

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50338518 (1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma | Bioorg Med Chem Lett 21: 1810-4 (2011) Article DOI: 10.1016/j.bmcl.2011.01.055 BindingDB Entry DOI: 10.7270/Q28W3DM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50338518 (1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 21: 1810-4 (2011) Article DOI: 10.1016/j.bmcl.2011.01.055 BindingDB Entry DOI: 10.7270/Q28W3DM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16452 ((4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta. | J Med Chem 34: 108-22 (1991) BindingDB Entry DOI: 10.7270/Q2513X6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50338518 (1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of POP | Bioorg Med Chem Lett 21: 1810-4 (2011) Article DOI: 10.1016/j.bmcl.2011.01.055 BindingDB Entry DOI: 10.7270/Q28W3DM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50338518 (1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of DPP9 | Bioorg Med Chem Lett 21: 1810-4 (2011) Article DOI: 10.1016/j.bmcl.2011.01.055 BindingDB Entry DOI: 10.7270/Q28W3DM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Homo sapiens (Human)) | BDBM50338518 (1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of DPP3 | Bioorg Med Chem Lett 21: 1810-4 (2011) Article DOI: 10.1016/j.bmcl.2011.01.055 BindingDB Entry DOI: 10.7270/Q28W3DM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM50338518 (1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of FAP | Bioorg Med Chem Lett 21: 1810-4 (2011) Article DOI: 10.1016/j.bmcl.2011.01.055 BindingDB Entry DOI: 10.7270/Q28W3DM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50338518 (1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of DPP2 | Bioorg Med Chem Lett 21: 1810-4 (2011) Article DOI: 10.1016/j.bmcl.2011.01.055 BindingDB Entry DOI: 10.7270/Q28W3DM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16315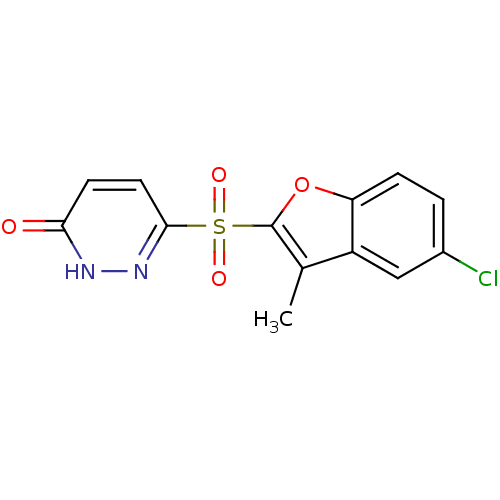 (6-[(5-chloro-3-methyl-1-benzofuran-2-)sulfonyl]-2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | 7.0 | 24 |
Pfizer | Assay Description The activity of the enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappearance ... | J Med Chem 46: 2283-6 (2003) Article DOI: 10.1021/jm034065z BindingDB Entry DOI: 10.7270/Q2NV9GGM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50009806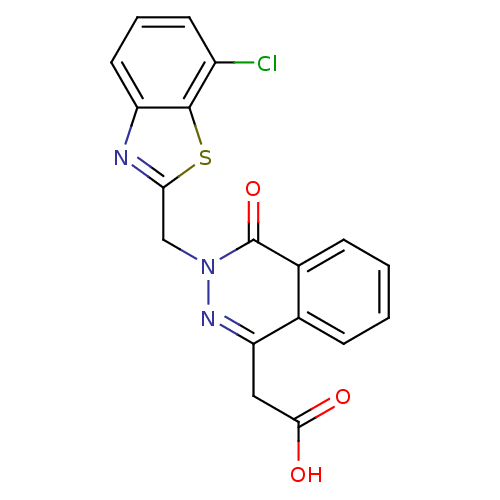 (CHEMBL20207 | [3-(7-Chloro-benzothiazol-2-ylmethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta. | J Med Chem 34: 108-22 (1991) BindingDB Entry DOI: 10.7270/Q2513X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16315 (6-[(5-chloro-3-methyl-1-benzofuran-2-)sulfonyl]-2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The activity of the enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappearance ... | J Med Chem 48: 6326-39 (2005) Article DOI: 10.1021/jm050462t BindingDB Entry DOI: 10.7270/Q2RN363Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50338507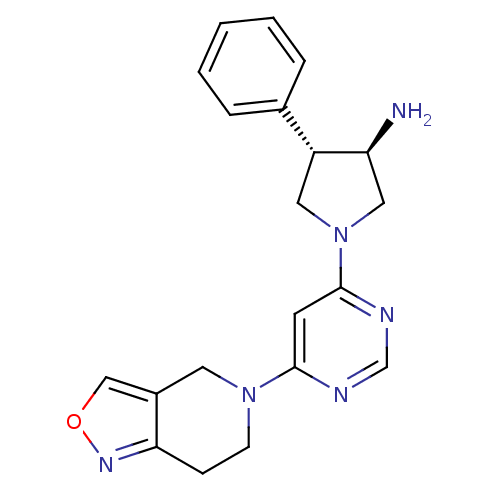 ((3R,4S)-1-(6-(6,7-dihydroisoxazolo[4,3-c]pyridin-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 21: 1810-4 (2011) Article DOI: 10.1016/j.bmcl.2011.01.055 BindingDB Entry DOI: 10.7270/Q28W3DM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16452 ((4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Compound was tested for the inhibition of the human placental aldose reductase using the substrate as glyceraldehyde. | J Med Chem 34: 108-22 (1991) BindingDB Entry DOI: 10.7270/Q2513X6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16452 ((4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Compound was tested for the rate of reduction of glyceraldehyde by human placental aldose reductase. | J Med Chem 34: 108-22 (1991) BindingDB Entry DOI: 10.7270/Q2513X6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50009763 (CHEMBL20024 | [3-(4-Fluoro-benzothiazol-2-ylmethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta. | J Med Chem 34: 108-22 (1991) BindingDB Entry DOI: 10.7270/Q2513X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50006469 (CHEMBL69956 | [8-Oxo-7-(5-trifluoromethyl-benzothi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibitory activity against aldose reductase isolated from human placenta | J Med Chem 35: 2155-62 (1992) BindingDB Entry DOI: 10.7270/Q2KK99RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16633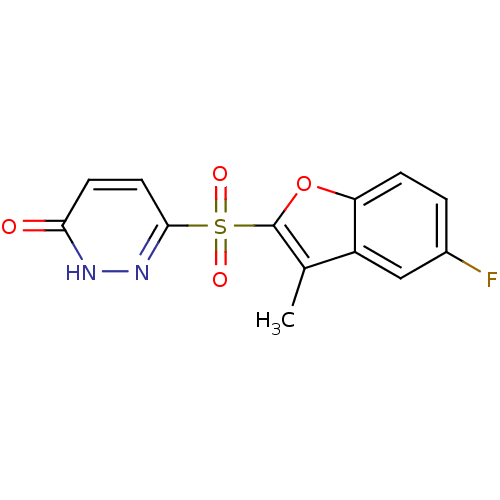 (6-[(5-fluoro-3-methyl-1-benzofuran-2-)sulfonyl]-2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The activity of the enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappearance ... | J Med Chem 48: 6326-39 (2005) Article DOI: 10.1021/jm050462t BindingDB Entry DOI: 10.7270/Q2RN363Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50009781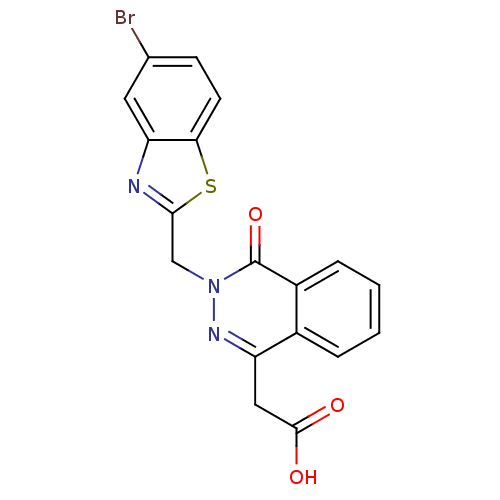 (CHEMBL20169 | [3-(5-Bromo-benzothiazol-2-ylmethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta. | J Med Chem 34: 108-22 (1991) BindingDB Entry DOI: 10.7270/Q2513X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16452 ((4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Compound was tested for the inhibition of the rat lens aldose reductase using the substrate as glucose. | J Med Chem 34: 108-22 (1991) BindingDB Entry DOI: 10.7270/Q2513X6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16452 ((4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description In vitro inhibitory activity against aldose reductase isolated from human placenta | J Med Chem 35: 457-65 (1992) BindingDB Entry DOI: 10.7270/Q28051K1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50008471 (CHEMBL143234 | [3-(5,7-Difluoro-benzooxazol-2-ylme...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description In vitro inhibitory activity against aldose reductase isolated from human placenta | J Med Chem 35: 457-65 (1992) BindingDB Entry DOI: 10.7270/Q28051K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50006475 (CHEMBL71966 | {1-[3-(2,3-Difluoro-phenyl)-[1,2,4]o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibitory activity against aldose reductase isolated from human placenta | J Med Chem 35: 2155-62 (1992) BindingDB Entry DOI: 10.7270/Q2KK99RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50009828 (CHEMBL20015 | [3-(4-Chloro-benzothiazol-2-ylmethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta. | J Med Chem 34: 108-22 (1991) BindingDB Entry DOI: 10.7270/Q2513X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50009784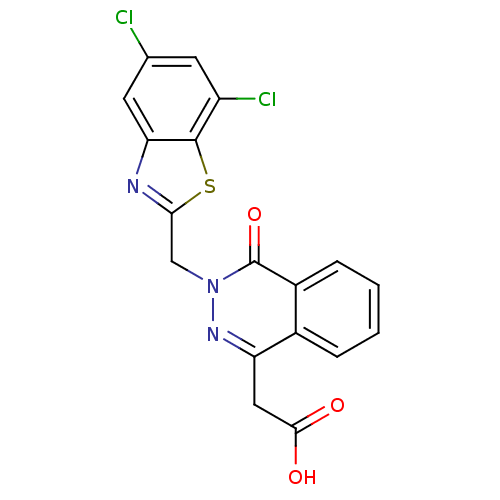 (CHEMBL278991 | [3-(5,7-Dichloro-benzothiazol-2-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta. | J Med Chem 34: 108-22 (1991) BindingDB Entry DOI: 10.7270/Q2513X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50006459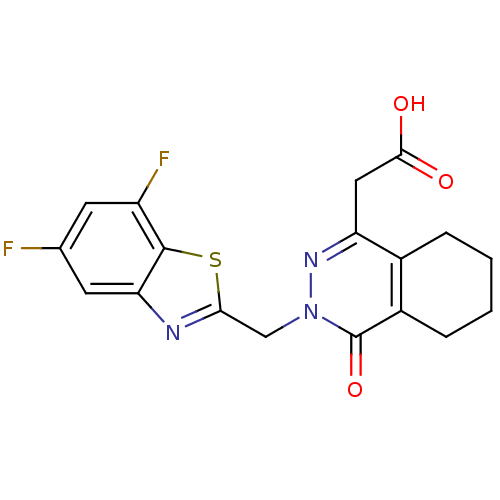 (CHEMBL304995 | [3-(5,7-Difluoro-benzothiazol-2-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibitory activity against aldose reductase isolated from human placenta | J Med Chem 35: 2155-62 (1992) BindingDB Entry DOI: 10.7270/Q2KK99RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16452 ((4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.0 | 24 |
Pfizer | Assay Description The activity of the enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappearance ... | J Med Chem 46: 2283-6 (2003) Article DOI: 10.1021/jm034065z BindingDB Entry DOI: 10.7270/Q2NV9GGM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sorbitol dehydrogenase (Homo sapiens (Human)) | BDBM50118710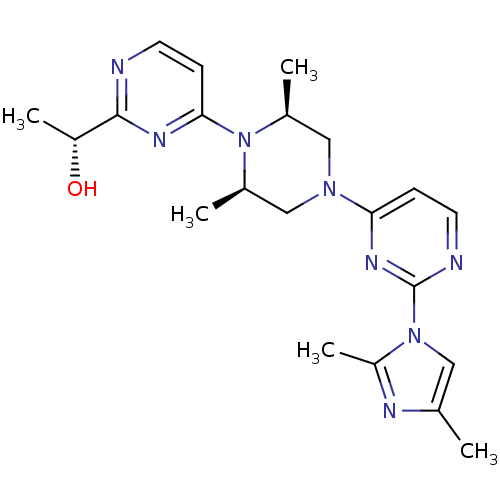 (1-(4-{4-[2-(2,4-Dimethyl-imidazol-1-yl)-pyrimidin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required for 50% for in vitro activity against human SDH (sorbitol dehydrogenase) | J Med Chem 45: 4398-401 (2002) BindingDB Entry DOI: 10.7270/Q2V69HZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sorbitol dehydrogenase (Homo sapiens (Human)) | BDBM50118706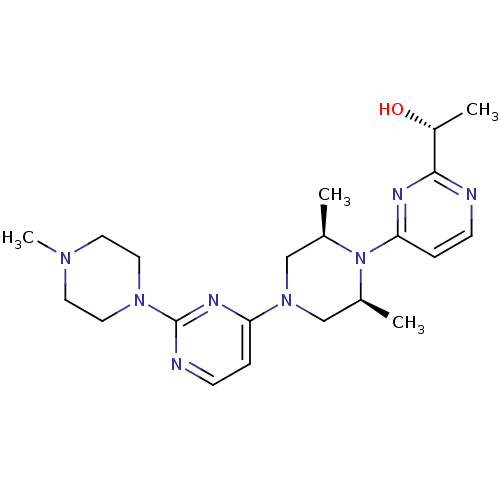 ((S)-1-(4-{2,6-Dimethyl-4-[2-(4-methyl-piperazin-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required for 50% for in vitro activity against human SDH (sorbitol dehydrogenase) | J Med Chem 45: 4398-401 (2002) BindingDB Entry DOI: 10.7270/Q2V69HZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50278106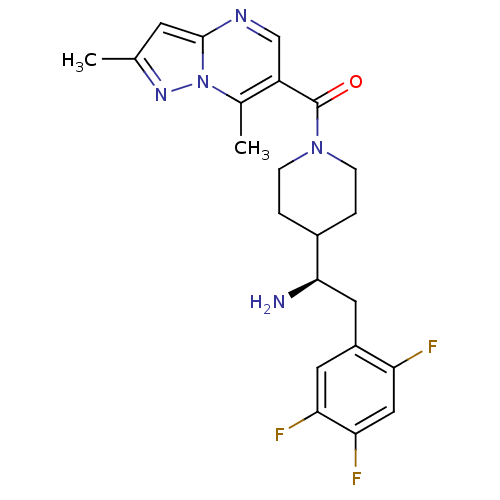 ((R)-(4-(1-amino-2-(2,4,5-trifluorophenyl)ethyl)pip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 19: 2220-3 (2009) Article DOI: 10.1016/j.bmcl.2009.02.099 BindingDB Entry DOI: 10.7270/Q2RN37QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50006470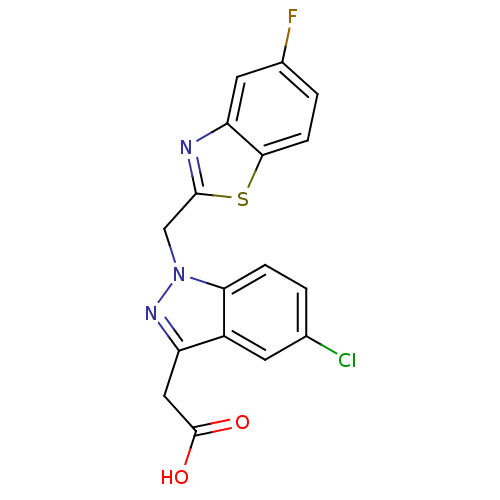 (CHEMBL73505 | [5-Chloro-1-(5-fluoro-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibitory activity against aldose reductase isolated from human placenta | J Med Chem 35: 2155-62 (1992) BindingDB Entry DOI: 10.7270/Q2KK99RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16452 ((4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibitory activity against aldose reductase isolated from human placenta | J Med Chem 35: 2155-62 (1992) BindingDB Entry DOI: 10.7270/Q2KK99RR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sorbitol dehydrogenase (Rattus norvegicus) | BDBM50118709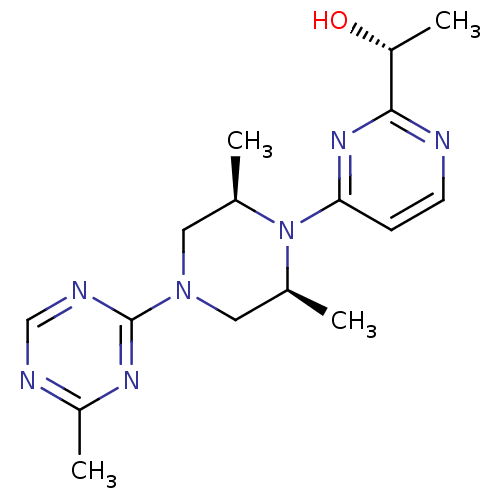 (1-{4-[2,6-Dimethyl-4-(4-methyl-[1,3,5]triazin-2-yl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required for 50% in vitro activity against rat SDH (sorbitol dehydrogenase) | J Med Chem 45: 4398-401 (2002) BindingDB Entry DOI: 10.7270/Q2V69HZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50009759 (CHEMBL20518 | [3-(5-Chloro-7-fluoro-benzothiazol-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta. | J Med Chem 34: 108-22 (1991) BindingDB Entry DOI: 10.7270/Q2513X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50006481 (CHEMBL73560 | [1-(5,7-Difluoro-benzothiazol-2-ylme...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibitory activity against aldose reductase isolated from human placenta | J Med Chem 35: 2155-62 (1992) BindingDB Entry DOI: 10.7270/Q2KK99RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16634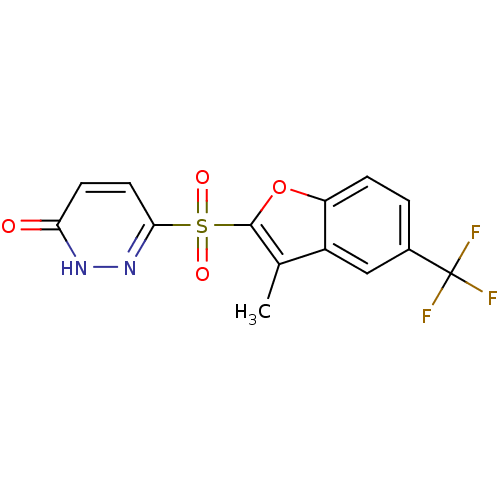 (6-{[3-methyl-5-(trifluoromethyl)-1-benzofuran-2-]s...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The activity of the enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappearance ... | J Med Chem 48: 6326-39 (2005) Article DOI: 10.1021/jm050462t BindingDB Entry DOI: 10.7270/Q2RN363Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50009750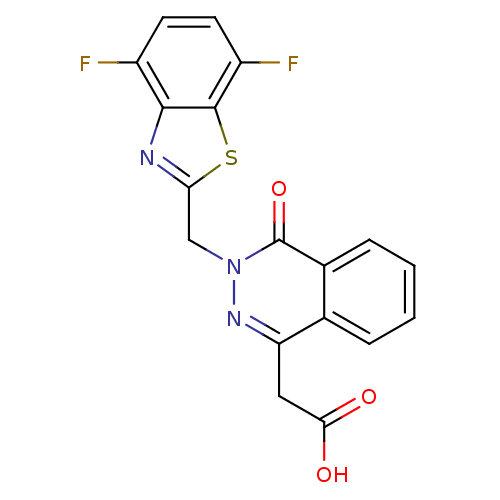 (CHEMBL282689 | [3-(4,7-Difluoro-benzothiazol-2-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta. | J Med Chem 34: 108-22 (1991) BindingDB Entry DOI: 10.7270/Q2513X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50006471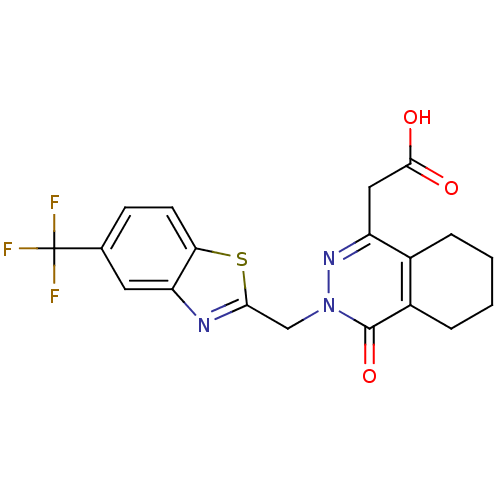 (CHEMBL68580 | [4-Oxo-3-(5-trifluoromethyl-benzothi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibitory activity against aldose reductase isolated from human placenta | J Med Chem 35: 2155-62 (1992) BindingDB Entry DOI: 10.7270/Q2KK99RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50009774 (CHEMBL20161 | [3-(5,7-Dimethyl-benzothiazol-2-ylme...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta. | J Med Chem 34: 108-22 (1991) BindingDB Entry DOI: 10.7270/Q2513X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16636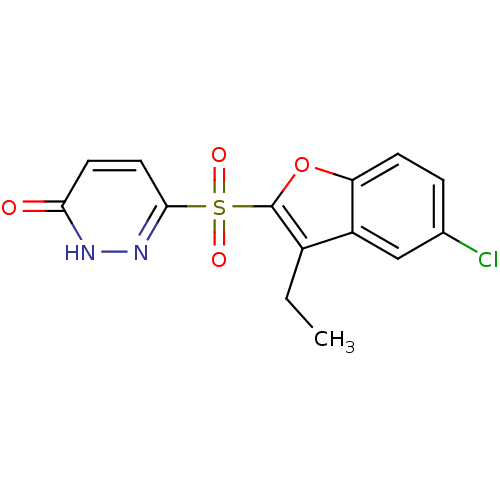 (6-[(5-chloro-3-ethyl-1-benzofuran-2-)sulfonyl]-2,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The activity of the enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappearance ... | J Med Chem 48: 6326-39 (2005) Article DOI: 10.1021/jm050462t BindingDB Entry DOI: 10.7270/Q2RN363Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sorbitol dehydrogenase (Rattus norvegicus) | BDBM50118706 ((S)-1-(4-{2,6-Dimethyl-4-[2-(4-methyl-piperazin-1-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required for 50% in vitro activity against rat SDH (sorbitol dehydrogenase) | J Med Chem 45: 4398-401 (2002) BindingDB Entry DOI: 10.7270/Q2V69HZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50009749 (CHEMBL416001 | [3-(7-Chloro-5-fluoro-benzothiazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta. | J Med Chem 34: 108-22 (1991) BindingDB Entry DOI: 10.7270/Q2513X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50009757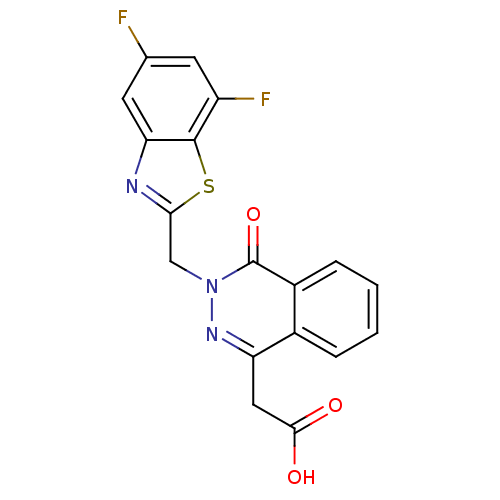 (CHEMBL20637 | [3-(5,7-Difluoro-benzothiazol-2-ylme...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta. | J Med Chem 34: 108-22 (1991) BindingDB Entry DOI: 10.7270/Q2513X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50008441 (CHEMBL422647 | [4-Oxo-3-(3-o-tolyl-[1,2,4]oxadiazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description In vitro inhibitory activity against aldose reductase isolated from human placenta | J Med Chem 35: 457-65 (1992) BindingDB Entry DOI: 10.7270/Q28051K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50008480 (CHEMBL341864 | {3-[3-(2-Bromo-phenyl)-[1,2,4]oxadi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description In vitro inhibitory activity against aldose reductase isolated from human placenta | J Med Chem 35: 457-65 (1992) BindingDB Entry DOI: 10.7270/Q28051K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50009804 (CHEMBL283996 | [3-(5-Fluoro-benzothiazol-2-ylmethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta. | J Med Chem 34: 108-22 (1991) BindingDB Entry DOI: 10.7270/Q2513X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sorbitol dehydrogenase (Homo sapiens (Human)) | BDBM50118709 (1-{4-[2,6-Dimethyl-4-(4-methyl-[1,3,5]triazin-2-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required for 50% for in vitro activity against human SDH (sorbitol dehydrogenase) | J Med Chem 45: 4398-401 (2002) BindingDB Entry DOI: 10.7270/Q2V69HZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sorbitol dehydrogenase (Rattus norvegicus) | BDBM50118710 (1-(4-{4-[2-(2,4-Dimethyl-imidazol-1-yl)-pyrimidin-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required for 50% in vitro activity against rat SDH (sorbitol dehydrogenase) | J Med Chem 45: 4398-401 (2002) BindingDB Entry DOI: 10.7270/Q2V69HZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sorbitol dehydrogenase (Homo sapiens (Human)) | BDBM50118708 (1-{4-[2,6-Dimethyl-4-(4-phenyl-[1,3,5]triazin-2-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required for 50% for in vitro activity against human SDH (sorbitol dehydrogenase) | J Med Chem 45: 4398-401 (2002) BindingDB Entry DOI: 10.7270/Q2V69HZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50009829 (CHEMBL20197 | [3-(5-Chloro-benzothiazol-2-ylmethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta. | J Med Chem 34: 108-22 (1991) BindingDB Entry DOI: 10.7270/Q2513X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50338517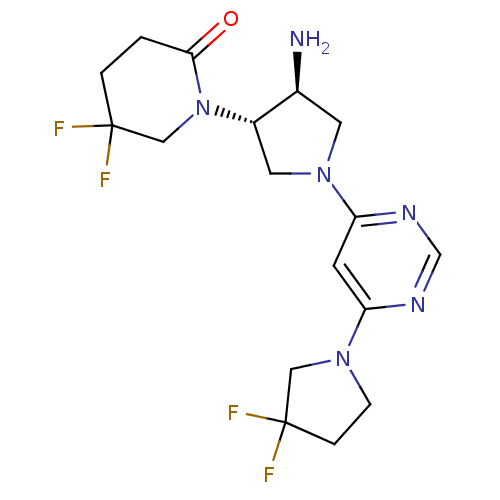 (1-((3S,4S)-4-amino-1-(6-(3,3-difluoropyrrolidin-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 21: 1810-4 (2011) Article DOI: 10.1016/j.bmcl.2011.01.055 BindingDB Entry DOI: 10.7270/Q28W3DM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 483 total ) | Next | Last >> |