 Found 11824 hits with Last Name = 'zhan' and Initial = 'p'
Found 11824 hits with Last Name = 'zhan' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50505279

(CHEMBL4436207)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(cc1)B(O)O |r| Show InChI InChI=1S/C27H37BN2O9S/c1-18(2)15-30(40(35,36)21-10-8-20(9-11-21)28(33)34)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)39-25-17-38-26-22(25)12-13-37-26/h3-11,18,22-26,31,33-34H,12-17H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease using RE(Edans)SGIFLETSK(Dabcyl)R as substrate by fluorescence method |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Binding affinity to wild type HIV1 protease |
J Med Chem 59: 2849-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00497
BindingDB Entry DOI: 10.7270/Q2JH3P3H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50144258

(4-[(8-Benzo[1,3]dioxol-5-ylmethyl-8-aza-bicyclo[3....)Show SMILES [#6]-[#6]-[#7](-[#6]-[#6])-[#6](=O)-c1ccc(cc1)-[#6](=[#6]-1/[#6]-[#6]-2-[#6]-[#6]-[#6](-[#6]-1)-[#7]-2-[#6]-c1ccc2-[#8]-[#6]-[#8]-c2c1)\c1ccccc1 Show InChI InChI=1S/C33H36N2O3/c1-3-34(4-2)33(36)26-13-11-25(12-14-26)32(24-8-6-5-7-9-24)27-19-28-15-16-29(20-27)35(28)21-23-10-17-30-31(18-23)38-22-37-30/h5-14,17-18,28-29H,3-4,15-16,19-22H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for delta opioid receptor |
Bioorg Med Chem Lett 14: 2109-12 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.051
BindingDB Entry DOI: 10.7270/Q25B01X4 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease using RE(Edans)SGIFLETSK(Dabcyl)R as substrate by fluorescence method |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142111
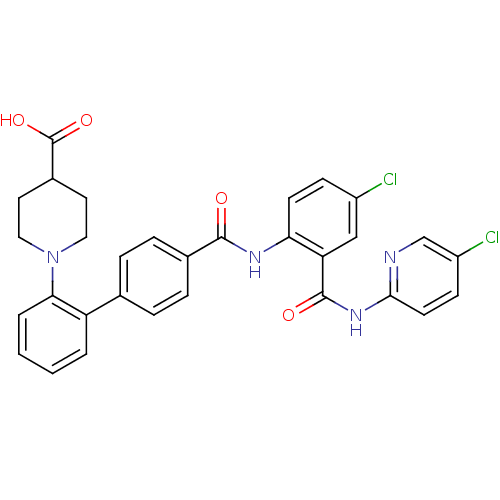
(1-{4'-[4-Chloro-2-(5-chloro-pyridin-2-ylcarbamoyl)...)Show SMILES OC(=O)C1CCN(CC1)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C31H26Cl2N4O4/c32-22-9-11-26(25(17-22)30(39)36-28-12-10-23(33)18-34-28)35-29(38)20-7-5-19(6-8-20)24-3-1-2-4-27(24)37-15-13-21(14-16-37)31(40)41/h1-12,17-18,21H,13-16H2,(H,35,38)(H,40,41)(H,34,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against coagulation factor Xa. |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12751

(1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-13-21(30(29-15)19-6-4-5-17(14-19)23(25)26)24(31)28-18-11-9-16(10-12-18)20-7-2-3-8-22(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Tested for binding affinity against human Coagulation factor Xa (trypsin-like serine protease) |
Bioorg Med Chem Lett 12: 1651-5 (2002)
BindingDB Entry DOI: 10.7270/Q2VT1RFZ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12751

(1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-13-21(30(29-15)19-6-4-5-17(14-19)23(25)26)24(31)28-18-11-9-16(10-12-18)20-7-2-3-8-22(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Coagulation factor Xa (serine protease) was determined |
Bioorg Med Chem Lett 12: 1511-5 (2002)
BindingDB Entry DOI: 10.7270/Q2P84B6X |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50144236

(4-[(R)-(S)-8-Aza-bicyclo[3.2.1]oct-(3Z)-ylidene-ph...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C(=C1\C[C@@H]2CC[C@H](C1)N2)\c1ccccc1 Show InChI InChI=1S/C25H30N2O/c1-3-27(4-2)25(28)20-12-10-19(11-13-20)24(18-8-6-5-7-9-18)21-16-22-14-15-23(17-21)26-22/h5-13,22-23,26H,3-4,14-17H2,1-2H3/b24-21-/t22-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for delta opioid receptor |
Bioorg Med Chem Lett 14: 2109-12 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.051
BindingDB Entry DOI: 10.7270/Q25B01X4 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50144229
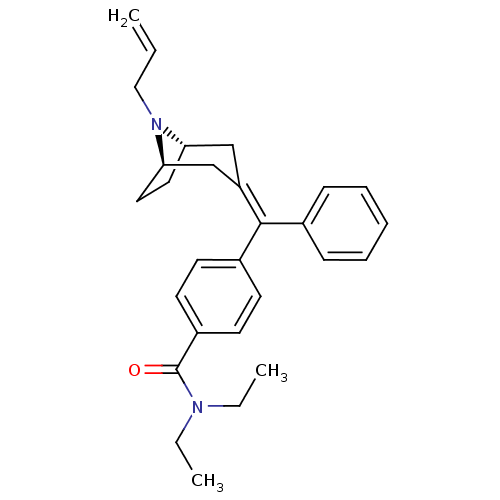
(4-{[(1S,5R)-8-Allyl-8-aza-bicyclo[3.2.1]oct-(3Z)-y...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C(=C1\C[C@@H]2CC[C@H](C1)N2CC=C)\c1ccccc1 |THB:22:21:14.15.20:17.18| Show InChI InChI=1S/C28H34N2O/c1-4-18-30-25-16-17-26(30)20-24(19-25)27(21-10-8-7-9-11-21)22-12-14-23(15-13-22)28(31)29(5-2)6-3/h4,7-15,25-26H,1,5-6,16-20H2,2-3H3/b27-24-/t25-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Effective concentration against stimulation of [35S]-GTP-gammaS, binding in CHO cells transfected with the human opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 2109-12 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.051
BindingDB Entry DOI: 10.7270/Q25B01X4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50393719
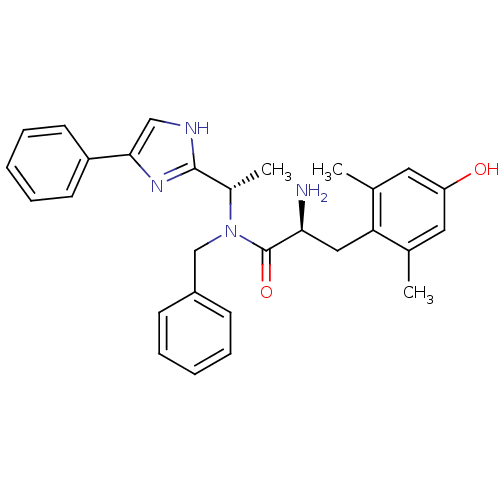
(CHEMBL2159118)Show SMILES C[C@H](N(Cc1ccccc1)C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C29H32N4O2/c1-19-14-24(34)15-20(2)25(19)16-26(30)29(35)33(18-22-10-6-4-7-11-22)21(3)28-31-17-27(32-28)23-12-8-5-9-13-23/h4-15,17,21,26,34H,16,18,30H2,1-3H3,(H,31,32)/t21-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to rat mu opioid receptor |
Bioorg Med Chem Lett 22: 4869-72 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.042
BindingDB Entry DOI: 10.7270/Q2F76DP8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50393725
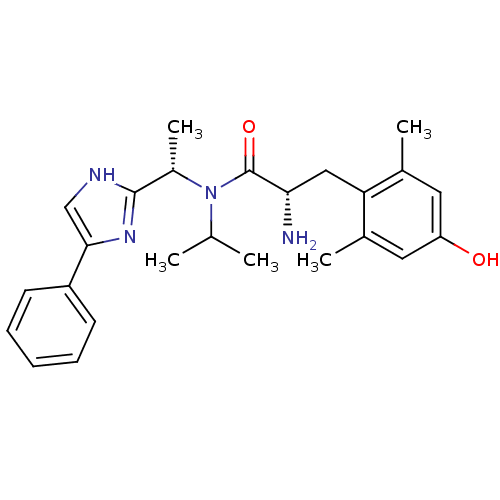
(CHEMBL2159117)Show SMILES CC(C)N([C@@H](C)c1nc(c[nH]1)-c1ccccc1)C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C |r| Show InChI InChI=1S/C25H32N4O2/c1-15(2)29(18(5)24-27-14-23(28-24)19-9-7-6-8-10-19)25(31)22(26)13-21-16(3)11-20(30)12-17(21)4/h6-12,14-15,18,22,30H,13,26H2,1-5H3,(H,27,28)/t18-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to rat mu opioid receptor |
Bioorg Med Chem Lett 22: 4869-72 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.042
BindingDB Entry DOI: 10.7270/Q2F76DP8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50193861
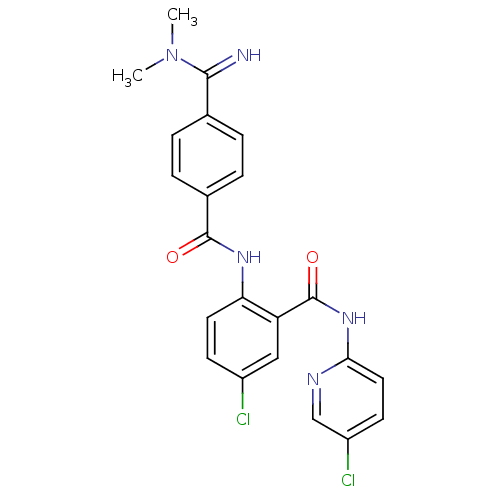
(5-chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N,N-dimet...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H19Cl2N5O2/c1-29(2)20(25)13-3-5-14(6-4-13)21(30)27-18-9-7-15(23)11-17(18)22(31)28-19-10-8-16(24)12-26-19/h3-12,25H,1-2H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50182939
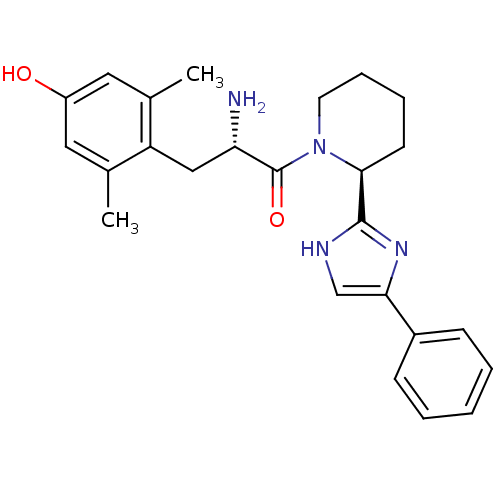
((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)-1-((S...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCCC[C@H]1c1nc(c[nH]1)-c1ccccc1 Show InChI InChI=1S/C25H30N4O2/c1-16-12-19(30)13-17(2)20(16)14-21(26)25(31)29-11-7-6-10-23(29)24-27-15-22(28-24)18-8-4-3-5-9-18/h3-5,8-9,12-13,15,21,23,30H,6-7,10-11,14,26H2,1-2H3,(H,27,28)/t21-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain |
Bioorg Med Chem Lett 16: 2505-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.082
BindingDB Entry DOI: 10.7270/Q29G5MDZ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50182939

((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)-1-((S...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCCC[C@H]1c1nc(c[nH]1)-c1ccccc1 Show InChI InChI=1S/C25H30N4O2/c1-16-12-19(30)13-17(2)20(16)14-21(26)25(31)29-11-7-6-10-23(29)24-27-15-22(28-24)18-8-4-3-5-9-18/h3-5,8-9,12-13,15,21,23,30H,6-7,10-11,14,26H2,1-2H3,(H,27,28)/t21-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to rat mu opioid receptor |
Bioorg Med Chem Lett 22: 4869-72 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.042
BindingDB Entry DOI: 10.7270/Q2F76DP8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50182943
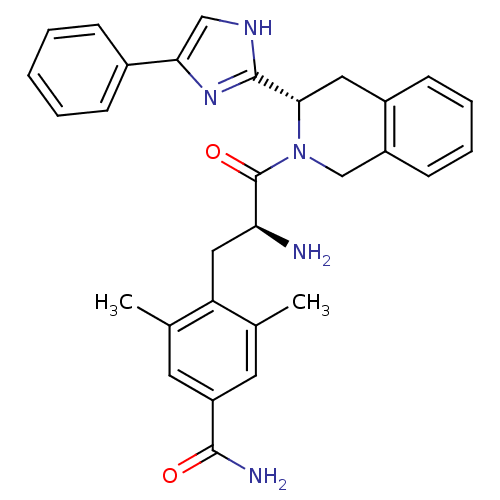
(4-((S)-2-amino-3-oxo-3-((S)-3-(4-phenyl-1H-imidazo...)Show SMILES Cc1cc(cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@H]1c1nc(c[nH]1)-c1ccccc1)C(N)=O Show InChI InChI=1S/C30H31N5O2/c1-18-12-23(28(32)36)13-19(2)24(18)15-25(31)30(37)35-17-22-11-7-6-10-21(22)14-27(35)29-33-16-26(34-29)20-8-4-3-5-9-20/h3-13,16,25,27H,14-15,17,31H2,1-2H3,(H2,32,36)(H,33,34)/t25-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to rat delta opioid receptor |
Bioorg Med Chem Lett 22: 4869-72 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.042
BindingDB Entry DOI: 10.7270/Q2F76DP8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50182943

(4-((S)-2-amino-3-oxo-3-((S)-3-(4-phenyl-1H-imidazo...)Show SMILES Cc1cc(cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@H]1c1nc(c[nH]1)-c1ccccc1)C(N)=O Show InChI InChI=1S/C30H31N5O2/c1-18-12-23(28(32)36)13-19(2)24(18)15-25(31)30(37)35-17-22-11-7-6-10-21(22)14-27(35)29-33-16-26(34-29)20-8-4-3-5-9-20/h3-13,16,25,27H,14-15,17,31H2,1-2H3,(H2,32,36)(H,33,34)/t25-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in rat brain |
Bioorg Med Chem Lett 16: 2505-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.082
BindingDB Entry DOI: 10.7270/Q29G5MDZ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249120
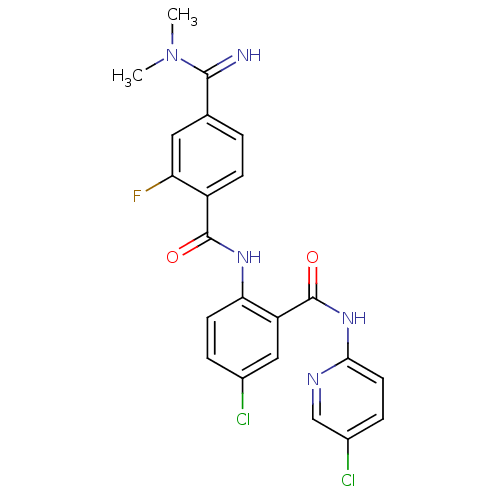
(CHEMBL472967 | N-(4-chloro-2-(5-chloropyridin-2-yl...)Show SMILES CN(C)C(=N)c1ccc(C(=O)Nc2ccc(Cl)cc2C(=O)Nc2ccc(Cl)cn2)c(F)c1 Show InChI InChI=1S/C22H18Cl2FN5O2/c1-30(2)20(26)12-3-6-15(17(25)9-12)21(31)28-18-7-4-13(23)10-16(18)22(32)29-19-8-5-14(24)11-27-19/h3-11,26H,1-2H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM19023

(1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...)Show SMILES COc1ccc(cc1)-n1nc(C(N)=O)c2CCN(C(=O)c12)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C25H25N5O4/c1-34-19-11-9-18(10-12-19)30-23-20(22(27-30)24(26)32)13-15-29(25(23)33)17-7-5-16(6-8-17)28-14-3-2-4-21(28)31/h5-12H,2-4,13-15H2,1H3,(H2,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142125
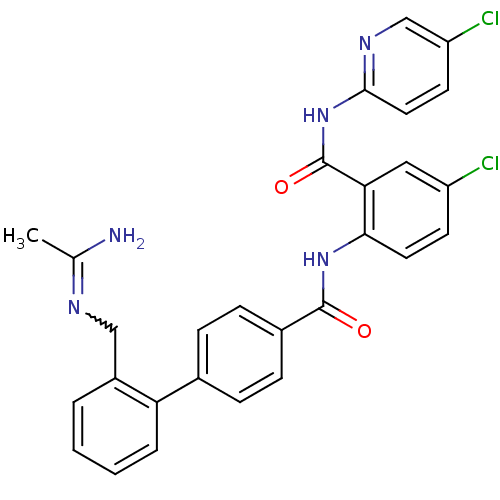
(2'-(Acetimidoylamino-methyl)-biphenyl-4-carboxylic...)Show SMILES CC(N)=NCc1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C28H23Cl2N5O2/c1-17(31)32-15-20-4-2-3-5-23(20)18-6-8-19(9-7-18)27(36)34-25-12-10-21(29)14-24(25)28(37)35-26-13-11-22(30)16-33-26/h2-14,16H,15H2,1H3,(H2,31,32)(H,34,36)(H,33,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against coagulation factor Xa. |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142090
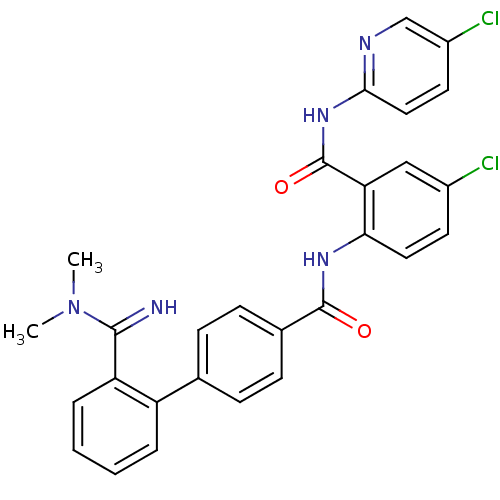
(2'-(N,N-Dimethyl-carbamimidoyl)-biphenyl-4-carboxy...)Show SMILES CN(C)C(=N)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C28H23Cl2N5O2/c1-35(2)26(31)22-6-4-3-5-21(22)17-7-9-18(10-8-17)27(36)33-24-13-11-19(29)15-23(24)28(37)34-25-14-12-20(30)16-32-25/h3-16,31H,1-2H3,(H,33,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142139
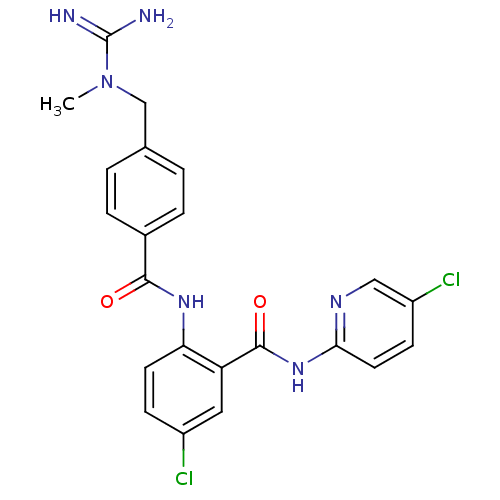
(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-{4-[(N-methyl...)Show SMILES CN(Cc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1)C(N)=N Show InChI InChI=1S/C22H20Cl2N6O2/c1-30(22(25)26)12-13-2-4-14(5-3-13)20(31)28-18-8-6-15(23)10-17(18)21(32)29-19-9-7-16(24)11-27-19/h2-11H,12H2,1H3,(H3,25,26)(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against coagulation factor X. |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50182942
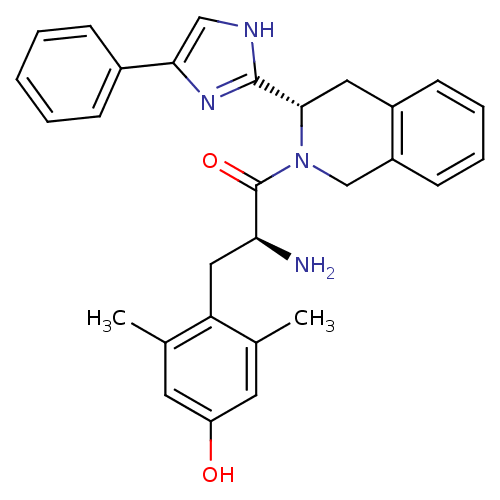
((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)-1-((S...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@H]1c1nc(c[nH]1)-c1ccccc1 Show InChI InChI=1S/C29H30N4O2/c1-18-12-23(34)13-19(2)24(18)15-25(30)29(35)33-17-22-11-7-6-10-21(22)14-27(33)28-31-16-26(32-28)20-8-4-3-5-9-20/h3-13,16,25,27,34H,14-15,17,30H2,1-2H3,(H,31,32)/t25-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to rat delta opioid receptor |
Bioorg Med Chem Lett 22: 4869-72 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.042
BindingDB Entry DOI: 10.7270/Q2F76DP8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142112
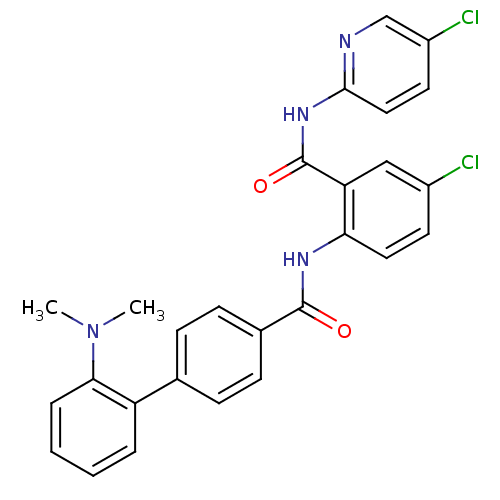
(2'-Dimethylamino-biphenyl-4-carboxylic acid [4-chl...)Show SMILES CN(C)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C27H22Cl2N4O2/c1-33(2)24-6-4-3-5-21(24)17-7-9-18(10-8-17)26(34)31-23-13-11-19(28)15-22(23)27(35)32-25-14-12-20(29)16-30-25/h3-16H,1-2H3,(H,31,34)(H,30,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50393717
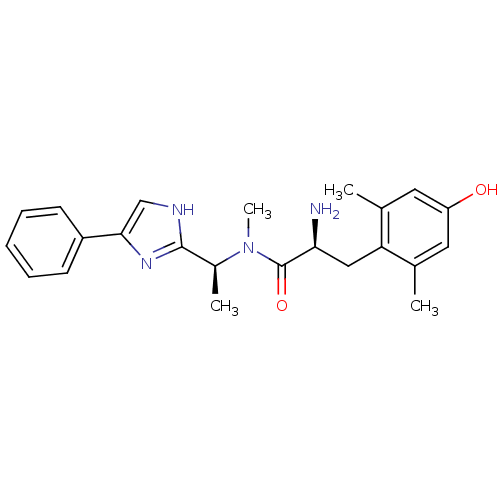
(CHEMBL2159116)Show SMILES C[C@H](N(C)C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C23H28N4O2/c1-14-10-18(28)11-15(2)19(14)12-20(24)23(29)27(4)16(3)22-25-13-21(26-22)17-8-6-5-7-9-17/h5-11,13,16,20,28H,12,24H2,1-4H3,(H,25,26)/t16-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to rat mu opioid receptor |
Bioorg Med Chem Lett 22: 4869-72 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.042
BindingDB Entry DOI: 10.7270/Q2F76DP8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50182954
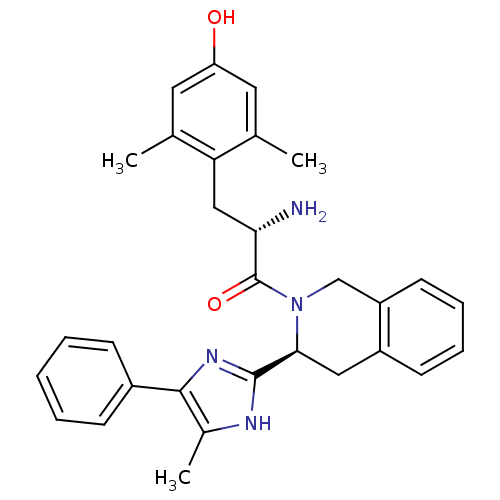
((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)-1-((S...)Show SMILES Cc1[nH]c(nc1-c1ccccc1)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C Show InChI InChI=1S/C30H32N4O2/c1-18-13-24(35)14-19(2)25(18)16-26(31)30(36)34-17-23-12-8-7-11-22(23)15-27(34)29-32-20(3)28(33-29)21-9-5-4-6-10-21/h4-14,26-27,35H,15-17,31H2,1-3H3,(H,32,33)/t26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in rat brain |
Bioorg Med Chem Lett 16: 2505-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.082
BindingDB Entry DOI: 10.7270/Q29G5MDZ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50393716
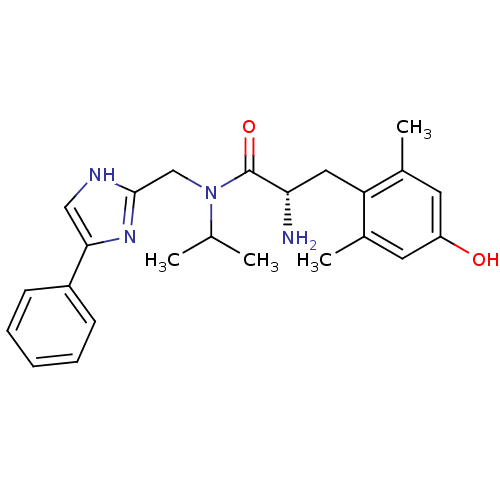
(CHEMBL2159115)Show SMILES CC(C)N(Cc1nc(c[nH]1)-c1ccccc1)C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C |r| Show InChI InChI=1S/C24H30N4O2/c1-15(2)28(14-23-26-13-22(27-23)18-8-6-5-7-9-18)24(30)21(25)12-20-16(3)10-19(29)11-17(20)4/h5-11,13,15,21,29H,12,14,25H2,1-4H3,(H,26,27)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to rat mu opioid receptor |
Bioorg Med Chem Lett 22: 4869-72 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.042
BindingDB Entry DOI: 10.7270/Q2F76DP8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50182942

((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)-1-((S...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@H]1c1nc(c[nH]1)-c1ccccc1 Show InChI InChI=1S/C29H30N4O2/c1-18-12-23(34)13-19(2)24(18)15-25(30)29(35)33-17-22-11-7-6-10-21(22)14-27(33)28-31-16-26(32-28)20-8-4-3-5-9-20/h3-13,16,25,27,34H,14-15,17,30H2,1-2H3,(H,31,32)/t25-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in rat brain |
Bioorg Med Chem Lett 16: 2505-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.082
BindingDB Entry DOI: 10.7270/Q29G5MDZ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249423
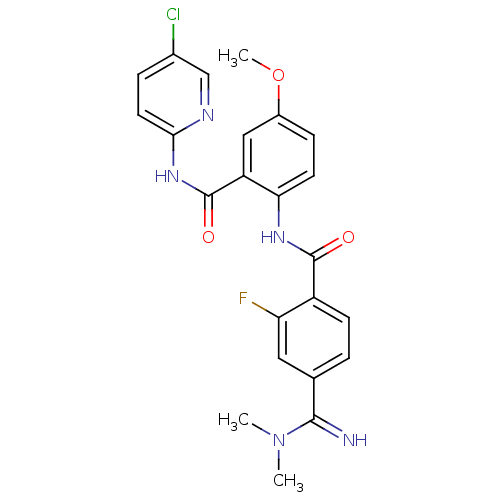
(CHEMBL515919 | N-(2-(5-chloropyridin-2-ylcarbamoyl...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2F)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H21ClFN5O3/c1-30(2)21(26)13-4-7-16(18(25)10-13)22(31)28-19-8-6-15(33-3)11-17(19)23(32)29-20-9-5-14(24)12-27-20/h4-12,26H,1-3H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50154039
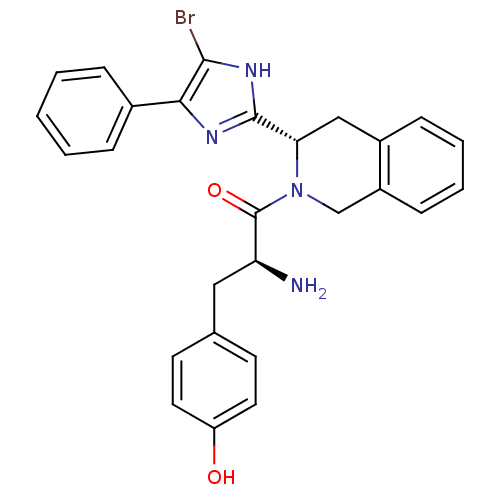
(2-Amino-1-[3-(5-bromo-4-phenyl-1H-imidazol-2-yl)-3...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1Cc2ccccc2C[C@H]1c1nc(c(Br)[nH]1)-c1ccccc1 Show InChI InChI=1S/C27H25BrN4O2/c28-25-24(18-6-2-1-3-7-18)30-26(31-25)23-15-19-8-4-5-9-20(19)16-32(23)27(34)22(29)14-17-10-12-21(33)13-11-17/h1-13,22-23,33H,14-16,29H2,(H,30,31)/t22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for delta opioid receptor of rat brain |
J Med Chem 47: 5009-20 (2004)
Article DOI: 10.1021/jm030548r
BindingDB Entry DOI: 10.7270/Q2H131GF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249298
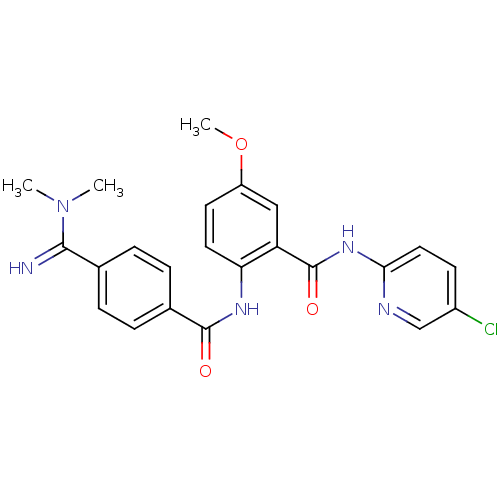
(BEVYXXA | CHEMBL512351 | N-(5-chloropyridin-2-yl)-...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H22ClN5O3/c1-29(2)21(25)14-4-6-15(7-5-14)22(30)27-19-10-9-17(32-3)12-18(19)23(31)28-20-11-8-16(24)13-26-20/h4-13,25H,1-3H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50182946
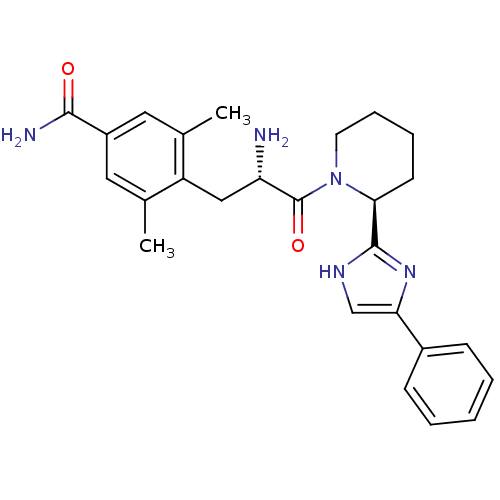
(4-((S)-2-amino-3-oxo-3-((S)-2-(4-phenyl-1H-imidazo...)Show SMILES Cc1cc(cc(C)c1C[C@H](N)C(=O)N1CCCC[C@H]1c1nc(c[nH]1)-c1ccccc1)C(N)=O Show InChI InChI=1S/C26H31N5O2/c1-16-12-19(24(28)32)13-17(2)20(16)14-21(27)26(33)31-11-7-6-10-23(31)25-29-15-22(30-25)18-8-4-3-5-9-18/h3-5,8-9,12-13,15,21,23H,6-7,10-11,14,27H2,1-2H3,(H2,28,32)(H,29,30)/t21-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain |
Bioorg Med Chem Lett 16: 2505-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.082
BindingDB Entry DOI: 10.7270/Q29G5MDZ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50182946

(4-((S)-2-amino-3-oxo-3-((S)-2-(4-phenyl-1H-imidazo...)Show SMILES Cc1cc(cc(C)c1C[C@H](N)C(=O)N1CCCC[C@H]1c1nc(c[nH]1)-c1ccccc1)C(N)=O Show InChI InChI=1S/C26H31N5O2/c1-16-12-19(24(28)32)13-17(2)20(16)14-21(27)26(33)31-11-7-6-10-23(31)25-29-15-22(30-25)18-8-4-3-5-9-18/h3-5,8-9,12-13,15,21,23H,6-7,10-11,14,27H2,1-2H3,(H2,28,32)(H,29,30)/t21-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to rat mu opioid receptor |
Bioorg Med Chem Lett 22: 4869-72 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.042
BindingDB Entry DOI: 10.7270/Q2F76DP8 |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-5/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50067441
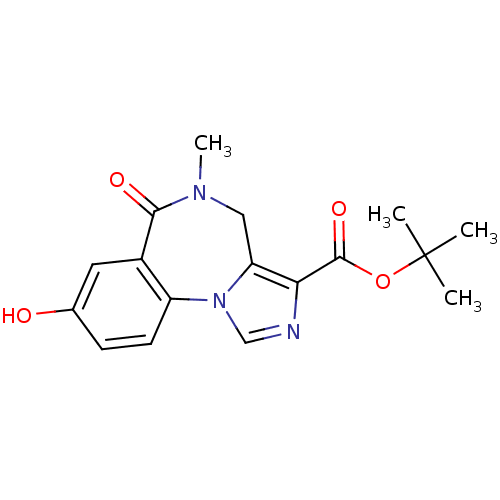
(8-Hydroxy-5-methyl-6-oxo-5,6-dihydro-4H-2,5,10b-tr...)Show SMILES CN1Cc2c(ncn2-c2ccc(O)cc2C1=O)C(=O)OC(C)(C)C Show InChI InChI=1S/C17H19N3O4/c1-17(2,3)24-16(23)14-13-8-19(4)15(22)11-7-10(21)5-6-12(11)20(13)9-18-14/h5-7,9,21H,8H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Binding affinity for Gamma-aminobutyric-acid A receptor alpha5-beta3-gamma2 |
J Med Chem 41: 4130-42 (1998)
Article DOI: 10.1021/jm980317y
BindingDB Entry DOI: 10.7270/Q2W37VFJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50144281
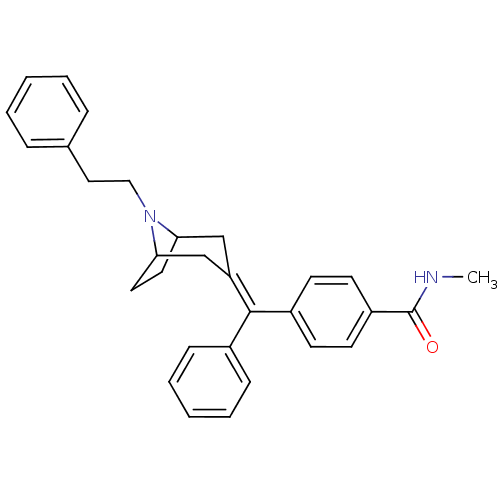
(CHEMBL63309 | N-Methyl-4-[(8-phenethyl-8-aza-bicyc...)Show SMILES [#6]-[#7]-[#6](=O)-c1ccc(cc1)-[#6](=[#6]-1\[#6]-[#6]-2-[#6]-[#6]-[#6](-[#6]-1)-[#7]-2-[#6]-[#6]-c1ccccc1)\c1ccccc1 Show InChI InChI=1S/C30H32N2O/c1-31-30(33)25-14-12-24(13-15-25)29(23-10-6-3-7-11-23)26-20-27-16-17-28(21-26)32(27)19-18-22-8-4-2-5-9-22/h2-15,27-28H,16-21H2,1H3,(H,31,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 2113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.052
BindingDB Entry DOI: 10.7270/Q21J997Q |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50144284

(CHEMBL68412 | N-Ethyl-4-[(3-hydroxy-phenyl)-(8-thi...)Show SMILES [#6]-[#6]-[#7]-[#6](=O)-c1ccc(cc1)-[#6](=[#6]-1\[#6]-[#6]-2-[#6]-[#6]-[#6](-[#6]-1)-[#7]-2-[#6]-c1cccs1)\c1cccc(-[#8])c1 Show InChI InChI=1S/C28H30N2O2S/c1-2-29-28(32)20-10-8-19(9-11-20)27(21-5-3-6-25(31)17-21)22-15-23-12-13-24(16-22)30(23)18-26-7-4-14-33-26/h3-11,14,17,23-24,31H,2,12-13,15-16,18H2,1H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 2113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.052
BindingDB Entry DOI: 10.7270/Q21J997Q |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50017698
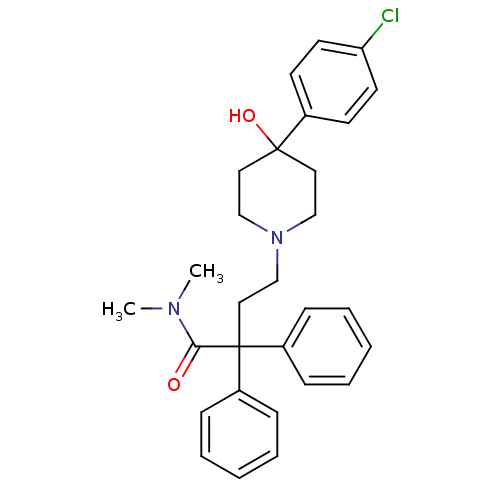
(4-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)-N,N...)Show SMILES CN(C)C(=O)C(CCN1CCC(O)(CC1)c1ccc(Cl)cc1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H33ClN2O2/c1-31(2)27(33)29(24-9-5-3-6-10-24,25-11-7-4-8-12-25)19-22-32-20-17-28(34,18-21-32)23-13-15-26(30)16-14-23/h3-16,34H,17-22H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for Mu opioid receptor of rat brain |
J Med Chem 47: 5009-20 (2004)
Article DOI: 10.1021/jm030548r
BindingDB Entry DOI: 10.7270/Q2H131GF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50113598
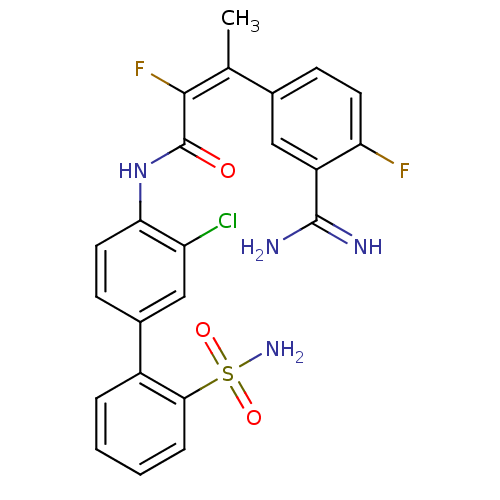
((Z)-3-(3-Carbamimidoyl-4-fluoro-phenyl)-2-fluoro-b...)Show SMILES C\C(=C(/F)C(=O)Nc1ccc(cc1Cl)-c1ccccc1S(N)(=O)=O)c1ccc(F)c(c1)C(N)=N Show InChI InChI=1S/C23H19ClF2N4O3S/c1-12(13-6-8-18(25)16(10-13)22(27)28)21(26)23(31)30-19-9-7-14(11-17(19)24)15-4-2-3-5-20(15)34(29,32)33/h2-11H,1H3,(H3,27,28)(H,30,31)(H2,29,32,33)/b21-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against factor Xa,activity expressed as Ki nM |
Bioorg Med Chem Lett 12: 1511-5 (2002)
BindingDB Entry DOI: 10.7270/Q2P84B6X |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50088712

(4-{[(1S,3S,5R)-8-(Benzo[1,3]dioxole-5-carbonyl)-8-...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)N([C@H]1C[C@@H]2CC[C@H](C1)N2C(=O)c1ccc2OCOc2c1)c1ccccc1 |THB:22:21:14.15.20:18.17| Show InChI InChI=1S/C32H35N3O4/c1-3-33(4-2)31(36)22-10-13-25(14-11-22)34(24-8-6-5-7-9-24)28-19-26-15-16-27(20-28)35(26)32(37)23-12-17-29-30(18-23)39-21-38-29/h5-14,17-18,26-28H,3-4,15-16,19-21H2,1-2H3/t26-,27+,28- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DPDPE radioligand binding to rat opioid receptor delta 1 site from rat brain membranes |
Bioorg Med Chem Lett 10: 1109-11 (2000)
BindingDB Entry DOI: 10.7270/Q2X0668B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142169
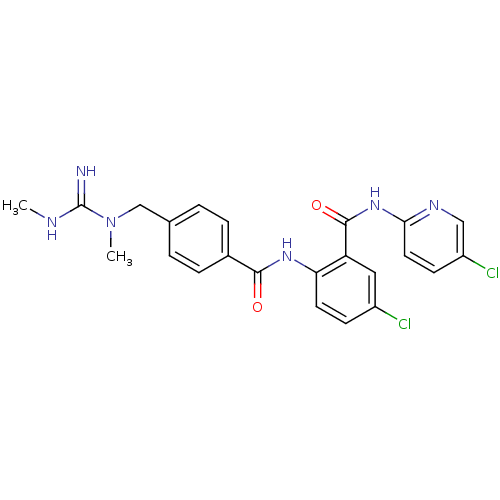
(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N,N'-dime...)Show SMILES CNC(=N)N(C)Cc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H22Cl2N6O2/c1-27-23(26)31(2)13-14-3-5-15(6-4-14)21(32)29-19-9-7-16(24)11-18(19)22(33)30-20-10-8-17(25)12-28-20/h3-12H,13H2,1-2H3,(H2,26,27)(H,29,32)(H,28,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50155124
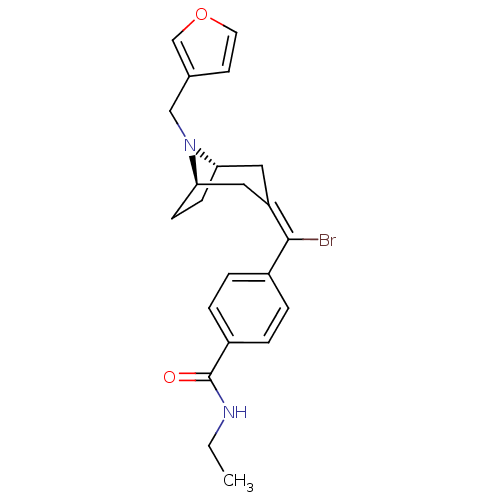
(4-{Bromo-[(1S,5R)-8-furan-3-ylmethyl-8-aza-bicyclo...)Show SMILES CCNC(=O)c1ccc(cc1)C(\Br)=C1\C[C@@H]2CC[C@H](C1)N2Cc1ccoc1 |THB:21:20:13.14.19:17.16| Show InChI InChI=1S/C22H25BrN2O2/c1-2-24-22(26)17-5-3-16(4-6-17)21(23)18-11-19-7-8-20(12-18)25(19)13-15-9-10-27-14-15/h3-6,9-10,14,19-20H,2,7-8,11-13H2,1H3,(H,24,26)/b21-18-/t19-,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against mu opioid receptor in mouse hot plate test |
Bioorg Med Chem Lett 14: 5493-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.004
BindingDB Entry DOI: 10.7270/Q2X34WZV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142129
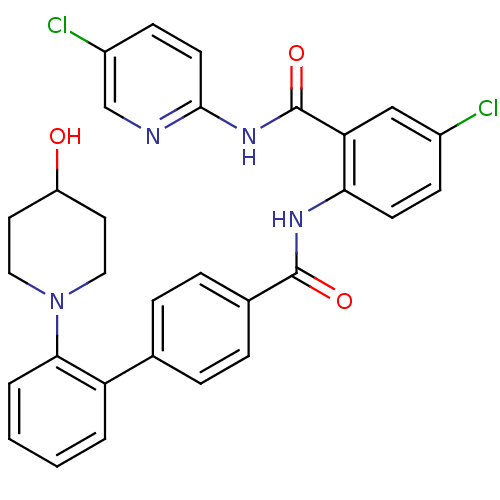
(2'-(4-Hydroxy-piperidin-1-yl)-biphenyl-4-carboxyli...)Show SMILES OC1CCN(CC1)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C30H26Cl2N4O3/c31-21-9-11-26(25(17-21)30(39)35-28-12-10-22(32)18-33-28)34-29(38)20-7-5-19(6-8-20)24-3-1-2-4-27(24)36-15-13-23(37)14-16-36/h1-12,17-18,23,37H,13-16H2,(H,34,38)(H,33,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142118
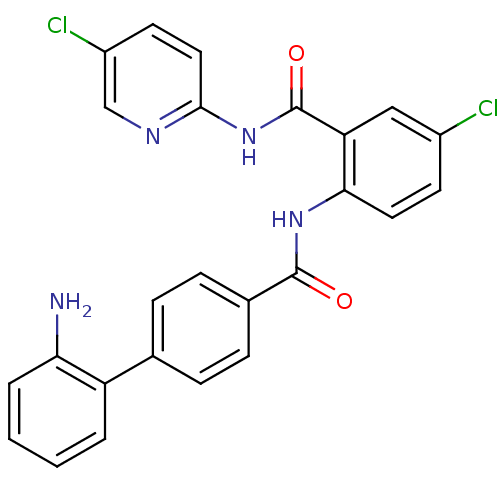
(2'-Amino-biphenyl-4-carboxylic acid [4-chloro-2-(5...)Show SMILES Nc1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C25H18Cl2N4O2/c26-17-9-11-22(20(13-17)25(33)31-23-12-10-18(27)14-29-23)30-24(32)16-7-5-15(6-8-16)19-3-1-2-4-21(19)28/h1-14H,28H2,(H,30,32)(H,29,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50144275
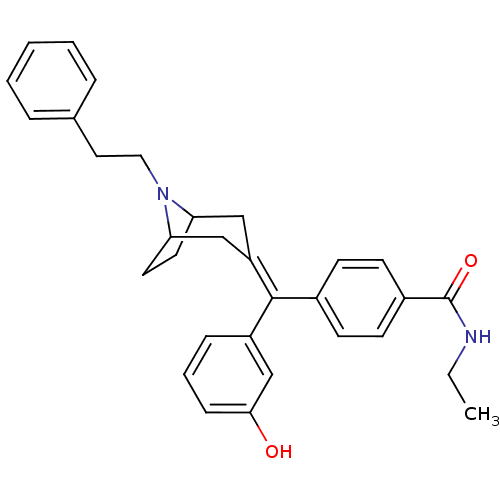
(CHEMBL419621 | N-Ethyl-4-[(3-hydroxy-phenyl)-(8-ph...)Show SMILES [#6]-[#6]-[#7]-[#6](=O)-c1ccc(cc1)-[#6](=[#6]-1/[#6]-[#6]-2-[#6]-[#6]-[#6](-[#6]-1)-[#7]-2-[#6]-[#6]-c1ccccc1)\c1cccc(-[#8])c1 Show InChI InChI=1S/C31H34N2O2/c1-2-32-31(35)24-13-11-23(12-14-24)30(25-9-6-10-29(34)21-25)26-19-27-15-16-28(20-26)33(27)18-17-22-7-4-3-5-8-22/h3-14,21,27-28,34H,2,15-20H2,1H3,(H,32,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.222 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 2113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.052
BindingDB Entry DOI: 10.7270/Q21J997Q |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50144249

(CHEMBL302801 | N,N-Diethyl-4-{[(1S,5R)-8-phenethyl...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C(=C1\C[C@@H]2CC[C@H](C1)N2CCc1ccccc1)\c1ccccc1 |THB:22:21:14.15.20:17.18| Show InChI InChI=1S/C33H38N2O/c1-3-34(4-2)33(36)28-17-15-27(16-18-28)32(26-13-9-6-10-14-26)29-23-30-19-20-31(24-29)35(30)22-21-25-11-7-5-8-12-25/h5-18,30-31H,3-4,19-24H2,1-2H3/b32-29-/t30-,31+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 2113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.052
BindingDB Entry DOI: 10.7270/Q21J997Q |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50144249

(CHEMBL302801 | N,N-Diethyl-4-{[(1S,5R)-8-phenethyl...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C(=C1\C[C@@H]2CC[C@H](C1)N2CCc1ccccc1)\c1ccccc1 |THB:22:21:14.15.20:17.18| Show InChI InChI=1S/C33H38N2O/c1-3-34(4-2)33(36)28-17-15-27(16-18-28)32(26-13-9-6-10-14-26)29-23-30-19-20-31(24-29)35(30)22-21-25-11-7-5-8-12-25/h5-18,30-31H,3-4,19-24H2,1-2H3/b32-29-/t30-,31+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for delta opioid receptor |
Bioorg Med Chem Lett 14: 2109-12 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.051
BindingDB Entry DOI: 10.7270/Q25B01X4 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50144291

(CHEMBL67790 | N-Ethyl-4-[(4-hydroxy-phenyl)-(8-thi...)Show SMILES [#6]-[#6]-[#7]-[#6](=O)-c1ccc(cc1)-[#6](=[#6]-1/[#6]-[#6]-2-[#6]-[#6]-[#6](-[#6]-1)-[#7]-2-[#6]-c1cccs1)\c1ccc(-[#8])cc1 Show InChI InChI=1S/C28H30N2O2S/c1-2-29-28(32)21-7-5-19(6-8-21)27(20-9-13-25(31)14-10-20)22-16-23-11-12-24(17-22)30(23)18-26-4-3-15-33-26/h3-10,13-15,23-24,31H,2,11-12,16-18H2,1H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 2113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.052
BindingDB Entry DOI: 10.7270/Q21J997Q |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50155085
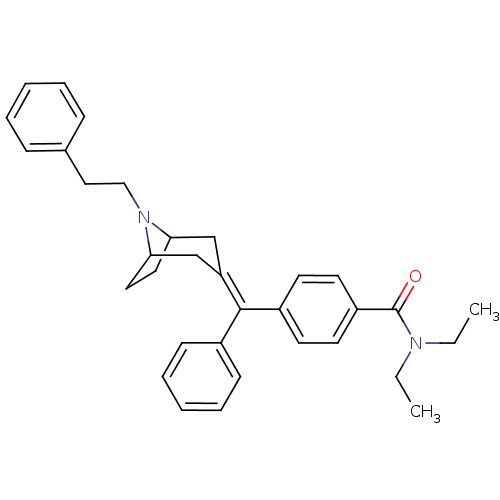
(CHEMBL362331 | N,N-Diethyl-4-[(8-phenethyl-8-aza-b...)Show SMILES [#6]-[#6]-[#7](-[#6]-[#6])-[#6](=O)-c1ccc(cc1)-[#6](=[#6]-1/[#6]-[#6]-2-[#6]-[#6]-[#6](-[#6]-1)-[#7]-2-[#6]-[#6]-c1ccccc1)\c1ccccc1 Show InChI InChI=1S/C33H38N2O/c1-3-34(4-2)33(36)28-17-15-27(16-18-28)32(26-13-9-6-10-14-26)29-23-30-19-20-31(24-29)35(30)22-21-25-11-7-5-8-12-25/h5-18,30-31H,3-4,19-24H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against mu opioid receptor in mouse hot plate test |
Bioorg Med Chem Lett 14: 5493-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.004
BindingDB Entry DOI: 10.7270/Q2X34WZV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50144276
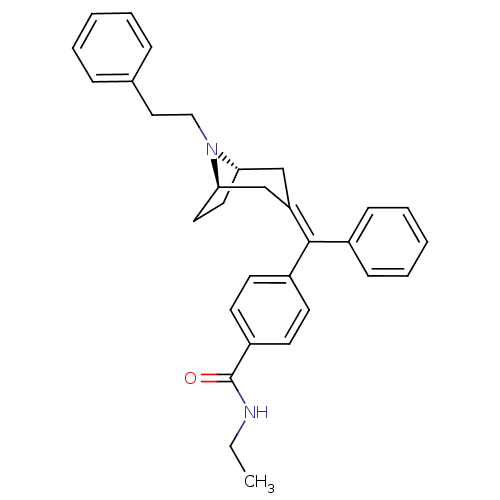
(CHEMBL63651 | N-Ethyl-4-{[(1S,5R)-8-phenethyl-8-az...)Show SMILES CCNC(=O)c1ccc(cc1)C(=C1\C[C@@H]2CC[C@H](C1)N2CCc1ccccc1)\c1ccccc1 |THB:20:19:12.18.13:15.16| Show InChI InChI=1S/C31H34N2O/c1-2-32-31(34)26-15-13-25(14-16-26)30(24-11-7-4-8-12-24)27-21-28-17-18-29(22-27)33(28)20-19-23-9-5-3-6-10-23/h3-16,28-29H,2,17-22H2,1H3,(H,32,34)/b30-27-/t28-,29+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 2113-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.052
BindingDB Entry DOI: 10.7270/Q21J997Q |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-5/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM26267
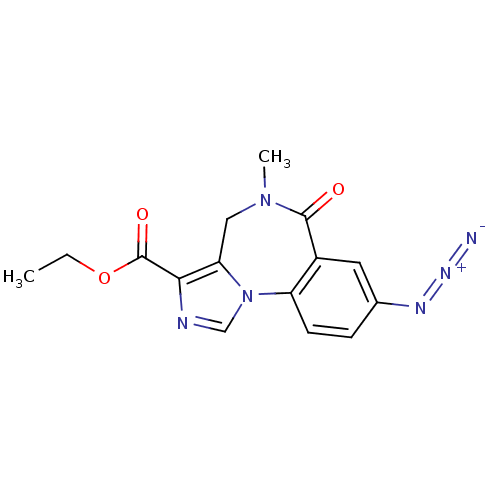
(RO-154513 | Ro15-4513 | [3H]Ro15-4513 | ethyl 12-a...)Show SMILES CCOC(=O)c1ncn-2c1CN(C)C(=O)c1cc(ccc-21)N=[N+]=[N-] Show InChI InChI=1S/C15H14N6O3/c1-3-24-15(23)13-12-7-20(2)14(22)10-6-9(18-19-16)4-5-11(10)21(12)8-17-13/h4-6,8H,3,7H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Binding affinity for Gamma-aminobutyric-acid A receptor alpha5-beta3-gamma2 |
J Med Chem 41: 4130-42 (1998)
Article DOI: 10.1021/jm980317y
BindingDB Entry DOI: 10.7270/Q2W37VFJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
GABA-A receptor; alpha-5/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50067429
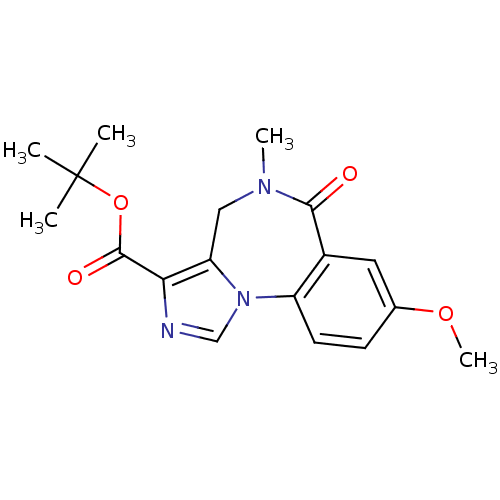
(8-Methoxy-5-methyl-6-oxo-5,6-dihydro-4H-2,5,10b-tr...)Show SMILES COc1ccc-2c(c1)C(=O)N(C)Cc1c(ncn-21)C(=O)OC(C)(C)C Show InChI InChI=1S/C18H21N3O4/c1-18(2,3)25-17(23)15-14-9-20(4)16(22)12-8-11(24-5)6-7-13(12)21(14)10-19-15/h6-8,10H,9H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Binding affinity for Gamma-aminobutyric-acid A receptor alpha5-beta3-gamma2 |
J Med Chem 41: 4130-42 (1998)
Article DOI: 10.1021/jm980317y
BindingDB Entry DOI: 10.7270/Q2W37VFJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data