

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433595 (CHEMBL2381827) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433577 (CHEMBL2381848) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433576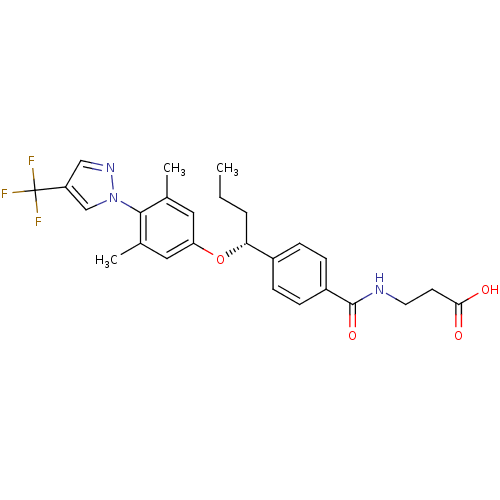 (CHEMBL2381849) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM100805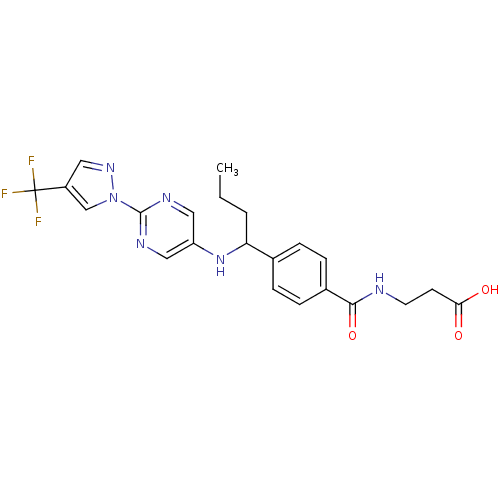 (US8507533, 113) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433598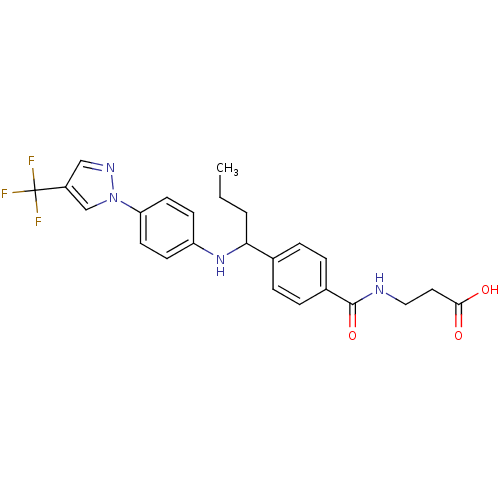 (CHEMBL2381821) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433589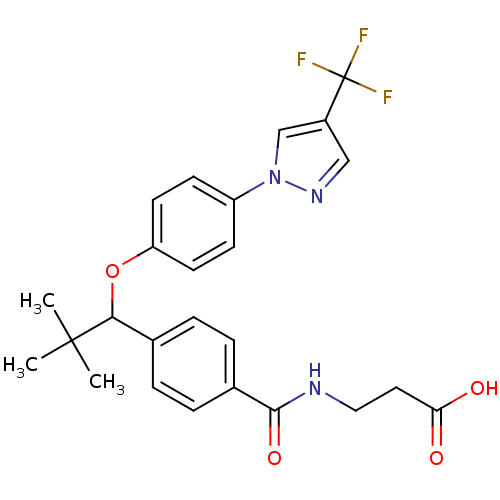 (CHEMBL2381834) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433597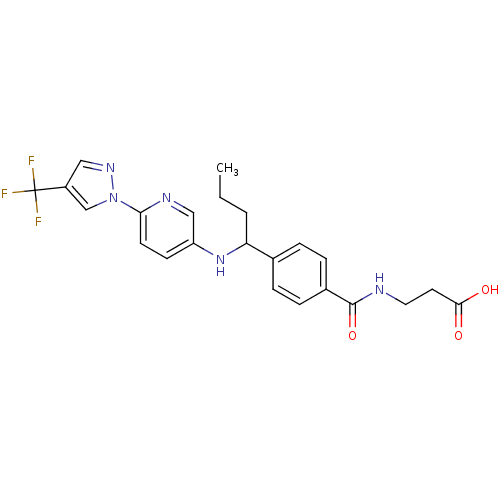 (CHEMBL2381822) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM100800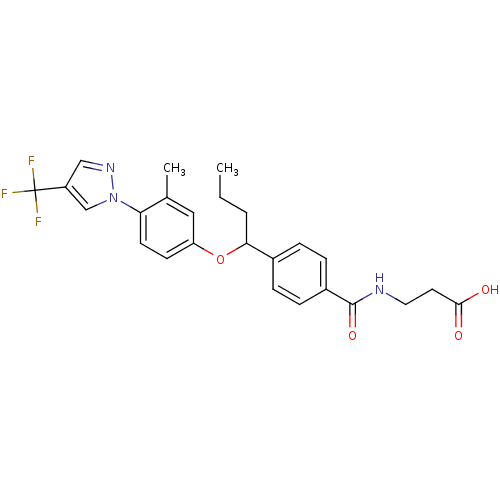 (US8507533, 108) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433594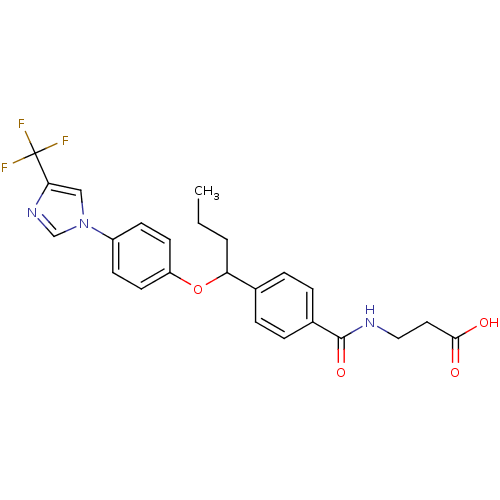 (CHEMBL2381828) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM100793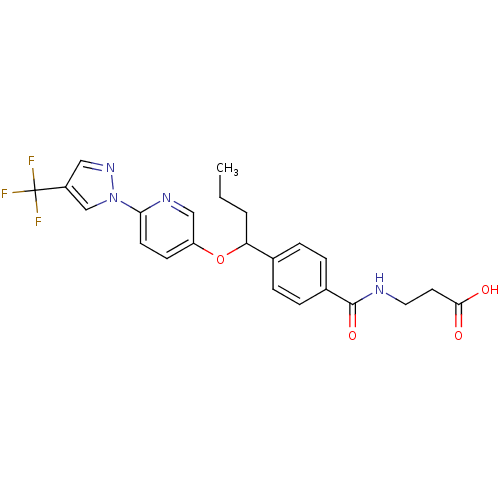 (US8507533, 86) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM100817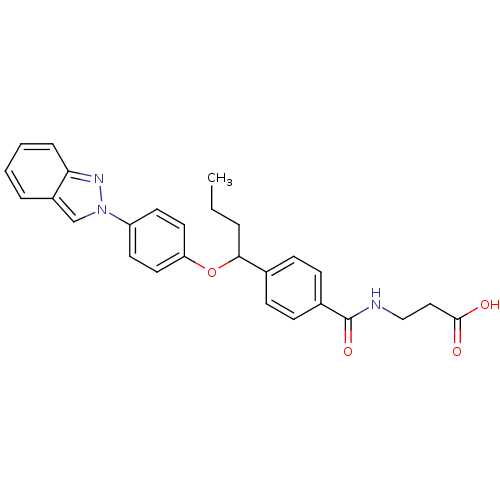 (US8507533, 124) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433590 (CHEMBL2381833) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433591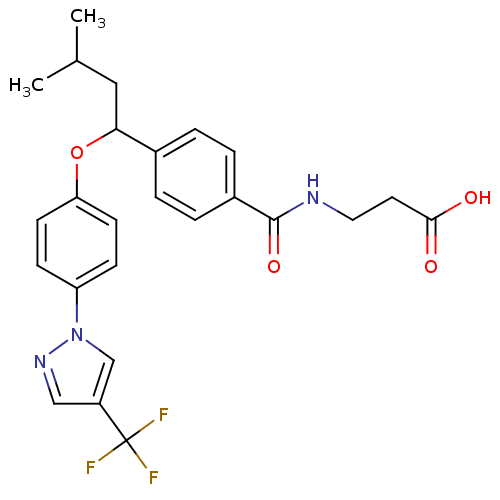 (CHEMBL2381832) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 549 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433596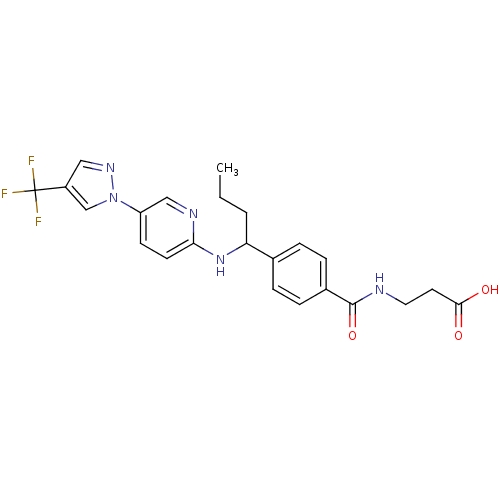 (CHEMBL2381826) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433599 (CHEMBL2381820) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433578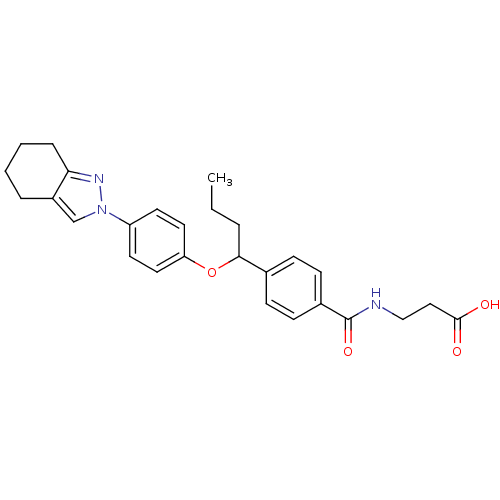 (CHEMBL2381846) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433592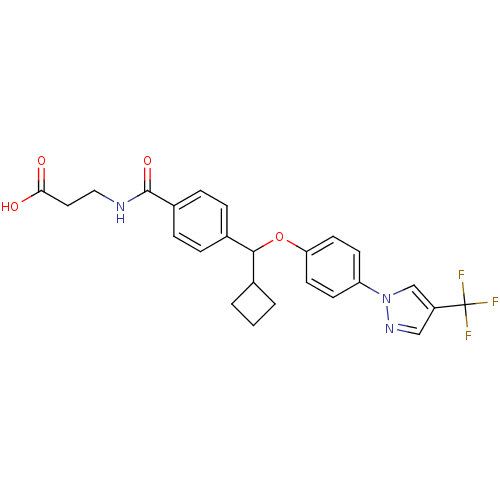 (CHEMBL2381831) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433588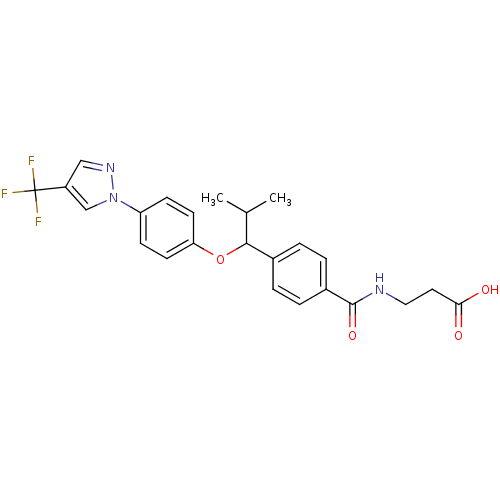 (CHEMBL2381835) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433593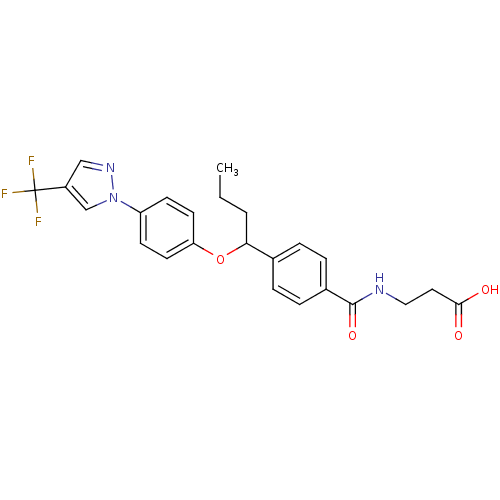 (CHEMBL2381824) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433585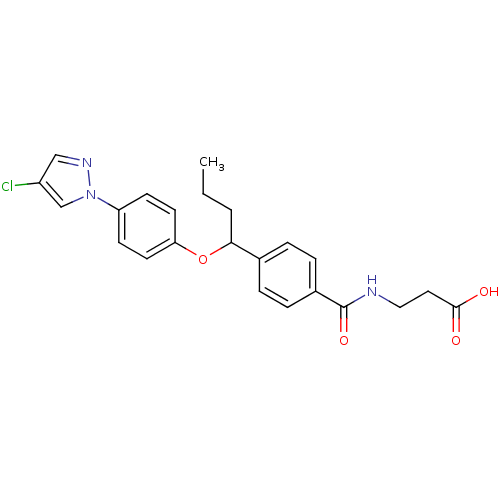 (CHEMBL2381838) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433579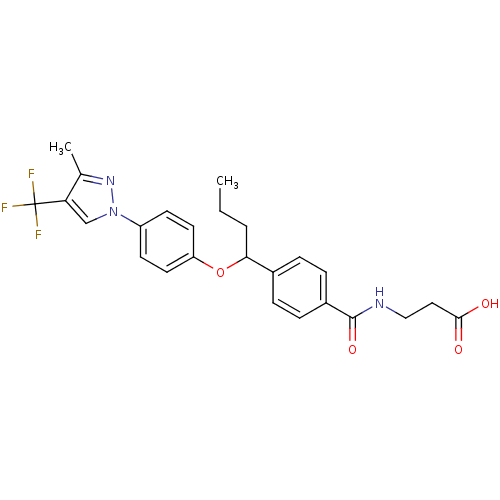 (CHEMBL2381845) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM100834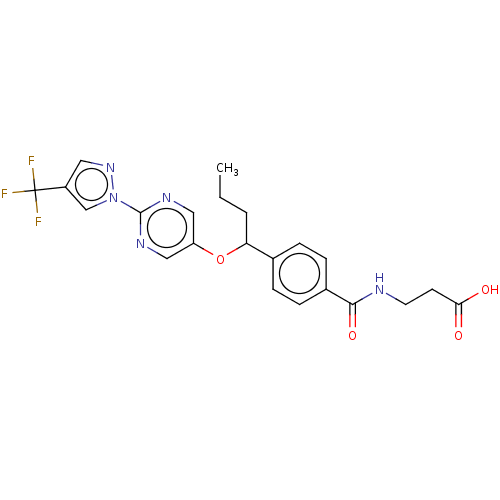 (CHEMBL2381830 | US8507533, 149) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433587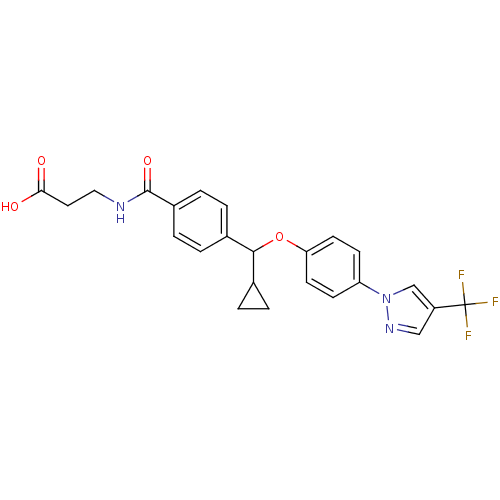 (CHEMBL2381836) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433586 (CHEMBL2381837) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433576 (CHEMBL2381849) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM100790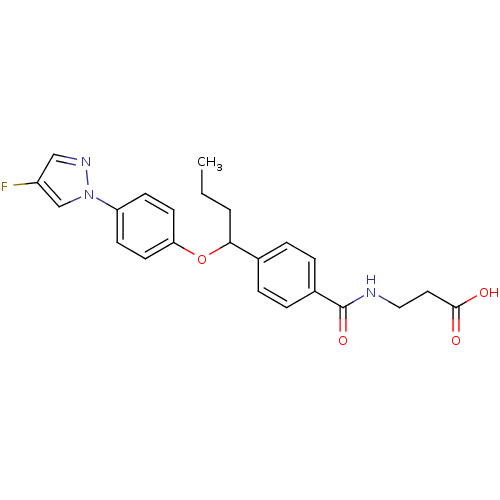 (US8507533, 28) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433584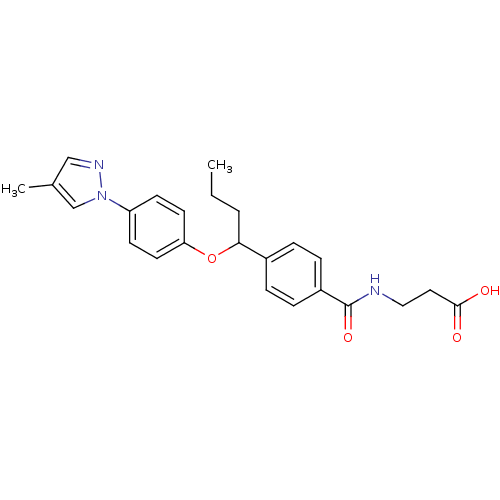 (CHEMBL2381839) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50433577 (CHEMBL2381848) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to GLP-1 receptor (unknown origin) | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433583 (CHEMBL2381841) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433582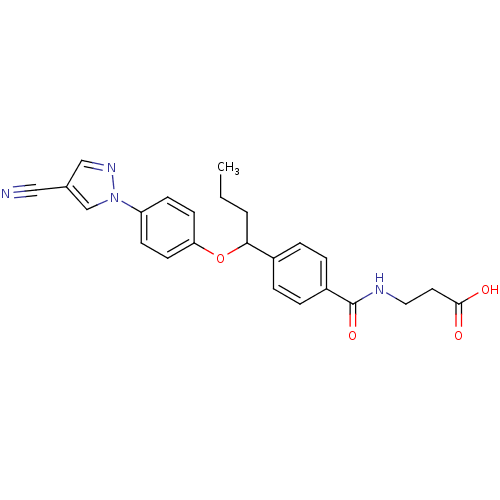 (CHEMBL2381842) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433581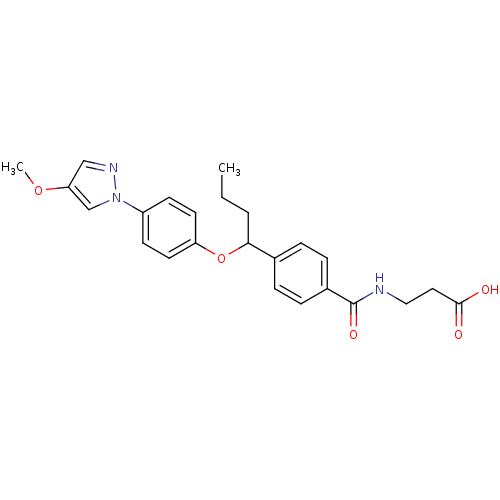 (CHEMBL2381843) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433580 (CHEMBL2381844) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C (Homo sapiens (Human)) | BDBM50259015 (5-(3-chlorobenzyl)-3-isopropyl-1H-pyrazolo[4,3-d]p...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of PDE1c | Bioorg Med Chem Lett 19: 2537-41 (2009) Article DOI: 10.1016/j.bmcl.2009.03.024 BindingDB Entry DOI: 10.7270/Q2RB74GG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50438698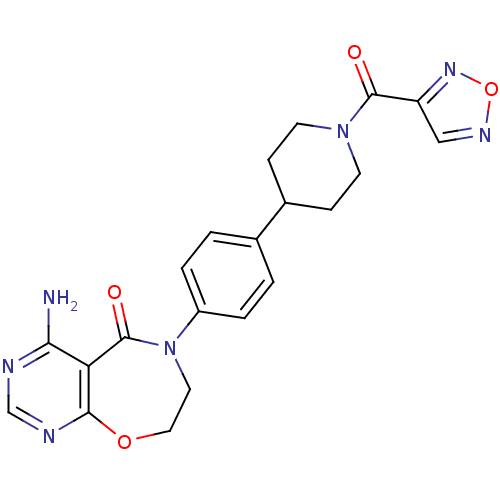 (CHEMBL2414663) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human full length DGAT-1 expressed in insect sf9 cells using [14C]decanoylCoA as substrate after 1.5 hrs by scintillation spectrometry | Bioorg Med Chem 21: 5081-97 (2013) Article DOI: 10.1016/j.bmc.2013.06.045 BindingDB Entry DOI: 10.7270/Q26111RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50355142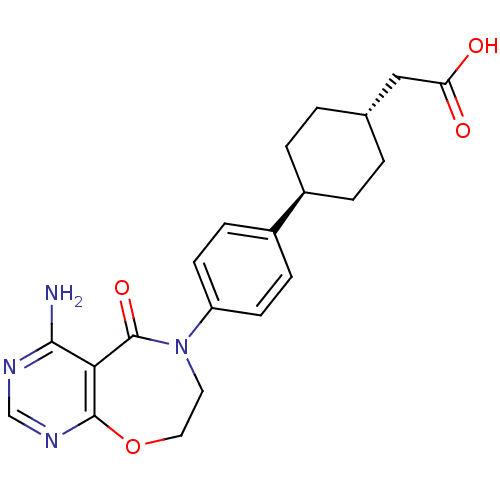 (CHEMBL1835919) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of DGAT-1-mediated triglyceride synthesis in human HT-29 cells using [3H]-glycerol as substrate incubated for 1 hr prior to substrate addi... | Bioorg Med Chem 21: 5081-97 (2013) Article DOI: 10.1016/j.bmc.2013.06.045 BindingDB Entry DOI: 10.7270/Q26111RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50438743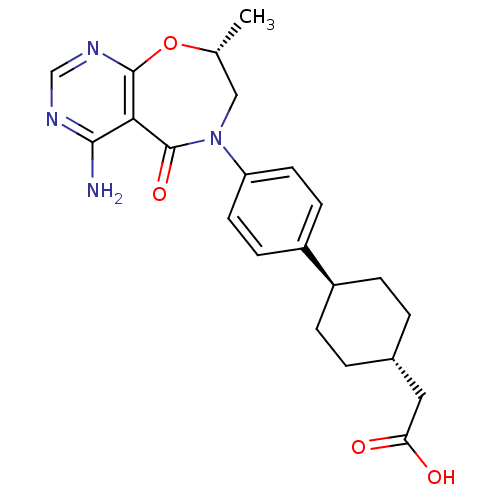 (CHEMBL2414687) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of DGAT-1-mediated triglyceride synthesis in human HT-29 cells using [3H]-glycerol as substrate incubated for 1 hr prior to substrate addi... | Bioorg Med Chem 21: 5081-97 (2013) Article DOI: 10.1016/j.bmc.2013.06.045 BindingDB Entry DOI: 10.7270/Q26111RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50355142 (CHEMBL1835919) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of DGAT1-mediated triglyceride synthesis in human HT-29 cells using [3H]glycerol as substrate after 6 hrs by beta counting | ACS Med Chem Lett 2: 407-412 (2011) Article DOI: 10.1021/ml200051p BindingDB Entry DOI: 10.7270/Q2MW2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50158278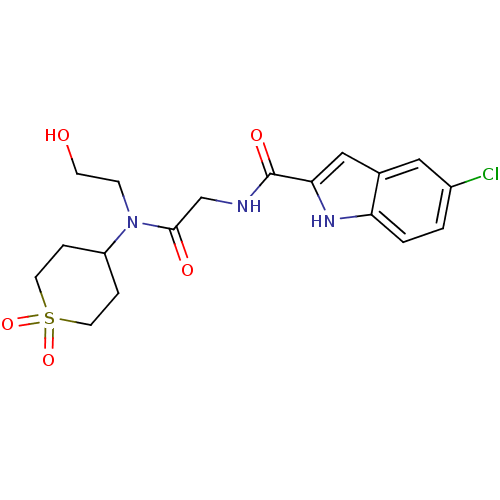 (5-Chloro-1H-indole-2-carboxylic acid {[(1,1-dioxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against glycogen phosphorylase | Bioorg Med Chem Lett 15: 459-65 (2004) Article DOI: 10.1016/j.bmcl.2004.10.048 BindingDB Entry DOI: 10.7270/Q26W99K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50438734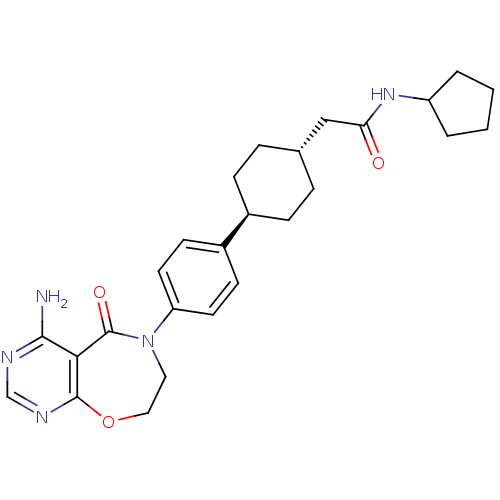 (CHEMBL2414751) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of DGAT-1-mediated triglyceride synthesis in human HT-29 cells using [3H]-glycerol as substrate incubated for 1 hr prior to substrate addi... | Bioorg Med Chem 21: 5081-97 (2013) Article DOI: 10.1016/j.bmc.2013.06.045 BindingDB Entry DOI: 10.7270/Q26111RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM27947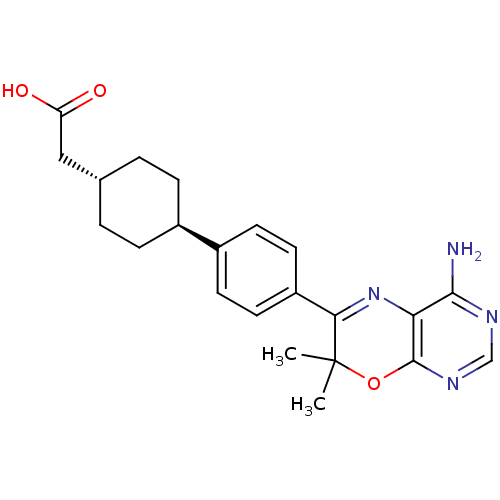 (2-[4-(4-{4-amino-7,7-dimethyl-7H-pyrimido[4,5-b][1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of DGAT1-mediated triglyceride synthesis in human HT-29 cells using [3H]glycerol as substrate after 6 hrs by beta counting | ACS Med Chem Lett 2: 407-412 (2011) Article DOI: 10.1021/ml200051p BindingDB Entry DOI: 10.7270/Q2MW2HM6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50158249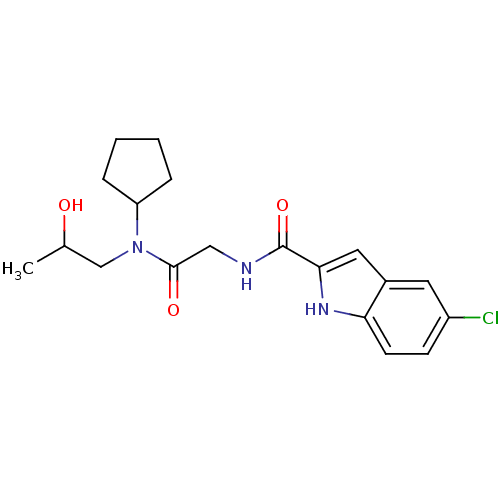 (5-Chloro-1H-indole-2-carboxylic acid {[cyclopentyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against glycogen phosphorylase | Bioorg Med Chem Lett 15: 459-65 (2004) Article DOI: 10.1016/j.bmcl.2004.10.048 BindingDB Entry DOI: 10.7270/Q26W99K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50355142 (CHEMBL1835919) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of full length human microsomal DGAT-1 expressed in baculovirus infected insect Sf9 cells using [14C]decanoylCoA as substrate after 1.5 hr... | ACS Med Chem Lett 2: 407-412 (2011) Article DOI: 10.1021/ml200051p BindingDB Entry DOI: 10.7270/Q2MW2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50438743 (CHEMBL2414687) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human full length DGAT-1 expressed in insect sf9 cells using [14C]decanoylCoA as substrate after 1.5 hrs by scintillation spectrometry | Bioorg Med Chem 21: 5081-97 (2013) Article DOI: 10.1016/j.bmc.2013.06.045 BindingDB Entry DOI: 10.7270/Q26111RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50355142 (CHEMBL1835919) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human full length DGAT-1 expressed in insect sf9 cells using [14C]decanoylCoA as substrate after 1.5 hrs by scintillation spectrometry | Bioorg Med Chem 21: 5081-97 (2013) Article DOI: 10.1016/j.bmc.2013.06.045 BindingDB Entry DOI: 10.7270/Q26111RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50438735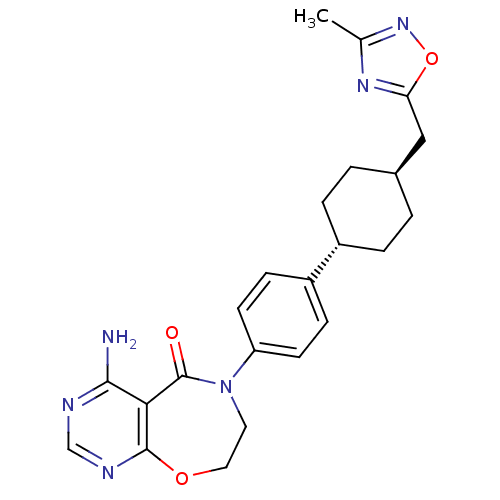 (CHEMBL2414670) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of DGAT-1-mediated triglyceride synthesis in human HT-29 cells using [3H]-glycerol as substrate incubated for 1 hr prior to substrate addi... | Bioorg Med Chem 21: 5081-97 (2013) Article DOI: 10.1016/j.bmc.2013.06.045 BindingDB Entry DOI: 10.7270/Q26111RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50438726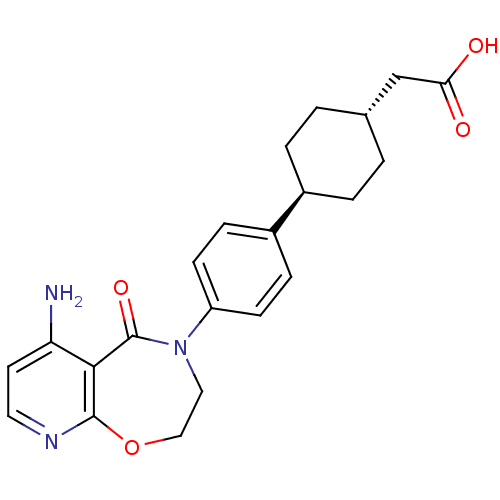 (CHEMBL2414684) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human full length DGAT-1 expressed in insect sf9 cells using [14C]decanoylCoA as substrate after 1.5 hrs by scintillation spectrometry | Bioorg Med Chem 21: 5081-97 (2013) Article DOI: 10.1016/j.bmc.2013.06.045 BindingDB Entry DOI: 10.7270/Q26111RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50438737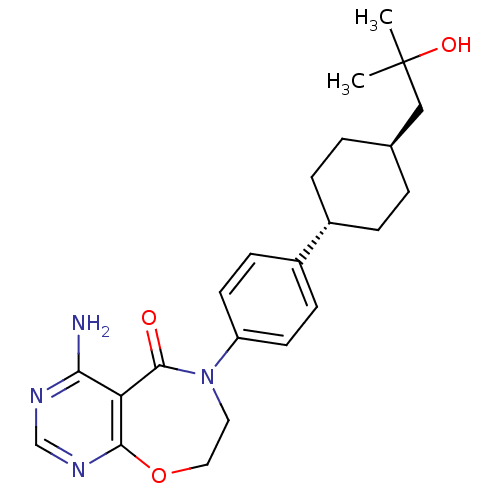 (CHEMBL2414668) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human full length DGAT-1 expressed in insect sf9 cells using [14C]decanoylCoA as substrate after 1.5 hrs by scintillation spectrometry | Bioorg Med Chem 21: 5081-97 (2013) Article DOI: 10.1016/j.bmc.2013.06.045 BindingDB Entry DOI: 10.7270/Q26111RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50438712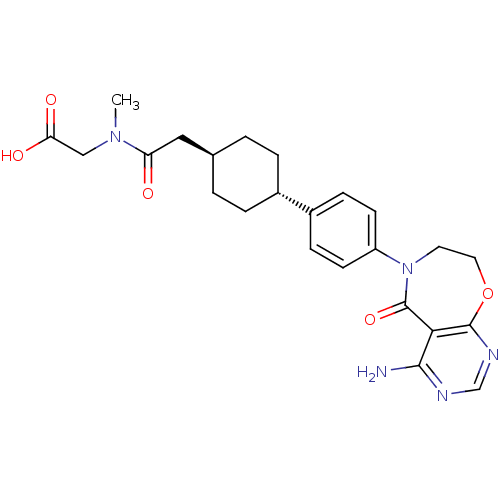 (CHEMBL2414697) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human full length DGAT-1 expressed in insect sf9 cells using [14C]decanoylCoA as substrate after 1.5 hrs by scintillation spectrometry | Bioorg Med Chem 21: 5081-97 (2013) Article DOI: 10.1016/j.bmc.2013.06.045 BindingDB Entry DOI: 10.7270/Q26111RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50158255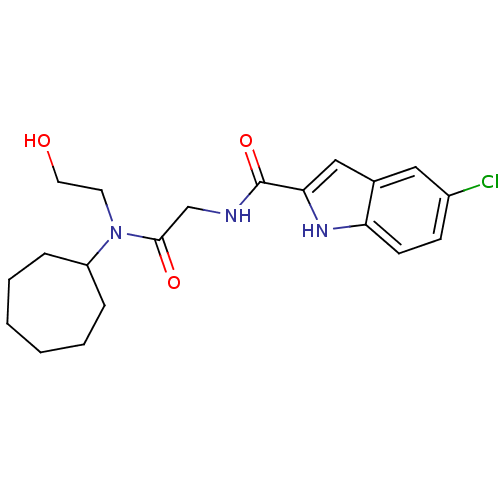 (5-Chloro-1H-indole-2-carboxylic acid {[cycloheptyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against glycogen phosphorylase | Bioorg Med Chem Lett 15: 459-65 (2004) Article DOI: 10.1016/j.bmcl.2004.10.048 BindingDB Entry DOI: 10.7270/Q26W99K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50158254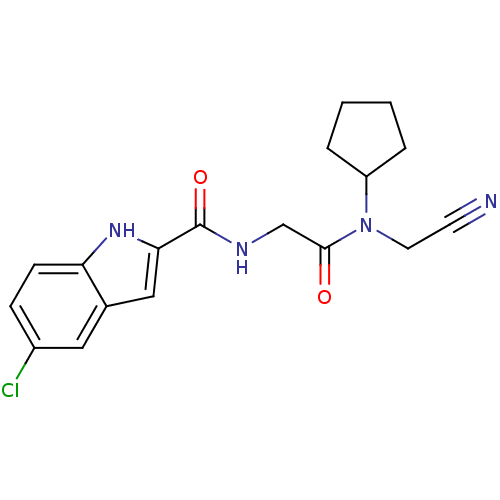 (5-Chloro-1H-indole-2-carboxylic acid [(cyanomethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against glycogen phosphorylase | Bioorg Med Chem Lett 15: 459-65 (2004) Article DOI: 10.1016/j.bmcl.2004.10.048 BindingDB Entry DOI: 10.7270/Q26W99K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 304 total ) | Next | Last >> |