

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343590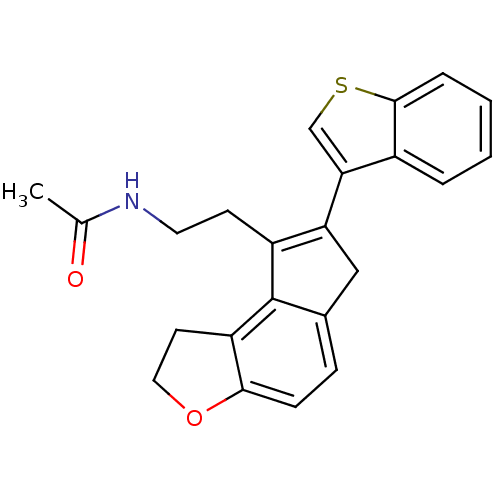 (CHEMBL1774531 | N-{2-[7-(1-Benzothien-3-yl)-1,6-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343598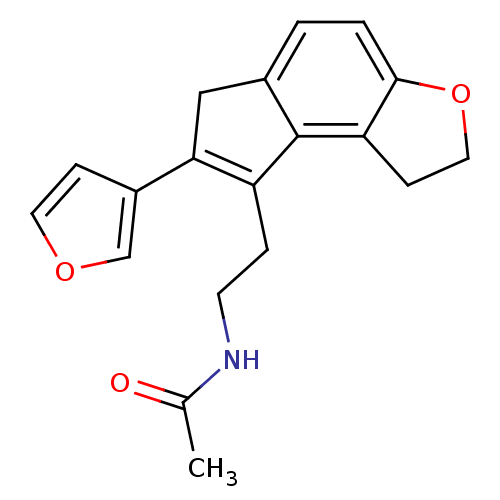 (CHEMBL1774523 | N-{2-[7-(3-Furyl)-1,6-dihydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343599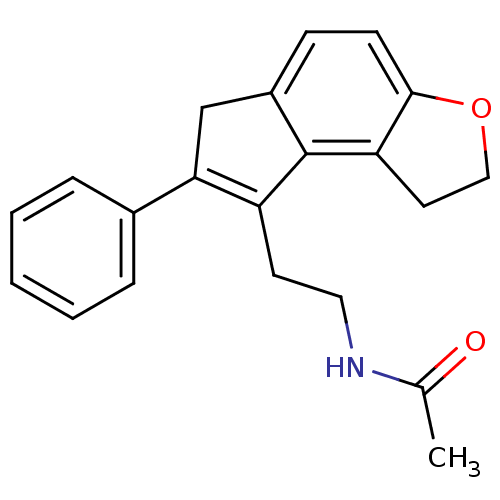 (CHEMBL1774522 | N-[2-(7-Phenyl-1,6-dihydro-2H-inde...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343601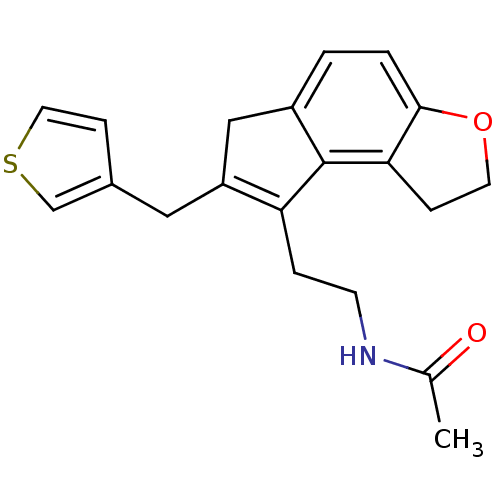 (CHEMBL1774520 | N-{2-[7-(3-Thienylmethyl)-1,6-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343599 (CHEMBL1774522 | N-[2-(7-Phenyl-1,6-dihydro-2H-inde...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343603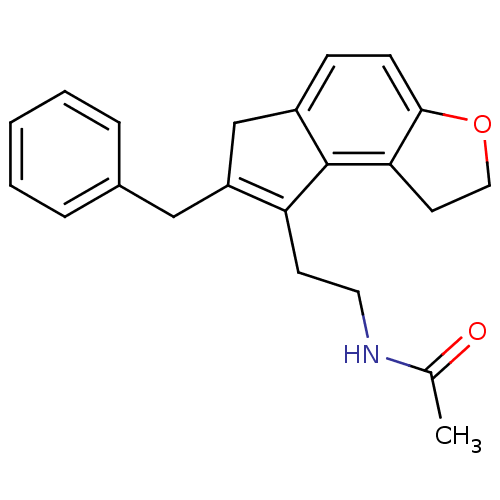 (CHEMBL1774518 | N-[2-(7-Benzyl-1,6-dihydro-2H-inde...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343600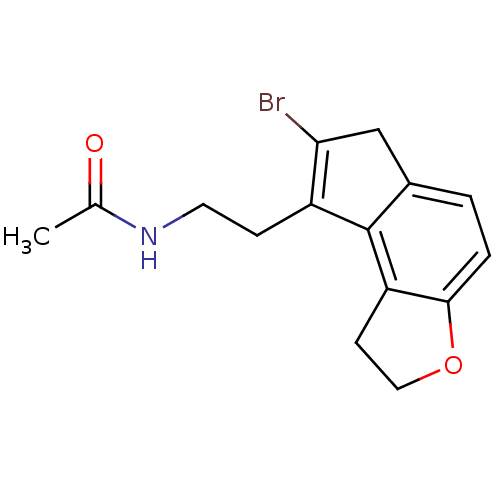 (CHEMBL1774521 | N-[2-(7-Bromo-1,6-dihydro-2H-inden...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343595 (CHEMBL1774526 | N-{2-[7-(3-Methoxyphenyl)-1,6-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343598 (CHEMBL1774523 | N-{2-[7-(3-Furyl)-1,6-dihydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343605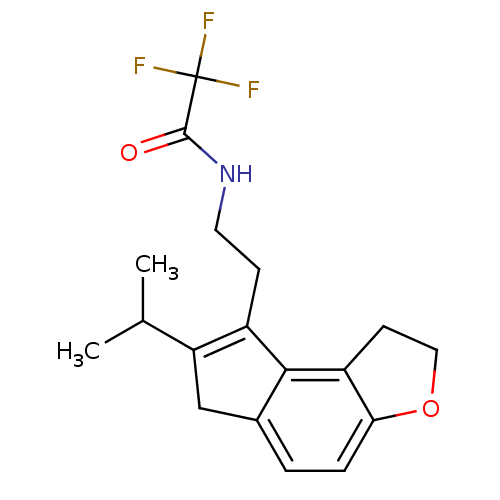 (2,2,2-Trifluoro-N-[2-(7-isopropyl-1,6-dihydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343592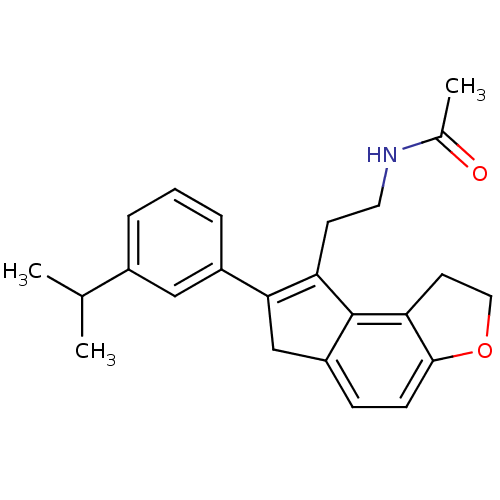 (CHEMBL1774529 | N-{2-[7-(3-Isopropylphenyl)-1,6-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343607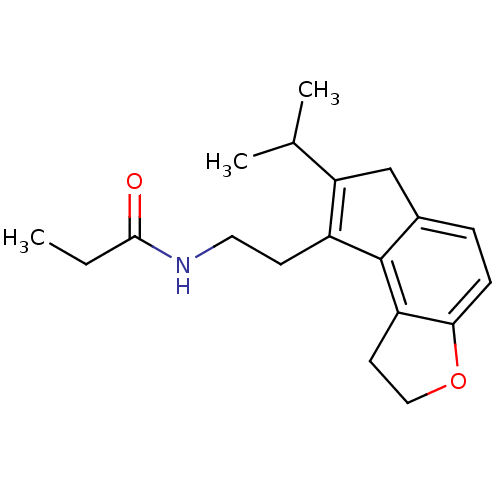 (CHEMBL1774514 | N-[2-(7-Isopropyl-1,6-dihydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343602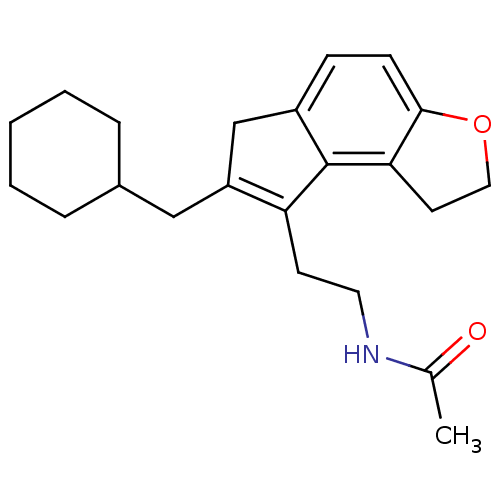 (CHEMBL1774519 | N-{2-[7-(Cyclohexylmethyl)-1,6-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343607 (CHEMBL1774514 | N-[2-(7-Isopropyl-1,6-dihydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343600 (CHEMBL1774521 | N-[2-(7-Bromo-1,6-dihydro-2H-inden...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343608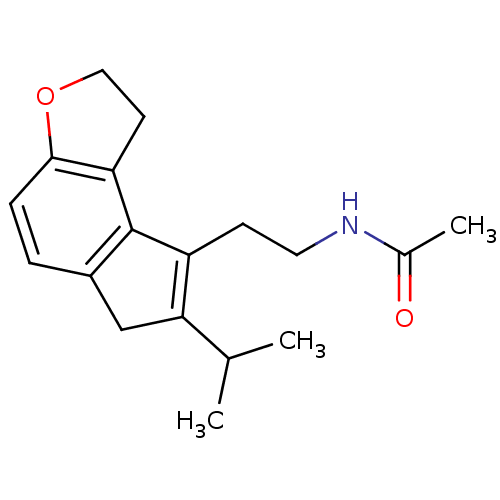 (CHEMBL1774513 | N-[2-(7-Isopropyl-1,6-dihydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343596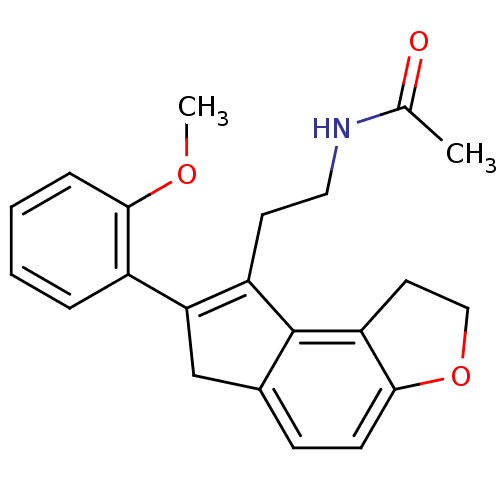 (CHEMBL1774525 | N-{2-[7-(2-Methoxyphenyl)-1,6-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343609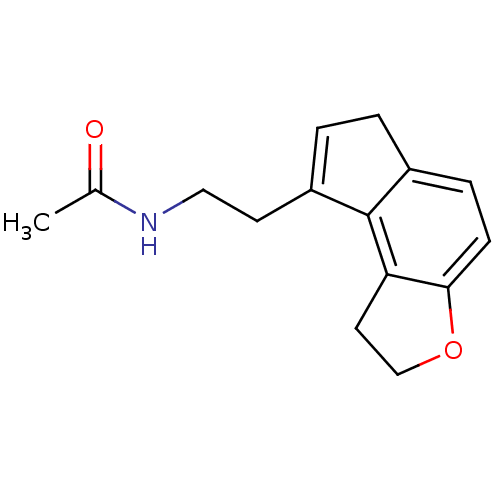 (CHEMBL1774512 | N-[2-(1,6-Dihydro-2H-indeno[5,4-b]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343608 (CHEMBL1774513 | N-[2-(7-Isopropyl-1,6-dihydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50330773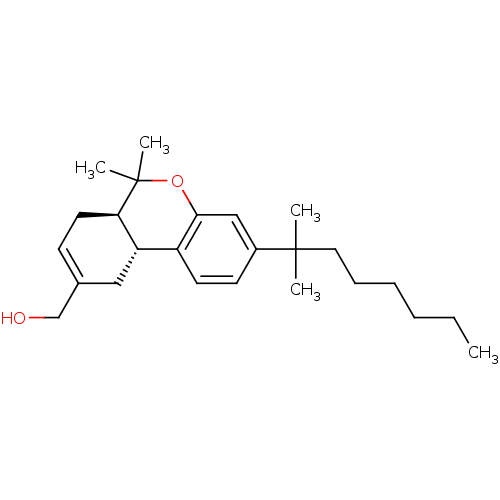 (((6aR,10aR)-6,6-dimethyl-3-(2-methyloctan-2-yl)-6a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clemson University Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor | Bioorg Med Chem 18: 7809-15 (2010) Article DOI: 10.1016/j.bmc.2010.09.061 BindingDB Entry DOI: 10.7270/Q2ZK5HP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343605 (2,2,2-Trifluoro-N-[2-(7-isopropyl-1,6-dihydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343597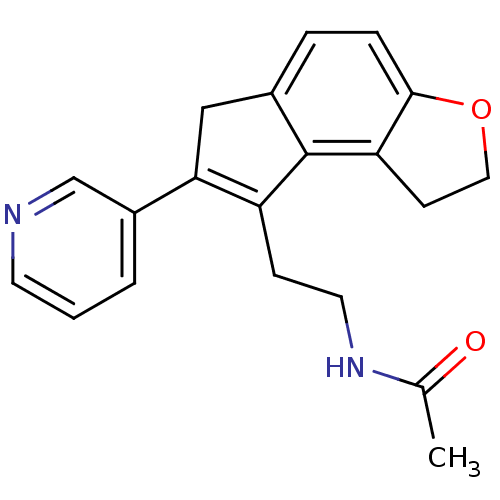 (CHEMBL1774524 | N-[2-(7-Pyridin-3-yl-1,6-dihydro-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343606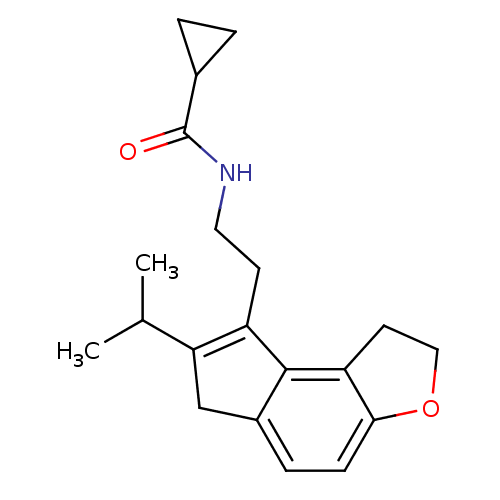 (CHEMBL1774515 | N-[2-(7-Isopropyl-1,6-dihydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343594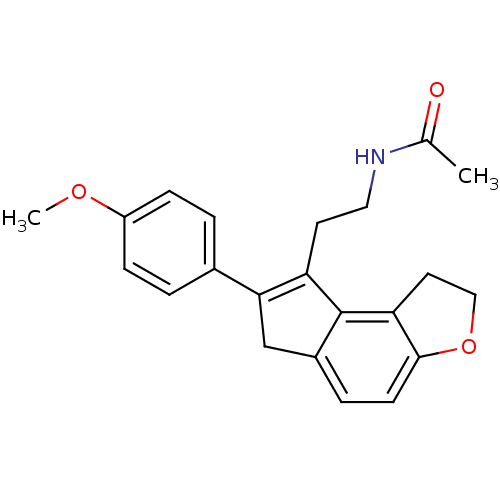 (CHEMBL1774527 | N-{2-[7-(4-Methoxyphenyl)-1,6-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343609 (CHEMBL1774512 | N-[2-(1,6-Dihydro-2H-indeno[5,4-b]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343595 (CHEMBL1774526 | N-{2-[7-(3-Methoxyphenyl)-1,6-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343597 (CHEMBL1774524 | N-[2-(7-Pyridin-3-yl-1,6-dihydro-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343604 (CHEMBL1774517 | N-Ethyl-N'-[2-(7-isopropyl-1,6-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Thailand/1(KAN-1)/2004(H5N1)) neuraminidase by by Michaelis Menten equation analysis | Antimicrob Agents Chemother 53: 3088-96 (2009) Article DOI: 10.1128/AAC.01667-08 BindingDB Entry DOI: 10.7270/Q2VM4CHC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343606 (CHEMBL1774515 | N-[2-(7-Isopropyl-1,6-dihydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343593 (CHEMBL1774528 | N-{2-[7-(2-Isopropoxyphenyl)-1,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/chicken/Yogjakarta/BBVet-IX/2004(H5N1)) neuraminidase by Michaelis Menten equation analysis | Antimicrob Agents Chemother 53: 3088-96 (2009) Article DOI: 10.1128/AAC.01667-08 BindingDB Entry DOI: 10.7270/Q2VM4CHC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Laos/25/2006(H5N1)) neuraminidase by Michaelis Menten equation analysis | Antimicrob Agents Chemother 53: 3088-96 (2009) Article DOI: 10.1128/AAC.01667-08 BindingDB Entry DOI: 10.7270/Q2VM4CHC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Thailand/1(KAN-1)/2004(H5N1)) neuraminidase by by Michaelis Menten equation analysis | Antimicrob Agents Chemother 53: 3088-96 (2009) Article DOI: 10.1128/AAC.01667-08 BindingDB Entry DOI: 10.7270/Q2VM4CHC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC6 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 5 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC5 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343591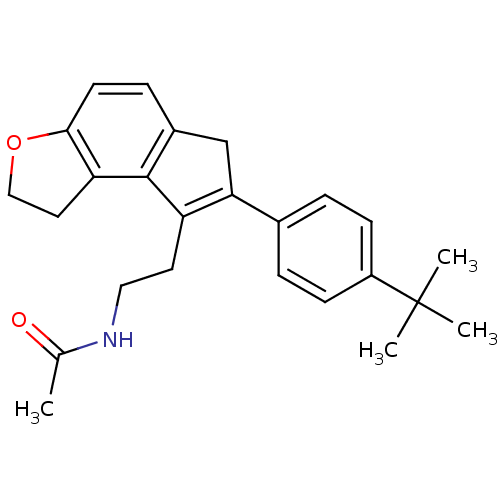 (CHEMBL1774530 | N-{2-[7-(4-tert-Butylphenyl)-1,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50135453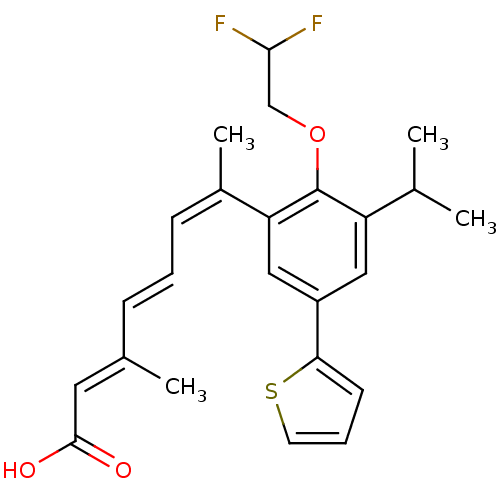 ((2E,4E,6Z)-7-[2-(2,2-Difluoro-ethoxy)-3-isopropyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc Curated by ChEMBL | Assay Description Binding affinity against RXR alpha receptor using [3H]-9-cis-RA as radioligand in CV-1 cells | Bioorg Med Chem Lett 13: 4071-5 (2003) BindingDB Entry DOI: 10.7270/Q2V987GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50135460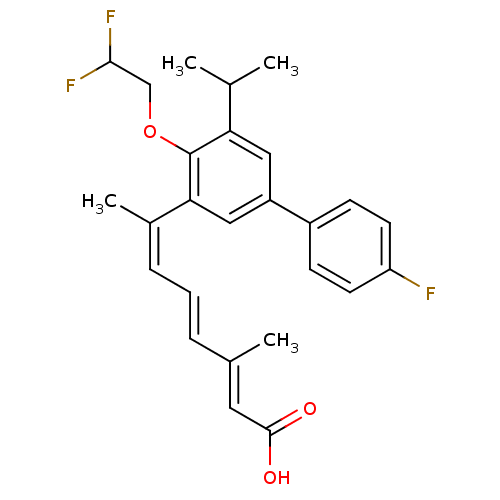 ((2E,4E,6Z)-7-[4-(2,2-Difluoro-ethoxy)-4'-fluoro-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc Curated by ChEMBL | Assay Description Binding affinity against RXR alpha receptor using [3H]-9-cis-RA as radioligand in CV-1 cells | Bioorg Med Chem Lett 13: 4071-5 (2003) BindingDB Entry DOI: 10.7270/Q2V987GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343601 (CHEMBL1774520 | N-{2-[7-(3-Thienylmethyl)-1,6-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Turkey/651242/2006(H5N1)) neuraminidase by Michaelis Menten equation analysis | Antimicrob Agents Chemother 53: 3088-96 (2009) Article DOI: 10.1128/AAC.01667-08 BindingDB Entry DOI: 10.7270/Q2VM4CHC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50135462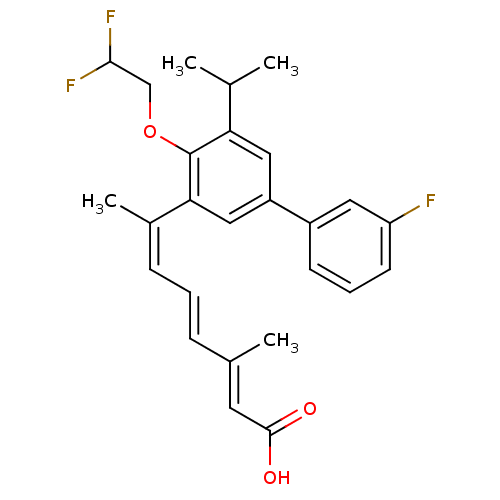 ((2E,4E,6Z)-7-[4-(2,2-Difluoro-ethoxy)-3'-fluoro-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc Curated by ChEMBL | Assay Description Binding affinity against RXR alpha receptor using [3H]-9-cis-RA as radioligand in CV-1 cells | Bioorg Med Chem Lett 13: 4071-5 (2003) BindingDB Entry DOI: 10.7270/Q2V987GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343596 (CHEMBL1774525 | N-{2-[7-(2-Methoxyphenyl)-1,6-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343604 (CHEMBL1774517 | N-Ethyl-N'-[2-(7-isopropyl-1,6-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50563846 (CHEMBL4787010) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-TAK-875 from human recombinant full-length GPR40 expressed in human HEK293 cell membrane incubated for 2 hrs by solid scintillat... | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00892 BindingDB Entry DOI: 10.7270/Q2GH9NPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Laos/25/2006(H5N1)) neuraminidase by Michaelis Menten equation analysis | Antimicrob Agents Chemother 53: 3088-96 (2009) Article DOI: 10.1128/AAC.01667-08 BindingDB Entry DOI: 10.7270/Q2VM4CHC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC9 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 13111 total ) | Next | Last >> |