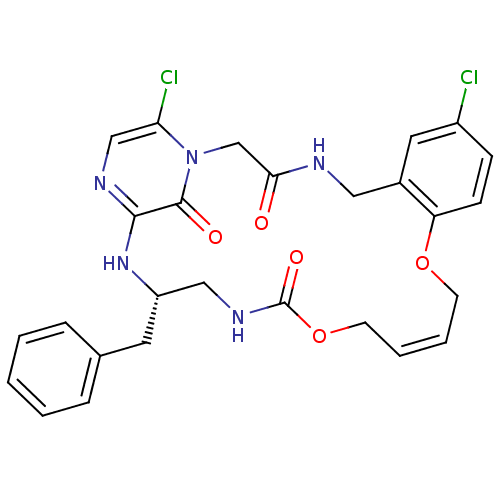 ((14Z,21S)-21-benzyl-8,26-dichloro-12,17-dioxa-1,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thrombin IIa | Bioorg Med Chem Lett 13: 2781-4 (2003) BindingDB Entry DOI: 10.7270/Q2GF0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM364562 (N-((4bR,6S,8aS,9R)-11- (cyclopropylmethyl)- 3-hydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122703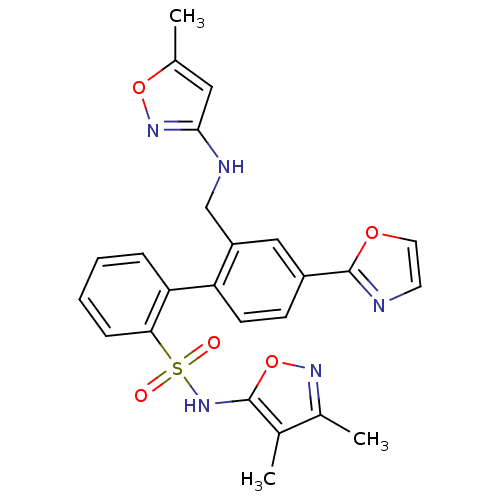 (2'-[(5-Methyl-isoxazol-3-ylamino)-methyl]-4'-oxazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147801 (CHEMBL102122 | N-(5-Chloro-2-[1,2,4]triazol-1-yl-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131470 ((11S)-11-BENZYL-6-CHLORO-1,2,10,11,12,13,14,15,16,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thrombin IIa | Bioorg Med Chem Lett 13: 2781-4 (2003) BindingDB Entry DOI: 10.7270/Q2GF0V2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147821 (2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM364549 ((4bR,6S,8aS,9R)-6-amino-11- (cyclopropylmethyl)-5,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb... | J Med Chem 50: 4255-9 (2007) BindingDB Entry DOI: 10.7270/Q2222X2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131473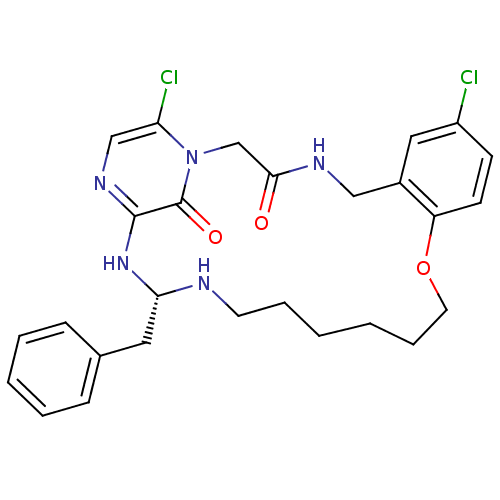 ((S)-20-Benzyl-8,25-dichloro-12-oxa-1,4,19,21,23-pe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thrombin IIa | Bioorg Med Chem Lett 13: 2781-4 (2003) BindingDB Entry DOI: 10.7270/Q2GF0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147822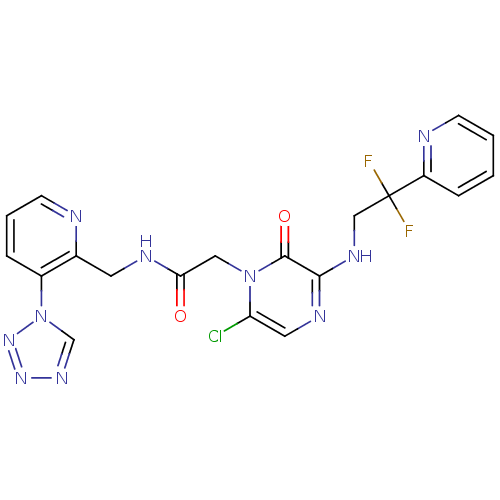 (2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122704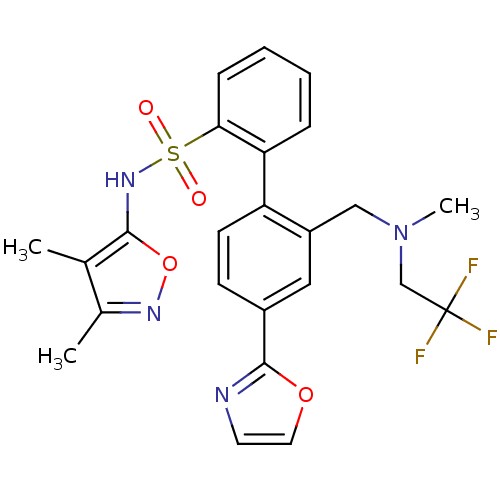 (2'-{[Methyl-(2,2,2-trifluoro-ethyl)-amino]-methyl}...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122711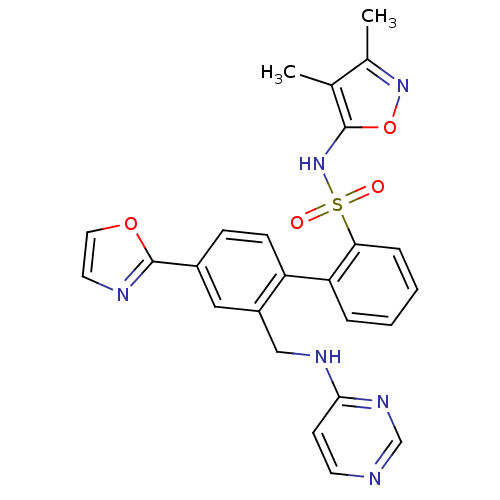 (4'-Oxazol-2-yl-2'-(pyrimidin-4-ylaminomethyl)-biph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147810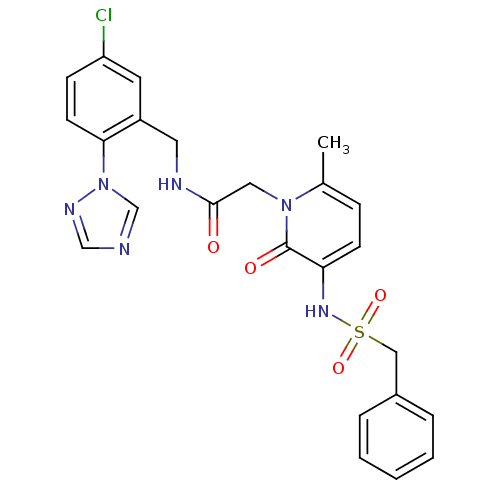 (CHEMBL100854 | N-(5-Chloro-2-[1,2,4]triazol-1-yl-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131458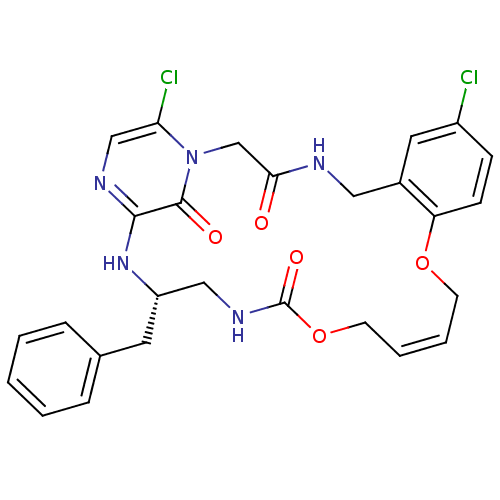 ((14E,21S)-21-benzyl-8,26-dichloro-12,17-dioxa-1,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thrombin IIa | Bioorg Med Chem Lett 13: 2781-4 (2003) BindingDB Entry DOI: 10.7270/Q2GF0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122678 (2'-Isobutoxymethyl-4'-oxazol-2-yl-biphenyl-2-sulfo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122710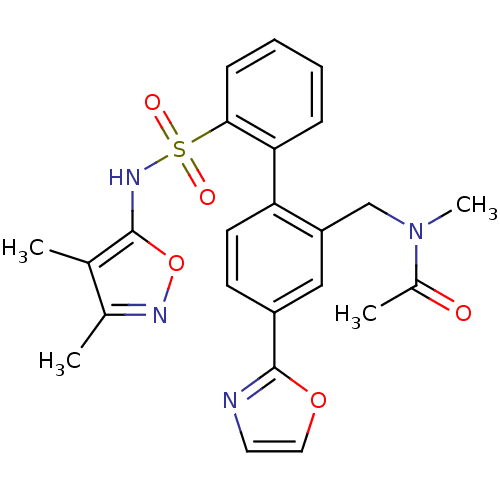 (CHEMBL284656 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50091104 (CHEMBL29422 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM151177 (BDBM151178 | US8987287, 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.200 | -55.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 ug membrane protein (recombinant... | US Patent US8987287 (2015) BindingDB Entry DOI: 10.7270/Q24F1PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4850 total ) | Next | Last >> |