 Found 325 hits with Last Name = 'zhang' and Initial = 'ch'
Found 325 hits with Last Name = 'zhang' and Initial = 'ch' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein arginine N-methyltransferase 1 [11-371]
(Homo sapiens (Human)) | BDBM50130929

(CHEMBL3633189)Show SMILES NC(=N)c1ccc(Nc2ncnc(Nc3ccc(cc3)C#N)c2[N+]([O-])=O)cc1 Show InChI InChI=1S/C18H14N8O2/c19-9-11-1-5-13(6-2-11)24-17-15(26(27)28)18(23-10-22-17)25-14-7-3-12(4-8-14)16(20)21/h1-8,10H,(H3,20,21)(H2,22,23,24,25) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-U-69,593 binding to Opioid receptor kappa 1 |
Bioorg Med Chem Lett 25: 5449-53 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.095
BindingDB Entry DOI: 10.7270/Q2R2136F |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50086441
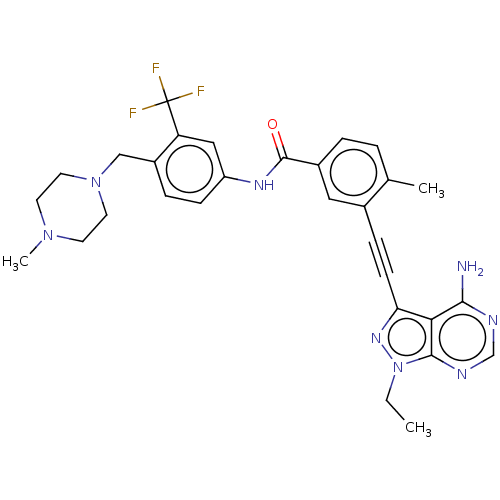
(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Yes |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086454
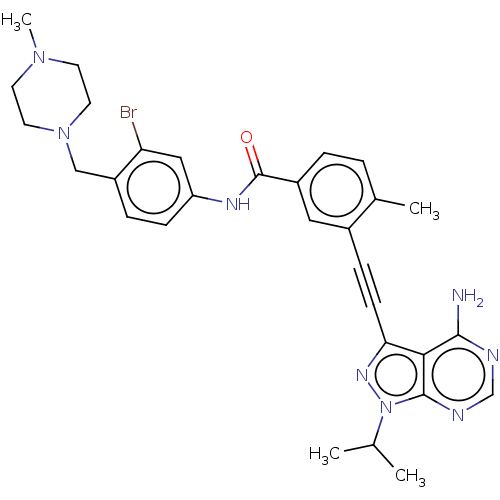
(CHEMBL3425518)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(Br)c2)c2c(N)ncnc12 Show InChI InChI=1S/C30H33BrN8O/c1-19(2)39-29-27(28(32)33-18-34-29)26(36-39)10-8-21-15-22(6-5-20(21)3)30(40)35-24-9-7-23(25(31)16-24)17-38-13-11-37(4)12-14-38/h5-7,9,15-16,18-19H,11-14,17H2,1-4H3,(H,35,40)(H2,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length recombinant YES using poly(Glu,Tyr)4:1 as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometri... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086455
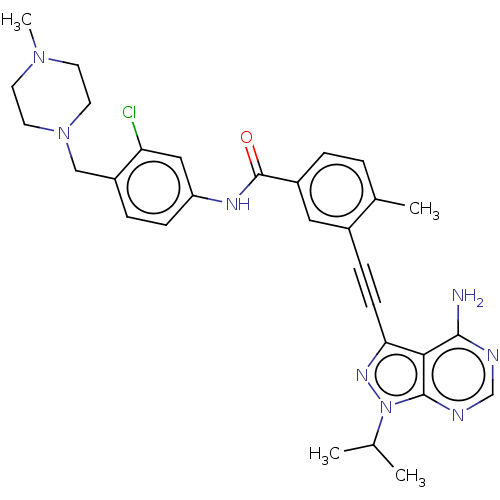
(CHEMBL3426219)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(Cl)c2)c2c(N)ncnc12 Show InChI InChI=1S/C30H33ClN8O/c1-19(2)39-29-27(28(32)33-18-34-29)26(36-39)10-8-21-15-22(6-5-20(21)3)30(40)35-24-9-7-23(25(31)16-24)17-38-13-11-37(4)12-14-38/h5-7,9,15-16,18-19H,11-14,17H2,1-4H3,(H,35,40)(H2,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086457
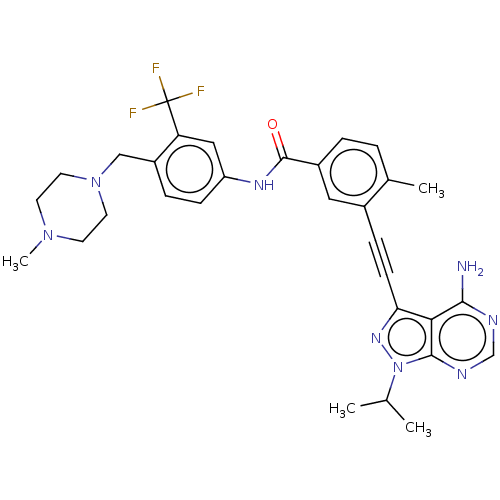
(CHEMBL3426217)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C31H33F3N8O/c1-19(2)42-29-27(28(35)36-18-37-29)26(39-42)10-8-21-15-22(6-5-20(21)3)30(43)38-24-9-7-23(25(16-24)31(32,33)34)17-41-13-11-40(4)12-14-41/h5-7,9,15-16,18-19H,11-14,17H2,1-4H3,(H,38,43)(H2,35,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086451
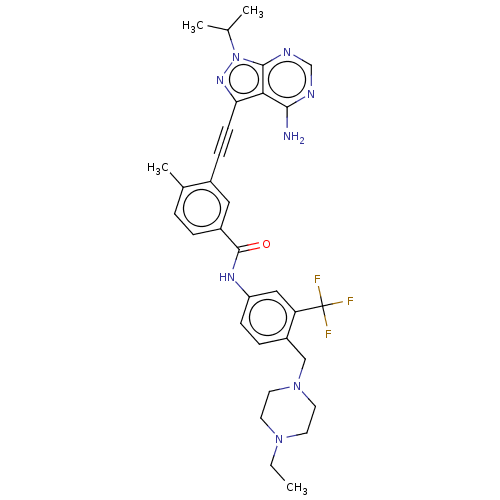
(CHEMBL3426222)Show SMILES CCN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn(C(C)C)c4ncnc(N)c34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C32H35F3N8O/c1-5-41-12-14-42(15-13-41)18-24-8-10-25(17-26(24)32(33,34)35)39-31(44)23-7-6-21(4)22(16-23)9-11-27-28-29(36)37-19-38-30(28)43(40-27)20(2)3/h6-8,10,16-17,19-20H,5,12-15,18H2,1-4H3,(H,39,44)(H2,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ABL (27 to end residues) using EAIYAAPFAKKK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radio... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HCK (230 to 497 residues) using GGMEDIYFEFMGGKKK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometric ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length recombinant Src using Cdc2 peptide as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometric sc... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50322535
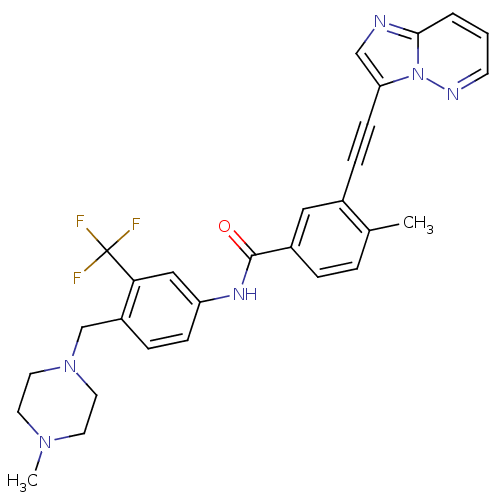
(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ABL T315I mutant (27 to end residues) using EAIYAAPFAKKK as substrate incubated for 40 mins in presence of [gamma33P-... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086453

(CHEMBL3426220 | US10266537, Compound 14)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2cccc(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C25H21F3N6O/c1-14(2)34-23-21(22(29)30-13-31-23)20(33-34)10-9-16-11-17(8-7-15(16)3)24(35)32-19-6-4-5-18(12-19)25(26,27)28/h4-8,11-14H,1-3H3,(H,32,35)(H2,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086450
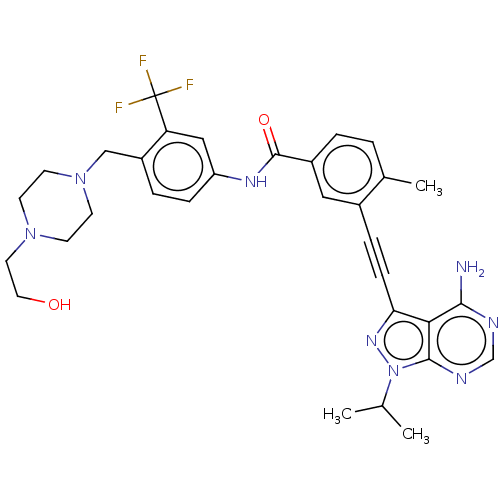
(CHEMBL3426223)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C32H35F3N8O2/c1-20(2)43-30-28(29(36)37-19-38-30)27(40-43)9-7-22-16-23(5-4-21(22)3)31(45)39-25-8-6-24(26(17-25)32(33,34)35)18-42-12-10-41(11-13-42)14-15-44/h4-6,8,16-17,19-20,44H,10-15,18H2,1-3H3,(H,39,45)(H2,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length recombinant LYN using poly(Glu,Tyr)4:1 as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometri... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50399676
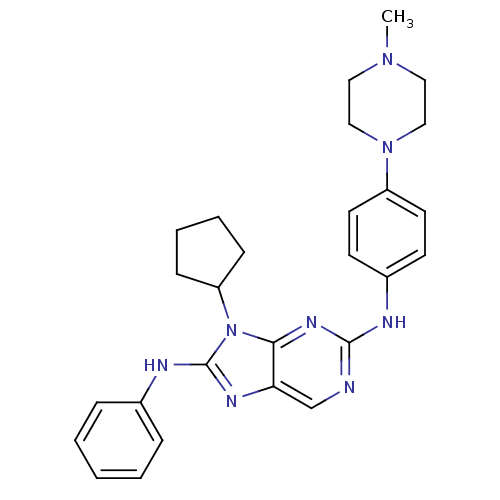
(CHEMBL2178352 | US9096601, 8-26)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc3nc(Nc4ccccc4)n(C4CCCC4)c3n2)cc1 Show InChI InChI=1S/C27H32N8/c1-33-15-17-34(18-16-33)22-13-11-21(12-14-22)29-26-28-19-24-25(32-26)35(23-9-5-6-10-23)27(31-24)30-20-7-3-2-4-8-20/h2-4,7-8,11-14,19,23H,5-6,9-10,15-18H2,1H3,(H,30,31)(H,28,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant EGFR by radiometric kinase assay |
J Med Chem 55: 10685-99 (2012)
Article DOI: 10.1021/jm301365e
BindingDB Entry DOI: 10.7270/Q2251KBV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086456

(CHEMBL3426218 | US10266537, Compound 17)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)cc2)c2c(N)ncnc12 Show InChI InChI=1S/C30H34N8O/c1-20(2)38-29-27(28(31)32-19-33-29)26(35-38)12-9-23-17-24(8-5-21(23)3)30(39)34-25-10-6-22(7-11-25)18-37-15-13-36(4)14-16-37/h5-8,10-11,17,19-20H,13-16,18H2,1-4H3,(H,34,39)(H2,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant RET (658 to end residues) using KKKSPGEYVNIEFG as substrate incubated for 40 mins in presence of [gamma33P-ATP] by ra... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG (38 to end residues) using EAIYAAPFAKKK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radio... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50399676

(CHEMBL2178352 | US9096601, 8-26)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc3nc(Nc4ccccc4)n(C4CCCC4)c3n2)cc1 Show InChI InChI=1S/C27H32N8/c1-33-15-17-34(18-16-33)22-13-11-21(12-14-22)29-26-28-19-24-25(32-26)35(23-9-5-6-10-23)27(31-24)30-20-7-3-2-4-8-20/h2-4,7-8,11-14,19,23H,5-6,9-10,15-18H2,1H3,(H,30,31)(H,28,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant EGFR by radiometric kinase assay |
J Med Chem 55: 10685-99 (2012)
Article DOI: 10.1021/jm301365e
BindingDB Entry DOI: 10.7270/Q2251KBV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Fyn |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086586

(CHEMBL3426234 | US10266537, Compound 29)Show SMILES CCn1nc(C#Cc2cccc(c2)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C29H29F3N8O/c1-3-40-27-25(26(33)34-18-35-27)24(37-40)10-7-19-5-4-6-20(15-19)28(41)36-22-9-8-21(23(16-22)29(30,31)32)17-39-13-11-38(2)12-14-39/h4-6,8-9,15-16,18H,3,11-14,17H2,1-2H3,(H,36,41)(H2,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length recombinant FYN using Cdc2 peptide as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometric sc... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase TXK
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant TXK (256 to end residues) using GEEPLYWSFPAKKK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by ra... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50386751

(CHEMBL2046884)Show SMILES O=C(Nc1nnc(Sc2ncnc3cc(OCCCN4CCOCC4)ccc23)s1)Nc1cccc(c1)C#C Show InChI InChI=1S/C26H25N7O3S2/c1-2-18-5-3-6-19(15-18)29-24(34)30-25-31-32-26(38-25)37-23-21-8-7-20(16-22(21)27-17-28-23)36-12-4-9-33-10-13-35-14-11-33/h1,3,5-8,15-17H,4,9-14H2,(H2,29,30,31,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
J Med Chem 55: 3852-66 (2012)
Article DOI: 10.1021/jm300042x
BindingDB Entry DOI: 10.7270/Q2M61M94 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant FGFR1 (456 to 765 residues) using KKKSPGEYVNIEFG as substrate incubated for 40 mins in presence of [gamma33P-ATP] by ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086449

(CHEMBL3426224 | US10266537, Compound 8)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn(C)c4ncnc(N)c34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H29F3N8O/c1-18-4-5-20(14-19(18)7-9-24-25-26(33)34-17-35-27(25)39(3)37-24)28(41)36-22-8-6-21(23(15-22)29(30,31)32)16-40-12-10-38(2)11-13-40/h4-6,8,14-15,17H,10-13,16H2,1-3H3,(H,36,41)(H2,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50386750

(CHEMBL2046699)Show InChI InChI=1S/C13H11N3O2S2/c1-17-10-5-8-9(6-11(10)18-2)15-7-16-12(8)20-13-14-3-4-19-13/h3-7H,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
J Med Chem 55: 3852-66 (2012)
Article DOI: 10.1021/jm300042x
BindingDB Entry DOI: 10.7270/Q2M61M94 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086444

(CHEMBL3426230 | US10266537, Compound 20)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn([C@@H]4CCOC4)c4ncnc(N)c34)cc2C(F)(F)F)CC1 |r| Show InChI InChI=1S/C32H33F3N8O2/c1-20-3-4-22(15-21(20)6-8-27-28-29(36)37-19-38-30(28)43(40-27)25-9-14-45-18-25)31(44)39-24-7-5-23(26(16-24)32(33,34)35)17-42-12-10-41(2)11-13-42/h3-5,7,15-16,19,25H,9-14,17-18H2,1-2H3,(H,39,44)(H2,36,37,38)/t25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086445

(CHEMBL3426229 | US10266537, Compound 21)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn([C@H]4CCOC4)c4ncnc(N)c34)cc2C(F)(F)F)CC1 |r| Show InChI InChI=1S/C32H33F3N8O2/c1-20-3-4-22(15-21(20)6-8-27-28-29(36)37-19-38-30(28)43(40-27)25-9-14-45-18-25)31(44)39-24-7-5-23(26(16-24)32(33,34)35)17-42-12-10-41(2)11-13-42/h3-5,7,15-16,19,25H,9-14,17-18H2,1-2H3,(H,39,44)(H2,36,37,38)/t25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086446
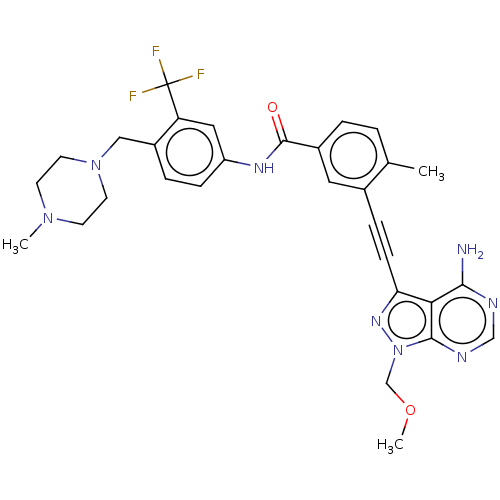
(CHEMBL3426228 | US10266537, Compound 19)Show SMILES COCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O2/c1-19-4-5-21(14-20(19)7-9-25-26-27(34)35-17-36-28(26)41(38-25)18-43-3)29(42)37-23-8-6-22(24(15-23)30(31,32)33)16-40-12-10-39(2)11-13-40/h4-6,8,14-15,17H,10-13,16,18H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086448

(CHEMBL3426226 | US10266537, Compound 6)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn(C4CCC4)c4ncnc(N)c34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C32H33F3N8O/c1-20-6-7-22(16-21(20)9-11-27-28-29(36)37-19-38-30(28)43(40-27)25-4-3-5-25)31(44)39-24-10-8-23(26(17-24)32(33,34)35)18-42-14-12-41(2)13-15-42/h6-8,10,16-17,19,25H,3-5,12-15,18H2,1-2H3,(H,39,44)(H2,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length recombinant CSK using poly(Glu,Tyr)4:1 as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometri... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50386748
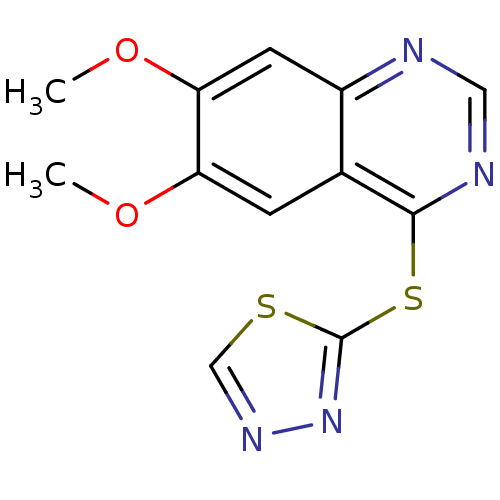
(CHEMBL2046726)Show InChI InChI=1S/C12H10N4O2S2/c1-17-9-3-7-8(4-10(9)18-2)13-5-14-11(7)20-12-16-15-6-19-12/h3-6H,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
J Med Chem 55: 3852-66 (2012)
Article DOI: 10.1021/jm300042x
BindingDB Entry DOI: 10.7270/Q2M61M94 |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant TIE2 Q939H/Q940H mutant (771 to end residues) using poly(Glu,Tyr)4:1 as substrate incubated for 40 mins in presence o... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50386749
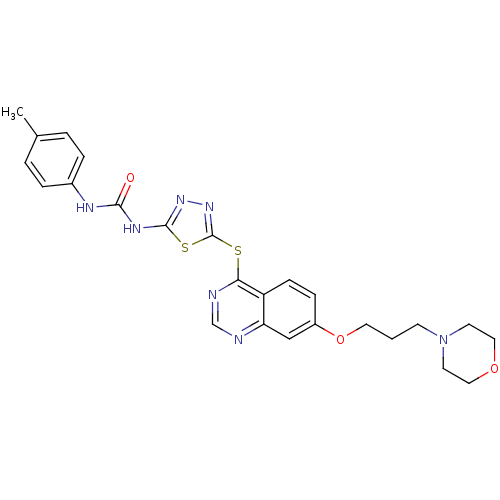
(CHEMBL2046883)Show SMILES Cc1ccc(NC(=O)Nc2nnc(Sc3ncnc4cc(OCCCN5CCOCC5)ccc34)s2)cc1 Show InChI InChI=1S/C25H27N7O3S2/c1-17-3-5-18(6-4-17)28-23(33)29-24-30-31-25(37-24)36-22-20-8-7-19(15-21(20)26-16-27-22)35-12-2-9-32-10-13-34-14-11-32/h3-8,15-16H,2,9-14H2,1H3,(H2,28,29,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
J Med Chem 55: 3852-66 (2012)
Article DOI: 10.1021/jm300042x
BindingDB Entry DOI: 10.7270/Q2M61M94 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086585
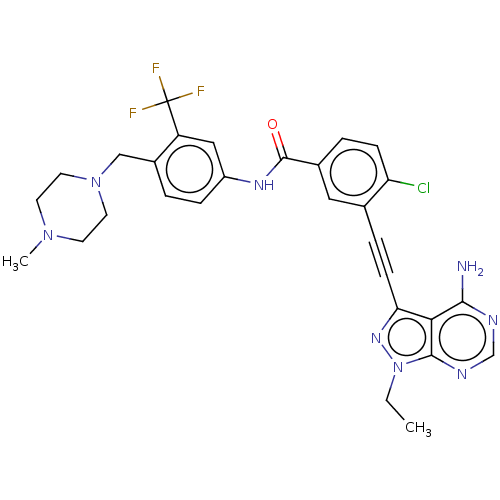
(CHEMBL3426235 | US10266537, Compound 26)Show SMILES CCn1nc(C#Cc2cc(ccc2Cl)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C29H28ClF3N8O/c1-3-41-27-25(26(34)35-17-36-27)24(38-41)9-6-18-14-19(5-8-23(18)30)28(42)37-21-7-4-20(22(15-21)29(31,32)33)16-40-12-10-39(2)11-13-40/h4-5,7-8,14-15,17H,3,10-13,16H2,1-2H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086443
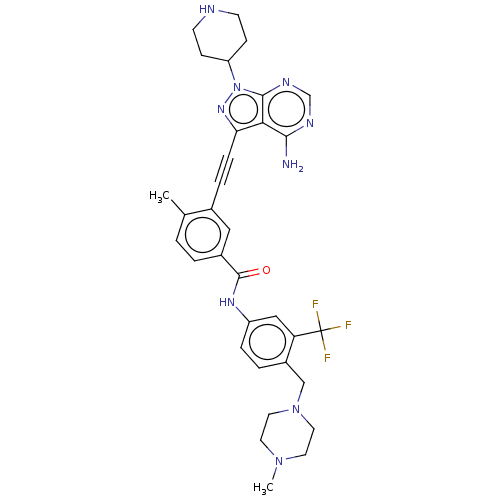
(CHEMBL3426232)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn(C4CCNCC4)c4ncnc(N)c34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C33H36F3N9O/c1-21-3-4-23(32(46)41-25-7-5-24(27(18-25)33(34,35)36)19-44-15-13-43(2)14-16-44)17-22(21)6-8-28-29-30(37)39-20-40-31(29)45(42-28)26-9-11-38-12-10-26/h3-5,7,17-18,20,26,38H,9-16,19H2,1-2H3,(H,41,46)(H2,37,39,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length recombinant LCK using KVEKIGEGTYGVVYK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometric... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human B-RAF V600E mutant |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant FLT1 (783 to end residues) using KKKSPGEYVNIEFG as substrate incubated for 40 mins in presence of [gamma33P-ATP] by r... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086442
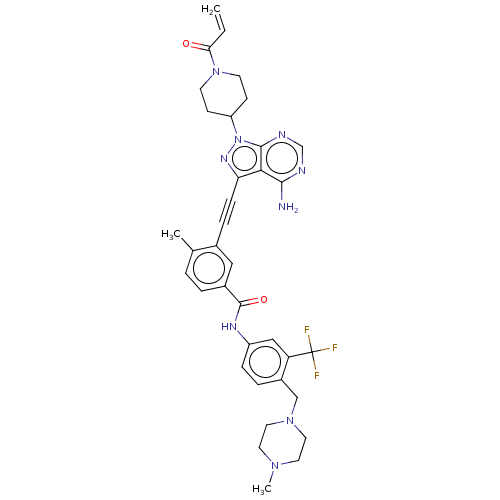
(CHEMBL3426233 | US10266537, Compound 121)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn(C4CCN(CC4)C(=O)C=C)c4ncnc(N)c34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C36H38F3N9O2/c1-4-31(49)47-13-11-28(12-14-47)48-34-32(33(40)41-22-42-34)30(44-48)10-8-24-19-25(6-5-23(24)2)35(50)43-27-9-7-26(29(20-27)36(37,38)39)21-46-17-15-45(3)16-18-46/h4-7,9,19-20,22,28H,1,11-18,21H2,2-3H3,(H,43,50)(H2,40,41,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length recombinant BLK M287V mutant using poly(Glu,Tyr)4:1 as substrate incubated for 40 mins in presence of [gamma33P-ATP] ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human EphA2 |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM448369
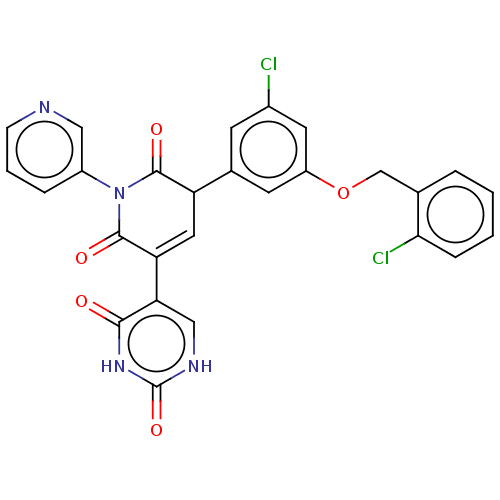
(Perampanel, 21)Show SMILES Clc1cc(OCc2ccccc2Cl)cc(c1)C1C=C(C(=O)N(C1=O)c1cccnc1)c1c[nH]c(=O)[nH]c1=O |c:19| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
| Assay Description
Inhibition of proteolytic activity was tested using recombinant SARS-CoV-2Mpro, which was expressed and purified as previously described.8,12 For the... |
ACS Cent Sci (2021)
Article DOI: 10.1021/acscentsci.1c00039
BindingDB Entry DOI: 10.7270/Q20K2CMV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase FRK
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant PTK5 (218 to end residues) using GGEEEEYFELVKKKK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086447
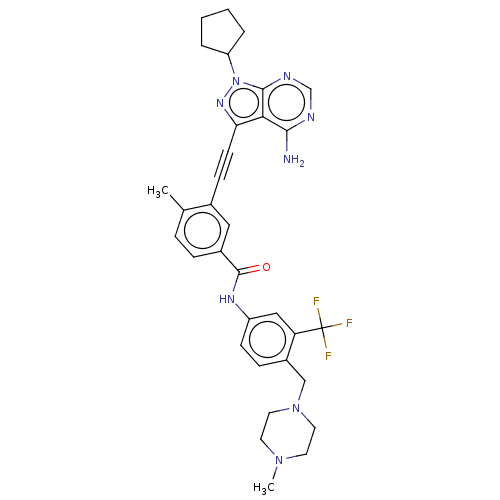
(CHEMBL3426227 | US10266537, Compound 7)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn(C4CCCC4)c4ncnc(N)c34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C33H35F3N8O/c1-21-7-8-23(17-22(21)10-12-28-29-30(37)38-20-39-31(29)44(41-28)26-5-3-4-6-26)32(45)40-25-11-9-24(27(18-25)33(34,35)36)19-43-15-13-42(2)14-16-43/h7-9,11,17-18,20,26H,3-6,13-16,19H2,1-2H3,(H,40,45)(H2,37,38,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Blk |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data