 Found 302 hits with Last Name = 'zeng' and Initial = 'z'
Found 302 hits with Last Name = 'zeng' and Initial = 'z' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50162774

(ABT-199 | US11420968, Example ABT-199 | Venetoclax)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)N(=O)=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50240339
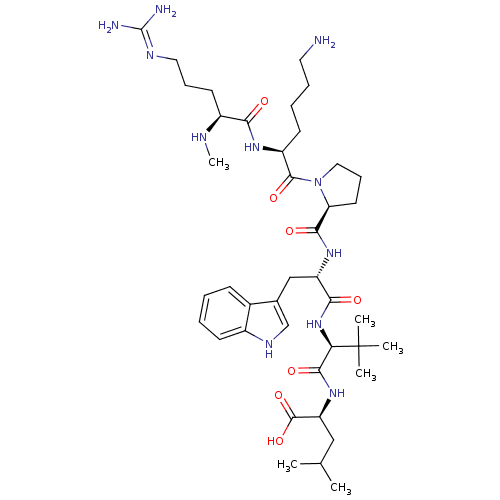
((S)-2-((S)-2-((S)-2-((S)-1-((S)-6-amino-2-((S)-5-g...)Show SMILES CN[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C(O)=O)C(C)(C)C |r,wU:2.1,43.45,wD:25.26,29.29,47.49,13.12,(-9.76,-17.57,;-8.43,-18.35,;-7.09,-17.6,;-7.09,-16.06,;-8.41,-15.28,;-8.4,-13.73,;-9.73,-12.95,;-9.72,-11.41,;-11.06,-10.63,;-8.38,-10.64,;-5.76,-18.38,;-5.77,-19.91,;-4.42,-17.61,;-3.09,-18.39,;-3.1,-19.93,;-4.44,-20.7,;-4.45,-22.24,;-5.78,-23,;-5.8,-24.54,;-1.74,-17.63,;-1.73,-16.1,;-.42,-18.41,;-0,-19.94,;1.55,-19.94,;2.08,-18.51,;.87,-17.56,;.94,-16,;-.36,-15.18,;2.3,-15.29,;3.6,-16.11,;3.53,-17.65,;4.83,-18.49,;6.26,-17.92,;7.23,-19.11,;6.4,-20.41,;6.81,-21.89,;5.72,-22.98,;4.24,-22.59,;3.84,-21.1,;4.92,-20.02,;4.97,-15.4,;5.05,-13.86,;6.27,-16.23,;7.64,-15.53,;8.93,-16.36,;8.86,-17.9,;10.3,-15.65,;11.59,-16.49,;11.52,-18.02,;12.82,-18.86,;12.82,-20.4,;14.19,-18.15,;12.96,-15.78,;14.25,-16.61,;13.04,-14.25,;7.72,-13.99,;7.71,-12.44,;9.26,-14.02,;6.18,-13.96,)| Show InChI InChI=1S/C41H67N11O7/c1-24(2)21-31(39(58)59)50-37(56)33(41(3,4)5)51-35(54)30(22-25-23-47-27-14-8-7-13-26(25)27)49-36(55)32-17-12-20-52(32)38(57)29(15-9-10-18-42)48-34(53)28(45-6)16-11-19-46-40(43)44/h7-8,13-14,23-24,28-33,45,47H,9-12,15-22,42H2,1-6H3,(H,48,53)(H,49,55)(H,50,56)(H,51,54)(H,58,59)(H4,43,44,46)/t28-,29-,30-,31-,32-,33+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 305-13 (2002)
Article DOI: 10.1124/jpet.300.1.305
BindingDB Entry DOI: 10.7270/Q2X34W09 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM85790
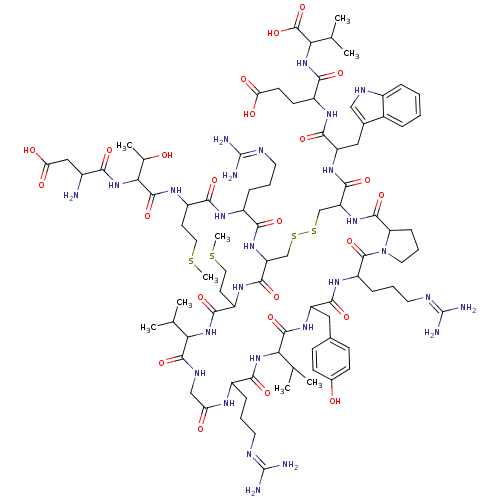
(Salmon MCH)Show SMILES CSCCC(NC(=O)C(NC(=O)C(N)CC(O)=O)C(C)O)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(NC(=O)C(CCSC)NC1=O)C(C)C)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(O)=O)C(=O)NC(C(C)C)C(O)=O |(16.32,3.32,;17.66,2.55,;18.99,3.32,;18.99,4.86,;20.32,5.63,;20.32,7.17,;21.66,7.94,;22.99,7.17,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;25.66,11.79,;24.33,14.1,;25.66,14.87,;25.66,16.41,;26.99,14.1,;20.32,10.25,;18.99,9.48,;20.32,11.79,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;26.99,-15.16,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;30.99,2.55,;32.33,3.32,;29.66,3.32,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;7.91,-1.3,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C89H139N27O24S4/c1-43(2)67-82(135)101-40-64(119)102-53(18-12-30-97-87(91)92)74(127)113-68(44(3)4)83(136)109-59(36-47-22-24-49(118)25-23-47)77(130)107-58(20-14-32-99-89(95)96)85(138)116-33-15-21-63(116)81(134)111-62(80(133)108-60(37-48-39-100-52-17-11-10-16-50(48)52)78(131)104-55(26-27-65(120)121)75(128)114-69(45(5)6)86(139)140)42-144-143-41-61(79(132)105-57(29-35-142-9)76(129)112-67)110-72(125)54(19-13-31-98-88(93)94)103-73(126)56(28-34-141-8)106-84(137)70(46(7)117)115-71(124)51(90)38-66(122)123/h10-11,16-17,22-25,39,43-46,51,53-63,67-70,100,117-118H,12-15,18-21,26-38,40-42,90H2,1-9H3,(H,101,135)(H,102,119)(H,103,126)(H,104,131)(H,105,132)(H,106,137)(H,107,130)(H,108,133)(H,109,136)(H,110,125)(H,111,134)(H,112,129)(H,113,127)(H,114,128)(H,115,124)(H,120,121)(H,122,123)(H,139,140)(H4,91,92,97)(H4,93,94,98)(H4,95,96,99) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM82078
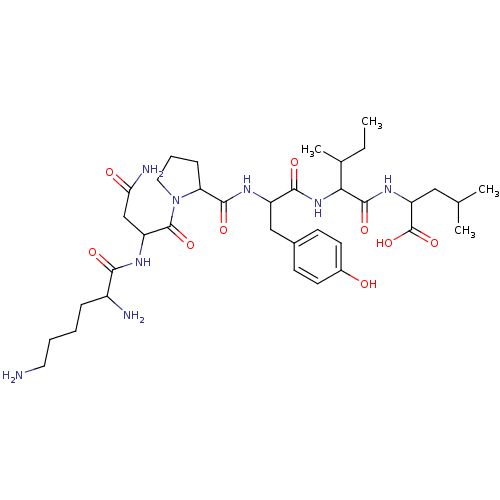
(CAS_55508-42-4 | NSC_128644 | Neurotensin)Show SMILES CCC(C)C(NC(=O)C(Cc1ccc(O)cc1)NC(=O)C1CCCN1C(=O)C(CC(N)=O)NC(=O)C(N)CCCCN)C(=O)NC(CC(C)C)C(O)=O Show InChI InChI=1S/C36H58N8O9/c1-5-21(4)30(34(50)42-27(36(52)53)17-20(2)3)43-32(48)25(18-22-11-13-23(45)14-12-22)40-33(49)28-10-8-16-44(28)35(51)26(19-29(39)46)41-31(47)24(38)9-6-7-15-37/h11-14,20-21,24-28,30,45H,5-10,15-19,37-38H2,1-4H3,(H2,39,46)(H,40,49)(H,41,47)(H,42,50)(H,43,48)(H,52,53) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 305-13 (2002)
Article DOI: 10.1124/jpet.300.1.305
BindingDB Entry DOI: 10.7270/Q2X34W09 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM85789
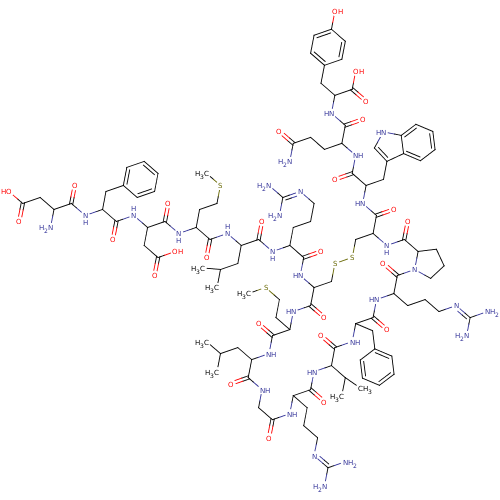
([Phe13,Tyr19]MCH)Show SMILES CSCCC(NC(=O)C(CC(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)C(N)CC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccccc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(CC(C)C)NC(=O)C(CCSC)NC1=O)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(N)=O)C(=O)NC(Cc1ccc(O)cc1)C(O)=O |(16.32,9.48,;17.66,10.25,;18.99,9.48,;20.32,10.25,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;24.33,14.1,;23.24,15.19,;23.63,16.68,;21.75,14.79,;25.66,11.79,;26.99,12.56,;26.99,14.1,;28.33,11.79,;28.33,10.25,;29.66,9.48,;29.66,7.94,;30.99,7.17,;32.33,7.94,;32.33,9.48,;30.99,10.25,;29.66,12.56,;31.15,12.16,;31.55,10.67,;32.48,12.93,;33.81,12.16,;32.48,14.47,;33.81,15.24,;33.81,16.78,;35.3,14.84,;21.66,7.94,;22.99,7.17,;20.32,7.17,;20.32,5.63,;18.99,4.86,;18.99,3.32,;17.66,2.55,;20.32,2.55,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;30.99,2.55,;32.33,3.32,;32.33,4.86,;33.66,2.55,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;5.24,.24,;3.91,1.01,;2.57,.24,;1.24,1.01,;2.57,-1.3,;3.91,-2.07,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C109H160N30O26S4/c1-57(2)45-74-90(148)122-54-85(142)123-68(27-17-39-118-107(112)113)95(153)138-88(59(5)6)104(162)134-77(48-61-23-13-10-14-24-61)97(155)128-73(29-19-41-120-109(116)117)105(163)139-42-20-30-83(139)103(161)137-82(102(160)132-78(50-63-53-121-67-26-16-15-25-65(63)67)99(157)125-70(35-36-84(111)141)92(150)135-80(106(164)165)49-62-31-33-64(140)34-32-62)56-169-168-55-81(101(159)127-72(38-44-167-8)93(151)130-74)136-91(149)69(28-18-40-119-108(114)115)124-96(154)75(46-58(3)4)131-94(152)71(37-43-166-7)126-100(158)79(52-87(145)146)133-98(156)76(47-60-21-11-9-12-22-60)129-89(147)66(110)51-86(143)144/h9-16,21-26,31-34,53,57-59,66,68-83,88,121,140H,17-20,27-30,35-52,54-56,110H2,1-8H3,(H2,111,141)(H,122,148)(H,123,142)(H,124,154)(H,125,157)(H,126,158)(H,127,159)(H,128,155)(H,129,147)(H,130,151)(H,131,152)(H,132,160)(H,133,156)(H,134,162)(H,135,150)(H,136,149)(H,137,161)(H,138,153)(H,143,144)(H,145,146)(H,164,165)(H4,112,113,118)(H4,114,115,119)(H4,116,117,120) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 2
(Homo sapiens (Human)) | BDBM85789

([Phe13,Tyr19]MCH)Show SMILES CSCCC(NC(=O)C(CC(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)C(N)CC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccccc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(CC(C)C)NC(=O)C(CCSC)NC1=O)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(N)=O)C(=O)NC(Cc1ccc(O)cc1)C(O)=O |(16.32,9.48,;17.66,10.25,;18.99,9.48,;20.32,10.25,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;24.33,14.1,;23.24,15.19,;23.63,16.68,;21.75,14.79,;25.66,11.79,;26.99,12.56,;26.99,14.1,;28.33,11.79,;28.33,10.25,;29.66,9.48,;29.66,7.94,;30.99,7.17,;32.33,7.94,;32.33,9.48,;30.99,10.25,;29.66,12.56,;31.15,12.16,;31.55,10.67,;32.48,12.93,;33.81,12.16,;32.48,14.47,;33.81,15.24,;33.81,16.78,;35.3,14.84,;21.66,7.94,;22.99,7.17,;20.32,7.17,;20.32,5.63,;18.99,4.86,;18.99,3.32,;17.66,2.55,;20.32,2.55,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;30.99,2.55,;32.33,3.32,;32.33,4.86,;33.66,2.55,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;5.24,.24,;3.91,1.01,;2.57,.24,;1.24,1.01,;2.57,-1.3,;3.91,-2.07,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C109H160N30O26S4/c1-57(2)45-74-90(148)122-54-85(142)123-68(27-17-39-118-107(112)113)95(153)138-88(59(5)6)104(162)134-77(48-61-23-13-10-14-24-61)97(155)128-73(29-19-41-120-109(116)117)105(163)139-42-20-30-83(139)103(161)137-82(102(160)132-78(50-63-53-121-67-26-16-15-25-65(63)67)99(157)125-70(35-36-84(111)141)92(150)135-80(106(164)165)49-62-31-33-64(140)34-32-62)56-169-168-55-81(101(159)127-72(38-44-167-8)93(151)130-74)136-91(149)69(28-18-40-119-108(114)115)124-96(154)75(46-58(3)4)131-94(152)71(37-43-166-7)126-100(158)79(52-87(145)146)133-98(156)76(47-60-21-11-9-12-22-60)129-89(147)66(110)51-86(143)144/h9-16,21-26,31-34,53,57-59,66,68-83,88,121,140H,17-20,27-30,35-52,54-56,110H2,1-8H3,(H2,111,141)(H,122,148)(H,123,142)(H,124,154)(H,125,157)(H,126,158)(H,127,159)(H,128,155)(H,129,147)(H,130,151)(H,131,152)(H,132,160)(H,133,156)(H,134,162)(H,135,150)(H,136,149)(H,137,161)(H,138,153)(H,143,144)(H,145,146)(H,164,165)(H4,112,113,118)(H4,114,115,119)(H4,116,117,120) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 2
(Homo sapiens (Human)) | BDBM85788
(MCH | hMCH)Show SMILES CSCCC(NC(=O)C(CC(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)C(N)CC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(CC(C)C)NC(=O)C(CCSC)NC1=O)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(N)=O)C(=O)NC(C(C)C)C(O)=O |(16.32,9.48,;17.66,10.25,;18.99,9.48,;20.32,10.25,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;24.33,14.1,;23.24,15.19,;23.63,16.68,;21.75,14.79,;25.66,11.79,;26.99,12.56,;26.99,14.1,;28.33,11.79,;28.33,10.25,;29.66,9.48,;29.66,7.94,;30.99,7.17,;32.33,7.94,;32.33,9.48,;30.99,10.25,;29.66,12.56,;31.15,12.16,;31.55,10.67,;32.48,12.93,;33.81,12.16,;32.48,14.47,;33.81,15.24,;33.81,16.78,;35.3,14.84,;21.66,7.94,;22.99,7.17,;20.32,7.17,;20.32,5.63,;18.99,4.86,;18.99,3.32,;17.66,2.55,;20.32,2.55,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;26.99,-15.16,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;30.99,2.55,;32.33,3.32,;32.33,4.86,;33.66,2.55,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;7.91,-1.3,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C105H160N30O26S4/c1-53(2)42-70-86(144)118-50-80(138)119-64(24-16-36-114-103(108)109)90(148)133-83(55(5)6)100(158)130-73(45-58-28-30-60(136)31-29-58)93(151)124-69(26-18-38-116-105(112)113)101(159)135-39-19-27-78(135)99(157)132-77(98(156)128-74(46-59-49-117-63-23-15-14-22-61(59)63)95(153)121-66(32-33-79(107)137)91(149)134-84(56(7)8)102(160)161)52-165-164-51-76(97(155)123-68(35-41-163-10)88(146)126-70)131-87(145)65(25-17-37-115-104(110)111)120-92(150)71(43-54(3)4)127-89(147)67(34-40-162-9)122-96(154)75(48-82(141)142)129-94(152)72(44-57-20-12-11-13-21-57)125-85(143)62(106)47-81(139)140/h11-15,20-23,28-31,49,53-56,62,64-78,83-84,117,136H,16-19,24-27,32-48,50-52,106H2,1-10H3,(H2,107,137)(H,118,144)(H,119,138)(H,120,150)(H,121,153)(H,122,154)(H,123,155)(H,124,151)(H,125,143)(H,126,146)(H,127,147)(H,128,156)(H,129,152)(H,130,158)(H,131,145)(H,132,157)(H,133,148)(H,134,149)(H,139,140)(H,141,142)(H,160,161)(H4,108,109,114)(H4,110,111,115)(H4,112,113,116) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 2
(Homo sapiens (Human)) | BDBM81801
(CAS_5283121 | LTC4 | NSC_5283121)Show SMILES CCCCCC=CCC=CC=CC=CC(SCC(NC(=O)CCC(N)C(O)=O)C(=O)NCC(O)=O)C(O)CCCC(O)=O |w:5.4,8.7,10.9,12.11| Show InChI InChI=1S/C30H47N3O9S/c1-2-3-4-5-6-7-8-9-10-11-12-13-16-25(24(34)15-14-17-27(36)37)43-21-23(29(40)32-20-28(38)39)33-26(35)19-18-22(31)30(41)42/h6-7,9-13,16,22-25,34H,2-5,8,14-15,17-21,31H2,1H3,(H,32,40)(H,33,35)(H,36,37)(H,38,39)(H,41,42) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by PDSP Ki Database
| |
J Biol Chem 275: 30531-6 (2000)
Article DOI: 10.1074/jbc.M003490200
BindingDB Entry DOI: 10.7270/Q2BP01BH |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 2
(Homo sapiens (Human)) | BDBM50292408
((R-(R*,S*-(E,E,Z,Z)))-N-(S-(1-(4-Carboxy-1-hydroxy...)Show SMILES CCCCC\C=C/C\C=C/C=C/C=C/[C@@H](SC[C@H](N)C(=O)NCC(O)=O)[C@@H](O)CCCC(O)=O |r| Show InChI InChI=1S/C25H40N2O6S/c1-2-3-4-5-6-7-8-9-10-11-12-13-16-22(21(28)15-14-17-23(29)30)34-19-20(26)25(33)27-18-24(31)32/h6-7,9-13,16,20-22,28H,2-5,8,14-15,17-19,26H2,1H3,(H,27,33)(H,29,30)(H,31,32)/b7-6-,10-9-,12-11+,16-13+/t20-,21-,22+/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by PDSP Ki Database
| |
J Biol Chem 275: 30531-6 (2000)
Article DOI: 10.1074/jbc.M003490200
BindingDB Entry DOI: 10.7270/Q2BP01BH |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(MOUSE) | BDBM50240339

((S)-2-((S)-2-((S)-2-((S)-1-((S)-6-amino-2-((S)-5-g...)Show SMILES CN[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C(O)=O)C(C)(C)C |r,wU:2.1,43.45,wD:25.26,29.29,47.49,13.12,(-9.76,-17.57,;-8.43,-18.35,;-7.09,-17.6,;-7.09,-16.06,;-8.41,-15.28,;-8.4,-13.73,;-9.73,-12.95,;-9.72,-11.41,;-11.06,-10.63,;-8.38,-10.64,;-5.76,-18.38,;-5.77,-19.91,;-4.42,-17.61,;-3.09,-18.39,;-3.1,-19.93,;-4.44,-20.7,;-4.45,-22.24,;-5.78,-23,;-5.8,-24.54,;-1.74,-17.63,;-1.73,-16.1,;-.42,-18.41,;-0,-19.94,;1.55,-19.94,;2.08,-18.51,;.87,-17.56,;.94,-16,;-.36,-15.18,;2.3,-15.29,;3.6,-16.11,;3.53,-17.65,;4.83,-18.49,;6.26,-17.92,;7.23,-19.11,;6.4,-20.41,;6.81,-21.89,;5.72,-22.98,;4.24,-22.59,;3.84,-21.1,;4.92,-20.02,;4.97,-15.4,;5.05,-13.86,;6.27,-16.23,;7.64,-15.53,;8.93,-16.36,;8.86,-17.9,;10.3,-15.65,;11.59,-16.49,;11.52,-18.02,;12.82,-18.86,;12.82,-20.4,;14.19,-18.15,;12.96,-15.78,;14.25,-16.61,;13.04,-14.25,;7.72,-13.99,;7.71,-12.44,;9.26,-14.02,;6.18,-13.96,)| Show InChI InChI=1S/C41H67N11O7/c1-24(2)21-31(39(58)59)50-37(56)33(41(3,4)5)51-35(54)30(22-25-23-47-27-14-8-7-13-26(25)27)49-36(55)32-17-12-20-52(32)38(57)29(15-9-10-18-42)48-34(53)28(45-6)16-11-19-46-40(43)44/h7-8,13-14,23-24,28-33,45,47H,9-12,15-22,42H2,1-6H3,(H,48,53)(H,49,55)(H,50,56)(H,51,54)(H,58,59)(H4,43,44,46)/t28-,29-,30-,31-,32-,33+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 305-13 (2002)
Article DOI: 10.1124/jpet.300.1.305
BindingDB Entry DOI: 10.7270/Q2X34W09 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(MOUSE) | BDBM82078

(CAS_55508-42-4 | NSC_128644 | Neurotensin)Show SMILES CCC(C)C(NC(=O)C(Cc1ccc(O)cc1)NC(=O)C1CCCN1C(=O)C(CC(N)=O)NC(=O)C(N)CCCCN)C(=O)NC(CC(C)C)C(O)=O Show InChI InChI=1S/C36H58N8O9/c1-5-21(4)30(34(50)42-27(36(52)53)17-20(2)3)43-32(48)25(18-22-11-13-23(45)14-12-22)40-33(49)28-10-8-16-44(28)35(51)26(19-29(39)46)41-31(47)24(38)9-6-7-15-37/h11-14,20-21,24-28,30,45H,5-10,15-19,37-38H2,1-4H3,(H2,39,46)(H,40,49)(H,41,47)(H,42,50)(H,43,48)(H,52,53) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 305-13 (2002)
Article DOI: 10.1124/jpet.300.1.305
BindingDB Entry DOI: 10.7270/Q2X34W09 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(MOUSE) | BDBM85844
(CAS_54385 | Levocabastine | NSC_54385)Show SMILES CC1CN(CCC1(C(O)=O)c1ccccc1)C1CCC(CC1)(C#N)c1ccc(F)cc1 |(1.11,-6.21,;-.22,-6.98,;-.22,-8.52,;-1.56,-9.29,;-2.89,-8.52,;-2.89,-6.98,;-1.56,-6.21,;-.84,-4.85,;-.73,-3.31,;.7,-4.8,;-2.28,-4.85,;-3.82,-4.8,;-4.54,-3.44,;-3.73,-2.13,;-2.19,-2.18,;-1.47,-3.54,;-1.56,-10.83,;-2.89,-11.6,;-2.89,-13.14,;-1.56,-13.91,;-.22,-13.14,;-.22,-11.6,;-2.28,-15.27,;-3,-16.63,;-.84,-15.27,;.7,-15.32,;1.43,-16.68,;.61,-17.99,;1.33,-19.35,;-.93,-17.93,;-1.65,-16.57,)| Show InChI InChI=1S/C26H29FN2O2/c1-19-17-29(16-15-26(19,24(30)31)21-5-3-2-4-6-21)23-11-13-25(18-28,14-12-23)20-7-9-22(27)10-8-20/h2-10,19,23H,11-17H2,1H3,(H,30,31) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 305-13 (2002)
Article DOI: 10.1124/jpet.300.1.305
BindingDB Entry DOI: 10.7270/Q2X34W09 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50162774

(ABT-199 | US11420968, Example ABT-199 | Venetoclax)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)N(=O)=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50511138
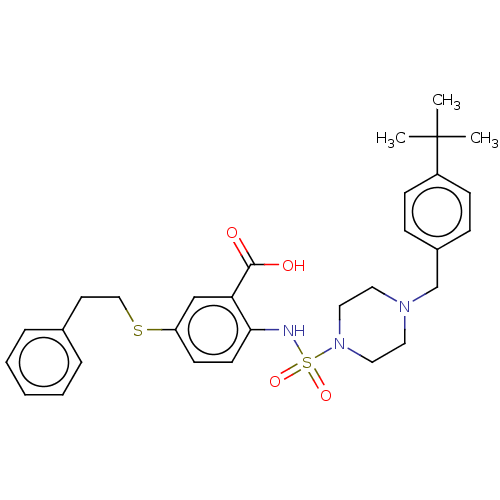
(CHEMBL4576854)Show SMILES CC(C)(C)c1ccc(CN2CCN(CC2)S(=O)(=O)Nc2ccc(SCCc3ccccc3)cc2C(O)=O)cc1 Show InChI InChI=1S/C30H37N3O4S2/c1-30(2,3)25-11-9-24(10-12-25)22-32-16-18-33(19-17-32)39(36,37)31-28-14-13-26(21-27(28)29(34)35)38-20-15-23-7-5-4-6-8-23/h4-14,21,31H,15-20,22H2,1-3H3,(H,34,35) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bcl-2-related protein A1
(Homo sapiens (Human)) | BDBM50511138

(CHEMBL4576854)Show SMILES CC(C)(C)c1ccc(CN2CCN(CC2)S(=O)(=O)Nc2ccc(SCCc3ccccc3)cc2C(O)=O)cc1 Show InChI InChI=1S/C30H37N3O4S2/c1-30(2,3)25-11-9-24(10-12-25)22-32-16-18-33(19-17-32)39(36,37)31-28-14-13-26(21-27(28)29(34)35)38-20-15-23-7-5-4-6-8-23/h4-14,21,31H,15-20,22H2,1-3H3,(H,34,35) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Carboxypeptidase A1
(Homo sapiens (Human)) | BDBM50373038
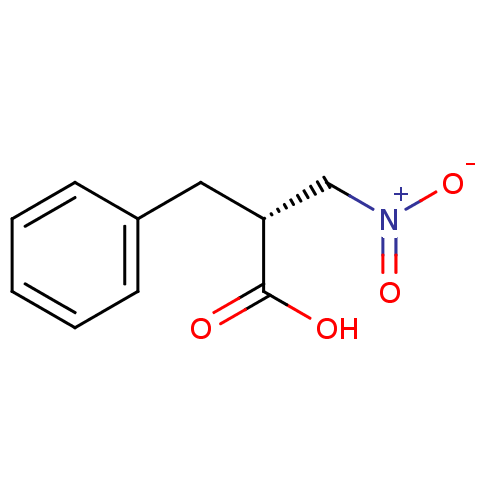
(CHEMBL407567)Show InChI InChI=1S/C10H11NO4/c12-10(13)9(7-11(14)15)6-8-4-2-1-3-5-8/h1-5,9H,6-7H2,(H,12,13)/t9-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yanbian University
Curated by ChEMBL
| Assay Description
Inhibition of CPA |
Bioorg Med Chem 16: 3596-601 (2008)
Article DOI: 10.1016/j.bmc.2008.02.010
BindingDB Entry DOI: 10.7270/Q25Q4WZ7 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 2
(Homo sapiens (Human)) | BDBM50162774

(ABT-199 | US11420968, Example ABT-199 | Venetoclax)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)N(=O)=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Bcl-2-related protein A1
(Homo sapiens (Human)) | BDBM34194
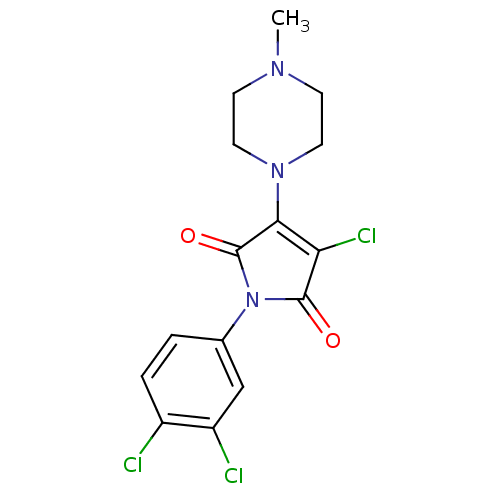
(3-chloranyl-1-(3,4-dichlorophenyl)-4-(4-methylpipe...)Show SMILES CN1CCN(CC1)C1=C(Cl)C(=O)N(C1=O)c1ccc(Cl)c(Cl)c1 |c:8| Show InChI InChI=1S/C15H14Cl3N3O2/c1-19-4-6-20(7-5-19)13-12(18)14(22)21(15(13)23)9-2-3-10(16)11(17)8-9/h2-3,8H,4-7H2,1H3 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Bcl-2-related protein A1
(Homo sapiens (Human)) | BDBM50604885
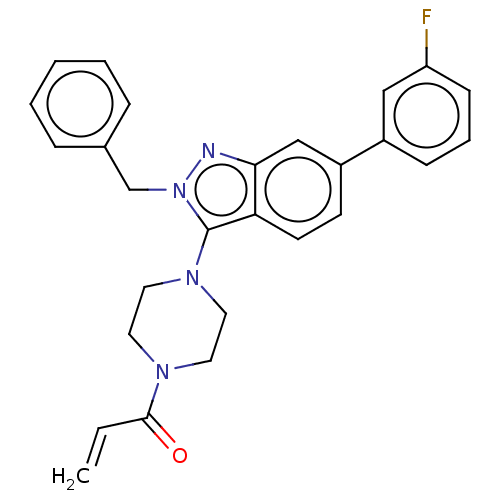
(CHEMBL5177177)Show SMILES Fc1cccc(c1)-c1ccc2c(N3CCN(CC3)C(=O)C=C)n(Cc3ccccc3)nc2c1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 2
(Homo sapiens (Human)) | BDBM85790

(Salmon MCH)Show SMILES CSCCC(NC(=O)C(NC(=O)C(N)CC(O)=O)C(C)O)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(NC(=O)C(CCSC)NC1=O)C(C)C)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(O)=O)C(=O)NC(C(C)C)C(O)=O |(16.32,3.32,;17.66,2.55,;18.99,3.32,;18.99,4.86,;20.32,5.63,;20.32,7.17,;21.66,7.94,;22.99,7.17,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;25.66,11.79,;24.33,14.1,;25.66,14.87,;25.66,16.41,;26.99,14.1,;20.32,10.25,;18.99,9.48,;20.32,11.79,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;26.99,-15.16,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;30.99,2.55,;32.33,3.32,;29.66,3.32,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;7.91,-1.3,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C89H139N27O24S4/c1-43(2)67-82(135)101-40-64(119)102-53(18-12-30-97-87(91)92)74(127)113-68(44(3)4)83(136)109-59(36-47-22-24-49(118)25-23-47)77(130)107-58(20-14-32-99-89(95)96)85(138)116-33-15-21-63(116)81(134)111-62(80(133)108-60(37-48-39-100-52-17-11-10-16-50(48)52)78(131)104-55(26-27-65(120)121)75(128)114-69(45(5)6)86(139)140)42-144-143-41-61(79(132)105-57(29-35-142-9)76(129)112-67)110-72(125)54(19-13-31-98-88(93)94)103-73(126)56(28-34-141-8)106-84(137)70(46(7)117)115-71(124)51(90)38-66(122)123/h10-11,16-17,22-25,39,43-46,51,53-63,67-70,100,117-118H,12-15,18-21,26-38,40-42,90H2,1-9H3,(H,101,135)(H,102,119)(H,103,126)(H,104,131)(H,105,132)(H,106,137)(H,107,130)(H,108,133)(H,109,136)(H,110,125)(H,111,134)(H,112,129)(H,113,127)(H,114,128)(H,115,124)(H,120,121)(H,122,123)(H,139,140)(H4,91,92,97)(H4,93,94,98)(H4,95,96,99) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 437 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50162774

(ABT-199 | US11420968, Example ABT-199 | Venetoclax)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)N(=O)=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >444 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Carboxypeptidase A1
(Homo sapiens (Human)) | BDBM50373036
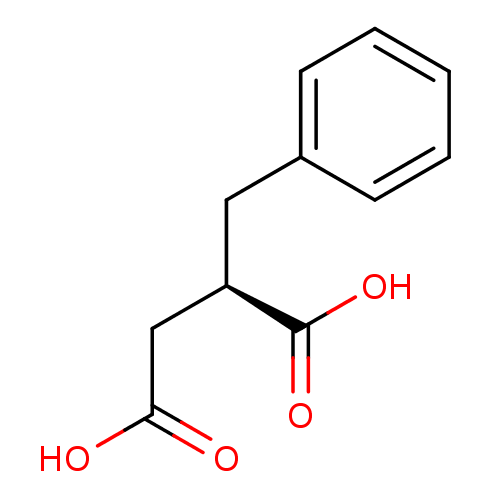
(CHEMBL259621)Show InChI InChI=1S/C11H12O4/c12-10(13)7-9(11(14)15)6-8-4-2-1-3-5-8/h1-5,9H,6-7H2,(H,12,13)(H,14,15)/t9-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yanbian University
Curated by ChEMBL
| Assay Description
Inhibition of CPA |
Bioorg Med Chem 16: 3596-601 (2008)
Article DOI: 10.1016/j.bmc.2008.02.010
BindingDB Entry DOI: 10.7270/Q25Q4WZ7 |
More data for this
Ligand-Target Pair | |
Bcl-2-related protein A1
(Homo sapiens (Human)) | BDBM50511138

(CHEMBL4576854)Show SMILES CC(C)(C)c1ccc(CN2CCN(CC2)S(=O)(=O)Nc2ccc(SCCc3ccccc3)cc2C(O)=O)cc1 Show InChI InChI=1S/C30H37N3O4S2/c1-30(2,3)25-11-9-24(10-12-25)22-32-16-18-33(19-17-32)39(36,37)31-28-14-13-26(21-27(28)29(34)35)38-20-15-23-7-5-4-6-8-23/h4-14,21,31H,15-20,22H2,1-3H3,(H,34,35) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 2
(Homo sapiens (Human)) | BDBM50297387
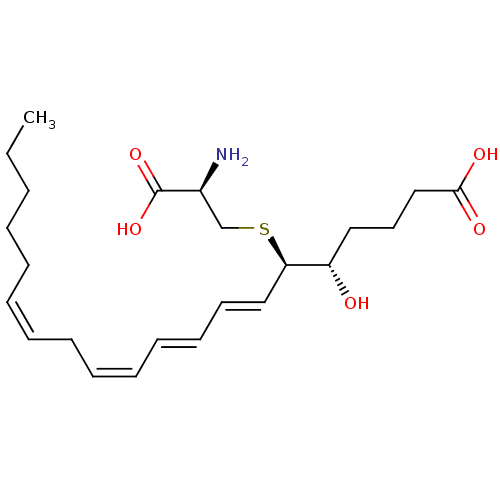
((5S,6R,7E,9E,11Z,14Z)-6-(cystein-S-yl)-5-hydroxyic...)Show SMILES CCCCC\C=C/C\C=C/C=C/C=C/[C@@H](SC[C@H](N)C(O)=O)[C@@H](O)CCCC(O)=O |r| Show InChI InChI=1S/C23H37NO5S/c1-2-3-4-5-6-7-8-9-10-11-12-13-16-21(30-18-19(24)23(28)29)20(25)15-14-17-22(26)27/h6-7,9-13,16,19-21,25H,2-5,8,14-15,17-18,24H2,1H3,(H,26,27)(H,28,29)/b7-6-,10-9-,12-11+,16-13+/t19-,20-,21+/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 693 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by PDSP Ki Database
| |
J Biol Chem 275: 30531-6 (2000)
Article DOI: 10.1074/jbc.M003490200
BindingDB Entry DOI: 10.7270/Q2BP01BH |
More data for this
Ligand-Target Pair | |
Carboxypeptidase A1
(Homo sapiens (Human)) | BDBM50373037
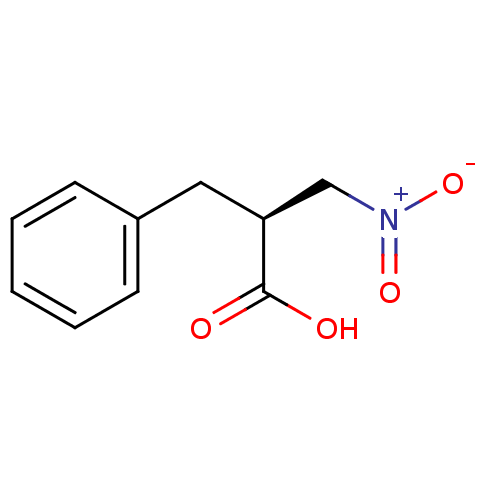
(CHEMBL261332)Show InChI InChI=1S/C10H11NO4/c12-10(13)9(7-11(14)15)6-8-4-2-1-3-5-8/h1-5,9H,6-7H2,(H,12,13)/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yanbian University
Curated by ChEMBL
| Assay Description
Inhibition of CPA |
Bioorg Med Chem 16: 3596-601 (2008)
Article DOI: 10.1016/j.bmc.2008.02.010
BindingDB Entry DOI: 10.7270/Q25Q4WZ7 |
More data for this
Ligand-Target Pair | |
Bcl-2-related protein A1
(Homo sapiens (Human)) | BDBM50604880

(CHEMBL5191993)Show SMILES CCCn1nc2cc(ccc2c1N1CCN(CC1)C(=O)C=C)-c1cccc(F)c1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Carboxypeptidase A1
(Homo sapiens (Human)) | BDBM50373039
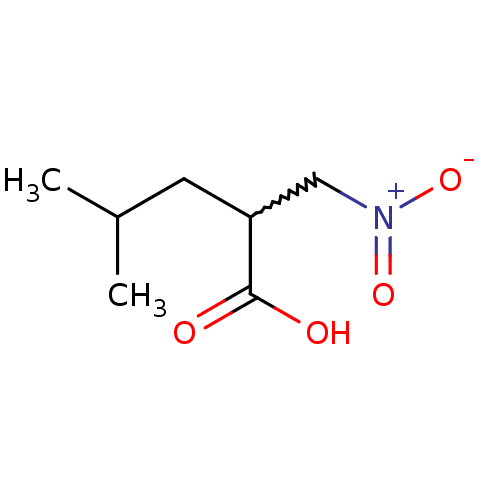
(CHEMBL407566)Show InChI InChI=1S/C7H13NO4/c1-5(2)3-6(7(9)10)4-8(11)12/h5-6H,3-4H2,1-2H3,(H,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yanbian University
Curated by ChEMBL
| Assay Description
Inhibition of CPA |
Bioorg Med Chem 16: 3596-601 (2008)
Article DOI: 10.1016/j.bmc.2008.02.010
BindingDB Entry DOI: 10.7270/Q25Q4WZ7 |
More data for this
Ligand-Target Pair | |
Bcl-2-related protein A1
(Homo sapiens (Human)) | BDBM50604882
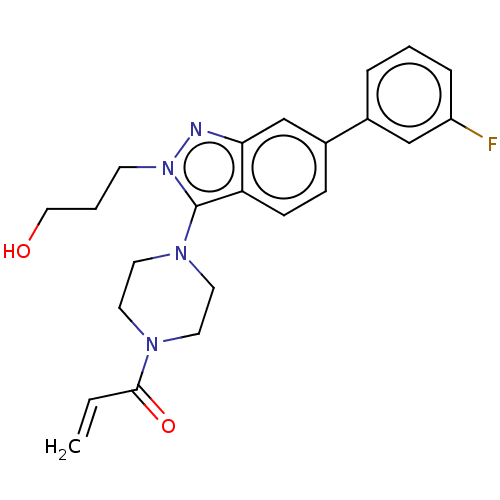
(CHEMBL5200494)Show SMILES OCCCn1nc2cc(ccc2c1N1CCN(CC1)C(=O)C=C)-c1cccc(F)c1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Bcl-2-related protein A1
(Homo sapiens (Human)) | BDBM50604879

(CHEMBL5187810)Show SMILES CCn1nc2cc(ccc2c1N1CCN(CC1)C(=O)C=C)-c1cccc(F)c1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Bcl-2-related protein A1
(Homo sapiens (Human)) | BDBM50604877
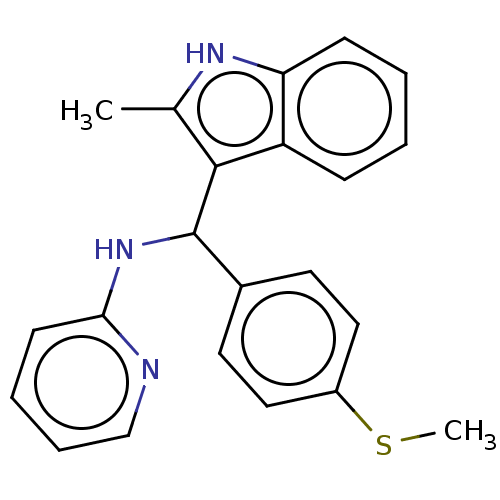
(CHEMBL5189912)Show SMILES CSc1ccc(cc1)C(Nc1ccccn1)c1c(C)[nH]c2ccccc12 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Bcl-2-related protein A1
(Homo sapiens (Human)) | BDBM50604888

(CHEMBL5172942)Show SMILES COc1cccc(Cn2nc3cc(ccc3c2N2CCNCC2)-c2cccc(F)c2)c1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Bcl-2-related protein A1
(Homo sapiens (Human)) | BDBM50604895
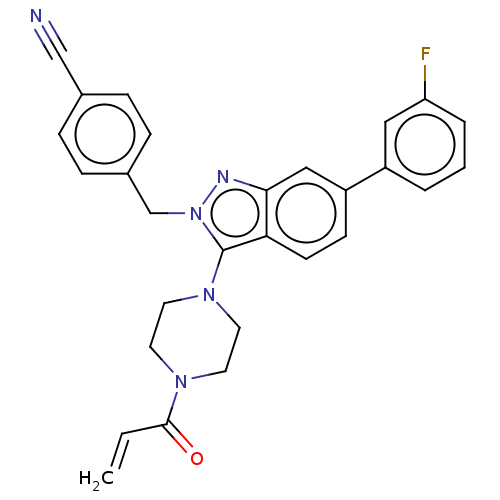
(CHEMBL5208811)Show SMILES Fc1cccc(c1)-c1ccc2c(N3CCN(CC3)C(=O)C=C)n(Cc3ccc(cc3)C#N)nc2c1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Bcl-2-related protein A1
(Homo sapiens (Human)) | BDBM50604894

(CHEMBL5207703)Show SMILES Fc1cccc(c1)-c1ccc2c(N3CCN(CC3)C(=O)C=C)n(Cc3cccc(c3)C#N)nc2c1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 2
(Homo sapiens (Human)) | BDBM85674
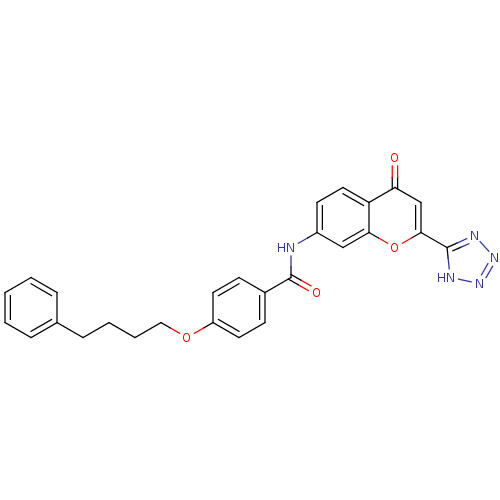
(CAS_103177-37-3 | NSC_115100 | Pranlukast)Show SMILES O=C(Nc1ccc2c(c1)oc(cc2=O)-c1nnn[nH]1)c1ccc(OCCCCc2ccccc2)cc1 Show InChI InChI=1S/C27H23N5O4/c33-23-17-25(26-29-31-32-30-26)36-24-16-20(11-14-22(23)24)28-27(34)19-9-12-21(13-10-19)35-15-5-4-8-18-6-2-1-3-7-18/h1-3,6-7,9-14,16-17H,4-5,8,15H2,(H,28,34)(H,29,30,31,32) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by PDSP Ki Database
| |
J Biol Chem 275: 30531-6 (2000)
Article DOI: 10.1074/jbc.M003490200
BindingDB Entry DOI: 10.7270/Q2BP01BH |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 2
(Homo sapiens (Human)) | BDBM50009073
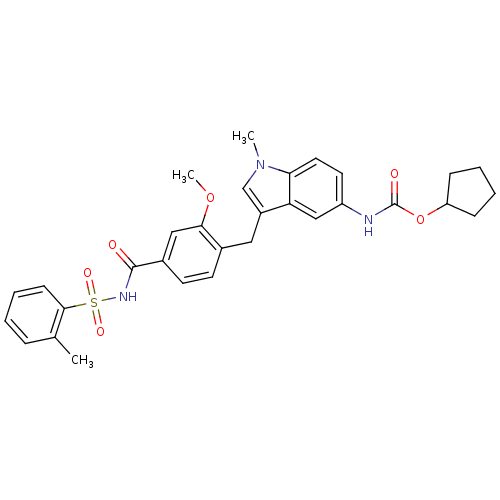
(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by PDSP Ki Database
| |
J Biol Chem 275: 30531-6 (2000)
Article DOI: 10.1074/jbc.M003490200
BindingDB Entry DOI: 10.7270/Q2BP01BH |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 2
(Homo sapiens (Human)) | BDBM85673
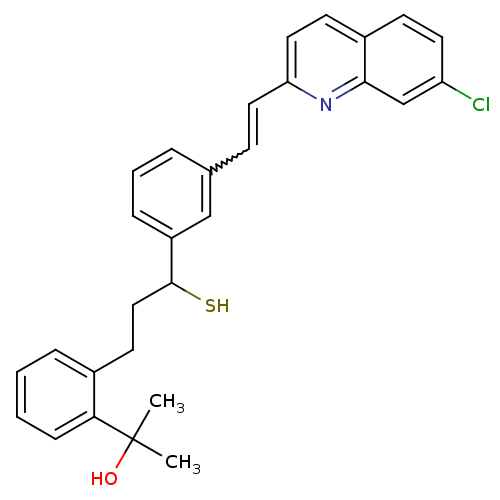
(CAS_5311297 | Montelukast | NSC_5311297)Show SMILES CC(C)(O)c1ccccc1CCC(S)c1cccc(C=Cc2ccc3ccc(Cl)cc3n2)c1 |w:19.19| Show InChI InChI=1S/C29H28ClNOS/c1-29(2,32)26-9-4-3-7-21(26)13-17-28(33)23-8-5-6-20(18-23)10-15-25-16-12-22-11-14-24(30)19-27(22)31-25/h3-12,14-16,18-19,28,32-33H,13,17H2,1-2H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by PDSP Ki Database
| |
J Biol Chem 275: 30531-6 (2000)
Article DOI: 10.1074/jbc.M003490200
BindingDB Entry DOI: 10.7270/Q2BP01BH |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 2
(Homo sapiens (Human)) | BDBM50013889
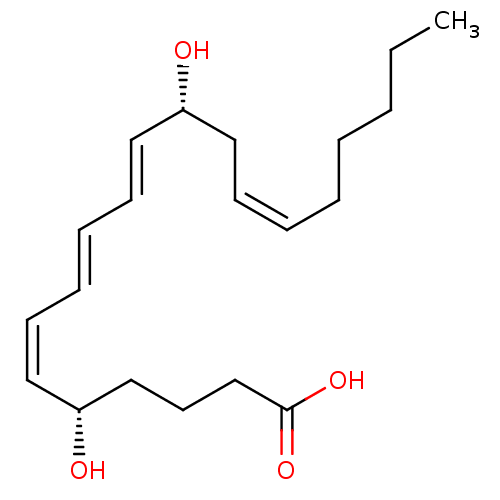
((5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxyicosa-6,8,10,...)Show SMILES CCCCC\C=C/C[C@@H](O)\C=C\C=C\C=C/[C@@H](O)CCCC(O)=O |r| Show InChI InChI=1S/C20H32O4/c1-2-3-4-5-6-9-13-18(21)14-10-7-8-11-15-19(22)16-12-17-20(23)24/h6-11,14-15,18-19,21-22H,2-5,12-13,16-17H2,1H3,(H,23,24)/b8-7+,9-6-,14-10+,15-11-/t18-,19-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by PDSP Ki Database
| |
J Biol Chem 275: 30531-6 (2000)
Article DOI: 10.1074/jbc.M003490200
BindingDB Entry DOI: 10.7270/Q2BP01BH |
More data for this
Ligand-Target Pair | |
Bcl-2-related protein A1
(Homo sapiens (Human)) | BDBM50604891
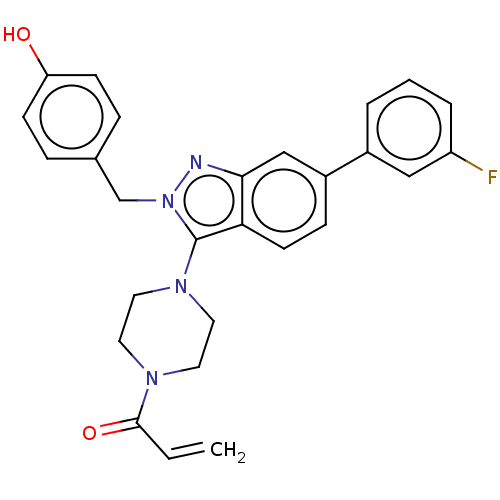
(CHEMBL5196465)Show SMILES Oc1ccc(Cn2nc3cc(ccc3c2N2CCN(CC2)C(=O)C=C)-c2cccc(F)c2)cc1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM34194

(3-chloranyl-1-(3,4-dichlorophenyl)-4-(4-methylpipe...)Show SMILES CN1CCN(CC1)C1=C(Cl)C(=O)N(C1=O)c1ccc(Cl)c(Cl)c1 |c:8| Show InChI InChI=1S/C15H14Cl3N3O2/c1-19-4-6-20(7-5-19)13-12(18)14(22)21(15(13)23)9-2-3-10(16)11(17)8-9/h2-3,8H,4-7H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 2
(Homo sapiens (Human)) | BDBM34194

(3-chloranyl-1-(3,4-dichlorophenyl)-4-(4-methylpipe...)Show SMILES CN1CCN(CC1)C1=C(Cl)C(=O)N(C1=O)c1ccc(Cl)c(Cl)c1 |c:8| Show InChI InChI=1S/C15H14Cl3N3O2/c1-19-4-6-20(7-5-19)13-12(18)14(22)21(15(13)23)9-2-3-10(16)11(17)8-9/h2-3,8H,4-7H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50604877

(CHEMBL5189912)Show SMILES CSc1ccc(cc1)C(Nc1ccccn1)c1c(C)[nH]c2ccccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Bcl-2-related protein A1
(Homo sapiens (Human)) | BDBM50604884

(CHEMBL5180166)Show SMILES CN(C)CCCn1nc2cc(ccc2c1N1CCN(CC1)C(=O)C=C)-c1cccc(F)c1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Bcl-2-related protein A1
(Homo sapiens (Human)) | BDBM50604890
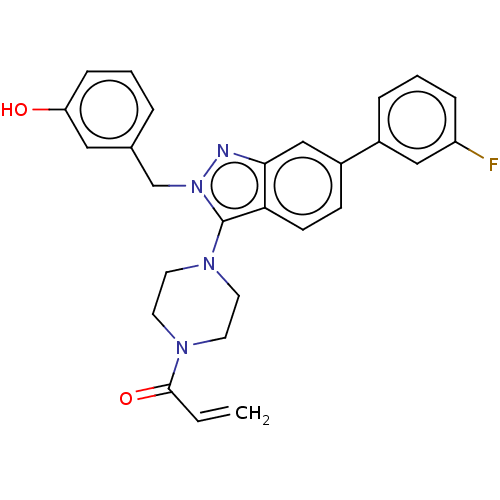
(CHEMBL5192473)Show SMILES Oc1cccc(Cn2nc3cc(ccc3c2N2CCN(CC2)C(=O)C=C)-c2cccc(F)c2)c1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Bcl-2-related protein A1
(Homo sapiens (Human)) | BDBM50604896

(CHEMBL5188901)Show SMILES CCC(=O)N1CCN(CC1)c1n(Cc2ccccc2)nc2cc(ccc12)-c1cccc(F)c1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM34194

(3-chloranyl-1-(3,4-dichlorophenyl)-4-(4-methylpipe...)Show SMILES CN1CCN(CC1)C1=C(Cl)C(=O)N(C1=O)c1ccc(Cl)c(Cl)c1 |c:8| Show InChI InChI=1S/C15H14Cl3N3O2/c1-19-4-6-20(7-5-19)13-12(18)14(22)21(15(13)23)9-2-3-10(16)11(17)8-9/h2-3,8H,4-7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Carboxypeptidase A1
(Homo sapiens (Human)) | BDBM50373035
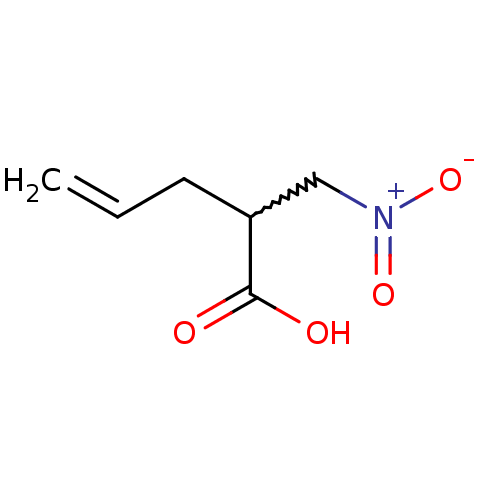
(CHEMBL259221)Show InChI InChI=1S/C6H9NO4/c1-2-3-5(6(8)9)4-7(10)11/h2,5H,1,3-4H2,(H,8,9) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yanbian University
Curated by ChEMBL
| Assay Description
Inhibition of CPA |
Bioorg Med Chem 16: 3596-601 (2008)
Article DOI: 10.1016/j.bmc.2008.02.010
BindingDB Entry DOI: 10.7270/Q25Q4WZ7 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase A1
(Homo sapiens (Human)) | BDBM50373037

(CHEMBL261332)Show InChI InChI=1S/C10H11NO4/c12-10(13)9(7-11(14)15)6-8-4-2-1-3-5-8/h1-5,9H,6-7H2,(H,12,13)/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yanbian University
Curated by ChEMBL
| Assay Description
Inhibition of CPA |
Bioorg Med Chem 16: 3596-601 (2008)
Article DOI: 10.1016/j.bmc.2008.02.010
BindingDB Entry DOI: 10.7270/Q25Q4WZ7 |
More data for this
Ligand-Target Pair | |
Bcl-2-related protein A1
(Homo sapiens (Human)) | BDBM50604881

(CHEMBL5175046)Show SMILES COCCCn1nc2cc(ccc2c1N1CCN(CC1)C(=O)C=C)-c1cccc(F)c1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Bcl-2-related protein A1
(Homo sapiens (Human)) | BDBM50604886
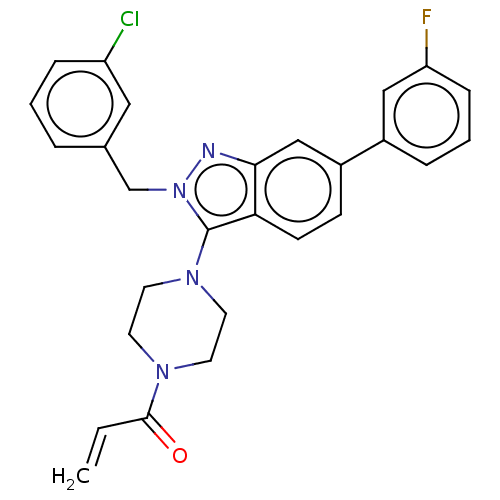
(CHEMBL5187123)Show SMILES Fc1cccc(c1)-c1ccc2c(N3CCN(CC3)C(=O)C=C)n(Cc3cccc(Cl)c3)nc2c1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Bcl-2-related protein A1
(Homo sapiens (Human)) | BDBM50604893
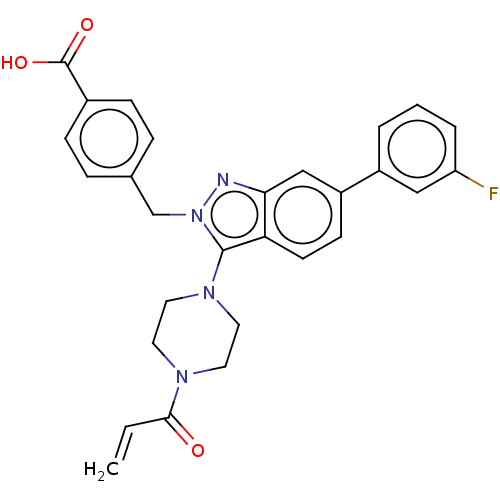
(CHEMBL5171576)Show SMILES OC(=O)c1ccc(Cn2nc3cc(ccc3c2N2CCN(CC2)C(=O)C=C)-c2cccc(F)c2)cc1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data