

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50109021 ((R)-8-(Benzylamino-methyl)-3,6,7,8-tetrahydro-1H-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity of compound for D2 receptor in rat striatal membrane determined for agonist state (high affinity state, D2 High) [3H]quinpirole as radioliga... | Bioorg Med Chem Lett 12: 271-4 (2002) BindingDB Entry DOI: 10.7270/Q22J6CDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50077581 ((R)-2-(Benzylamino-methyl)-3,4,7,9-tetrahydro-2H-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity of compound for D2 receptor in rat striatal membrane determined for agonist state (high affinity state, D2 High) [3H]quinpirole as radioliga... | Bioorg Med Chem Lett 12: 271-4 (2002) BindingDB Entry DOI: 10.7270/Q22J6CDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50109020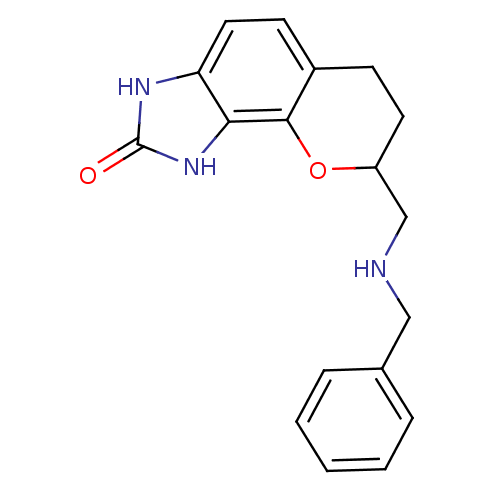 (8-(Benzylamino-methyl)-3,6,7,8-tetrahydro-1H-chrom...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity of compound for D2 receptor in rat striatal membrane determined for agonist state (high affinity state, D2 High) [3H]quinpirole as radioliga... | Bioorg Med Chem Lett 12: 271-4 (2002) BindingDB Entry DOI: 10.7270/Q22J6CDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50061669 ((R)-2-(Benzylamino-methyl)-chroman-7-ol; oxalic ac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity of compound for D2 receptor in rat striatal membrane determined for agonist state (high affinity state, D2 High) [3H]quinpirole as radioliga... | Bioorg Med Chem Lett 12: 271-4 (2002) BindingDB Entry DOI: 10.7270/Q22J6CDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]Spiprone from human dopamine D2 receptor expressed in HEK293 cells | Bioorg Med Chem 20: 4862-71 (2012) Article DOI: 10.1016/j.bmc.2012.05.057 BindingDB Entry DOI: 10.7270/Q28P61K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50109021 ((R)-8-(Benzylamino-methyl)-3,6,7,8-tetrahydro-1H-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity for the Human Dopamine receptor D3 was determined using membranes from CHO cells labeled with [3H]spiperone | Bioorg Med Chem Lett 12: 271-4 (2002) BindingDB Entry DOI: 10.7270/Q22J6CDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in HEK293 cells | Bioorg Med Chem 20: 4862-71 (2012) Article DOI: 10.1016/j.bmc.2012.05.057 BindingDB Entry DOI: 10.7270/Q28P61K9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50109020 (8-(Benzylamino-methyl)-3,6,7,8-tetrahydro-1H-chrom...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity for the Human Dopamine receptor D3 was determined using membranes from CHO cells labeled with [3H]spiperone | Bioorg Med Chem Lett 12: 271-4 (2002) BindingDB Entry DOI: 10.7270/Q22J6CDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human dopamine D1 receptor expressed in HEK293 cells | Bioorg Med Chem 20: 4862-71 (2012) Article DOI: 10.1016/j.bmc.2012.05.057 BindingDB Entry DOI: 10.7270/Q28P61K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50206243 (CHEMBL3918431) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [125I]alpha-bungarotoxin from human alpha7 nAChR expressed in HEK293 cell membranes incubated for 2 hrs and measured by gamma countin... | ACS Med Chem Lett 8: 366-371 (2017) Article DOI: 10.1021/acsmedchemlett.7b00032 BindingDB Entry DOI: 10.7270/Q2S46V8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50077569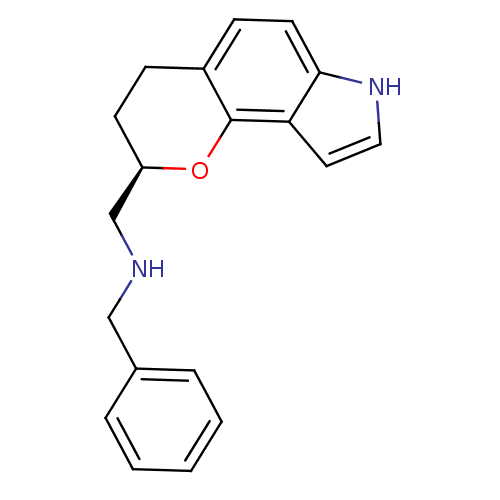 (Benzyl-[(R)-1-(2,3,4,7-tetrahydro-pyrano[2,3-e]ind...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity of compound for D2 receptor in rat striatal membrane determined for agonist state (high affinity state, D2 High) [3H]quinpirole as radioliga... | Bioorg Med Chem Lett 12: 271-4 (2002) BindingDB Entry DOI: 10.7270/Q22J6CDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]Ketanserin from human 5HT2A receptor expressed in HEK293 cells | Bioorg Med Chem 20: 4862-71 (2012) Article DOI: 10.1016/j.bmc.2012.05.057 BindingDB Entry DOI: 10.7270/Q28P61K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50061669 ((R)-2-(Benzylamino-methyl)-chroman-7-ol; oxalic ac...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity for the Human Dopamine receptor D3 was determined using membranes from CHO cells labeled with [3H]spiperone | Bioorg Med Chem Lett 12: 271-4 (2002) BindingDB Entry DOI: 10.7270/Q22J6CDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50077581 ((R)-2-(Benzylamino-methyl)-3,4,7,9-tetrahydro-2H-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity for the Human Dopamine receptor D3 was determined using membranes from CHO cells labeled with [3H]spiperone | Bioorg Med Chem Lett 12: 271-4 (2002) BindingDB Entry DOI: 10.7270/Q22J6CDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50387561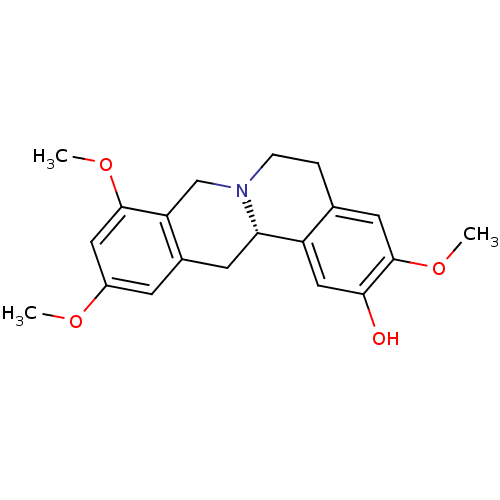 (CHEMBL2057455 | US9359372, DC037079) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human dopamine D1 receptor expressed in HEK293 cells | Bioorg Med Chem 20: 4862-71 (2012) Article DOI: 10.1016/j.bmc.2012.05.057 BindingDB Entry DOI: 10.7270/Q28P61K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50109021 ((R)-8-(Benzylamino-methyl)-3,6,7,8-tetrahydro-1H-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity for the Dopamine receptor D2S was determined using membranes from CHO cells labeled with [3H]spiperone | Bioorg Med Chem Lett 12: 271-4 (2002) BindingDB Entry DOI: 10.7270/Q22J6CDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50235306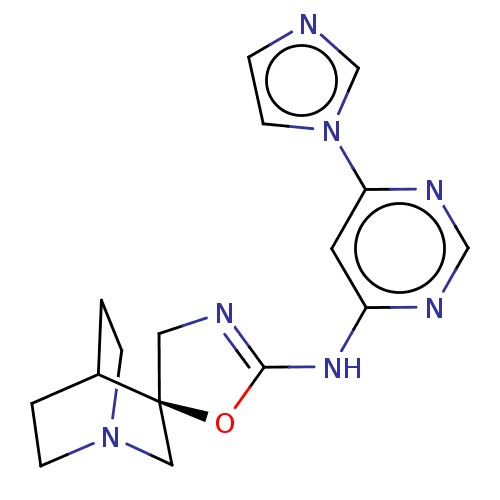 (CHEMBL4084621) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [125I]alpha-bungarotoxin from rat alpha7 nAChR expressed in HEK293 cell membranes incubated for 2 hrs and measured by gamma counting ... | ACS Med Chem Lett 8: 366-371 (2017) Article DOI: 10.1021/acsmedchemlett.7b00032 BindingDB Entry DOI: 10.7270/Q2S46V8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50061669 ((R)-2-(Benzylamino-methyl)-chroman-7-ol; oxalic ac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity of compound for Dopamine receptor D2 in rat striatal membrane determined for antagonist state (low affinity state, D2 Low) with [3H]spiperon... | Bioorg Med Chem Lett 12: 271-4 (2002) BindingDB Entry DOI: 10.7270/Q22J6CDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity against human Cannabinoid receptor 2 expressed in CHO cells by using WIN 55,2122Mesylate [5,73H] as Radioactive tracer | Bioorg Med Chem Lett 12: 2399-402 (2002) BindingDB Entry DOI: 10.7270/Q2TM79FG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50206243 (CHEMBL3918431) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [125I]Tyr54-alpha-bungarotoxin from rat alpha7 nAChR expressed in HEK293 cell membranes co-expressing human RIC3 measured after 2 hrs... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50109021 ((R)-8-(Benzylamino-methyl)-3,6,7,8-tetrahydro-1H-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity of compound for Dopamine receptor D2 in rat striatal membrane determined for antagonist state (low affinity state, D2 Low) with [3H]spiperon... | Bioorg Med Chem Lett 12: 271-4 (2002) BindingDB Entry DOI: 10.7270/Q22J6CDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50206243 (CHEMBL3918431) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [125I]alpha-bungarotoxin from rat alpha7 nAChR expressed in HEK293 cell membranes incubated for 2 hrs and measured by gamma counting ... | ACS Med Chem Lett 8: 366-371 (2017) Article DOI: 10.1021/acsmedchemlett.7b00032 BindingDB Entry DOI: 10.7270/Q2S46V8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50109020 (8-(Benzylamino-methyl)-3,6,7,8-tetrahydro-1H-chrom...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity for the Dopamine receptor D2S was determined using membranes from CHO cells labeled with [3H]spiperone | Bioorg Med Chem Lett 12: 271-4 (2002) BindingDB Entry DOI: 10.7270/Q22J6CDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50451435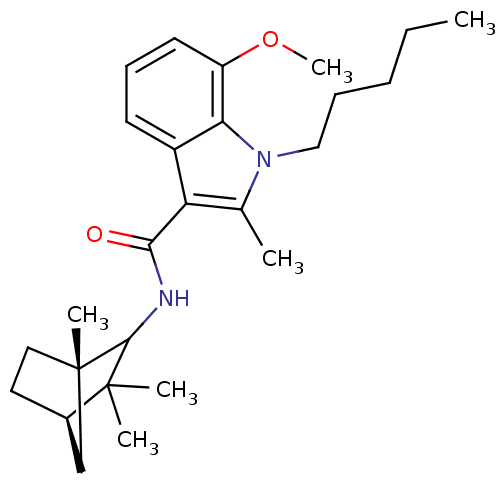 (CHEMBL2112289) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity against human CB2 receptor expressed in CHO cells by using WIN55,2122 Mesylate [5,73H] as Radioactive tracer | Bioorg Med Chem Lett 12: 2399-402 (2002) BindingDB Entry DOI: 10.7270/Q2TM79FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50109024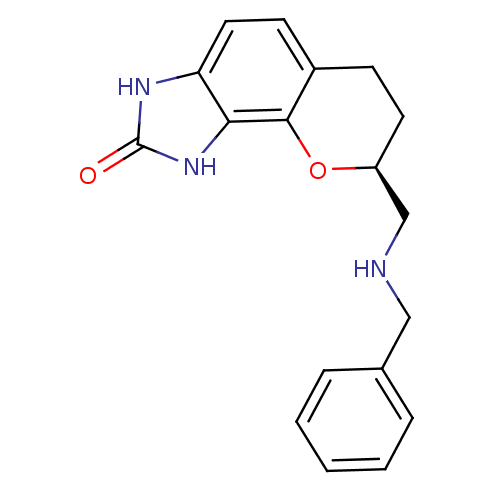 ((S)-8-(Benzylamino-methyl)-3,6,7,8-tetrahydro-1H-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity of compound for D2 receptor in rat striatal membrane determined for agonist state (high affinity state, D2 High) [3H]quinpirole as radioliga... | Bioorg Med Chem Lett 12: 271-4 (2002) BindingDB Entry DOI: 10.7270/Q22J6CDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50109019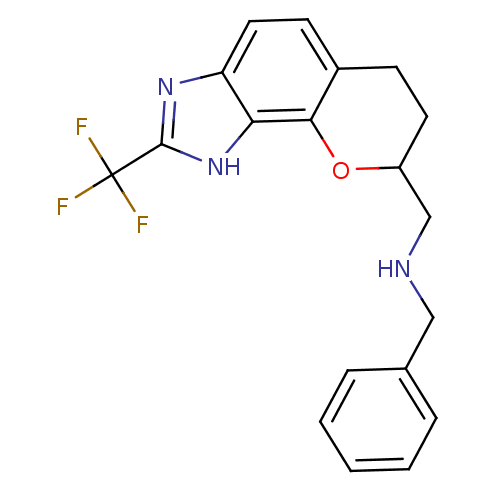 (Benzyl-(2-trifluoromethyl-3,6,7,8-tetrahydro-chrom...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity of compound for D2 receptor in rat striatal membrane determined for agonist state (high affinity state, D2 High) [3H]quinpirole as radioliga... | Bioorg Med Chem Lett 12: 271-4 (2002) BindingDB Entry DOI: 10.7270/Q22J6CDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50077581 ((R)-2-(Benzylamino-methyl)-3,4,7,9-tetrahydro-2H-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity of compound for Dopamine receptor D2 in rat striatal membrane determined for antagonist state (low affinity state, D2 Low) with [3H]spiperon... | Bioorg Med Chem Lett 12: 271-4 (2002) BindingDB Entry DOI: 10.7270/Q22J6CDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50378584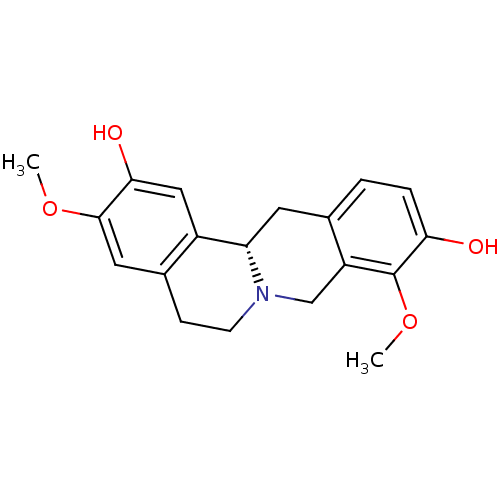 (STEPHOLIDINE) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 6.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human dopamine D1 receptor expressed in HEK293 cells | Bioorg Med Chem 20: 4862-71 (2012) Article DOI: 10.1016/j.bmc.2012.05.057 BindingDB Entry DOI: 10.7270/Q28P61K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50109021 ((R)-8-(Benzylamino-methyl)-3,6,7,8-tetrahydro-1H-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity for the Human Dopamine receptor D4 was determined using membranes from CHO cells labeled with [3H]spiperone | Bioorg Med Chem Lett 12: 271-4 (2002) BindingDB Entry DOI: 10.7270/Q22J6CDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50109020 (8-(Benzylamino-methyl)-3,6,7,8-tetrahydro-1H-chrom...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity of compound for Dopamine receptor D2 in rat striatal membrane determined for antagonist state (low affinity state, D2 Low) with [3H]spiperon... | Bioorg Med Chem Lett 12: 271-4 (2002) BindingDB Entry DOI: 10.7270/Q22J6CDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50387564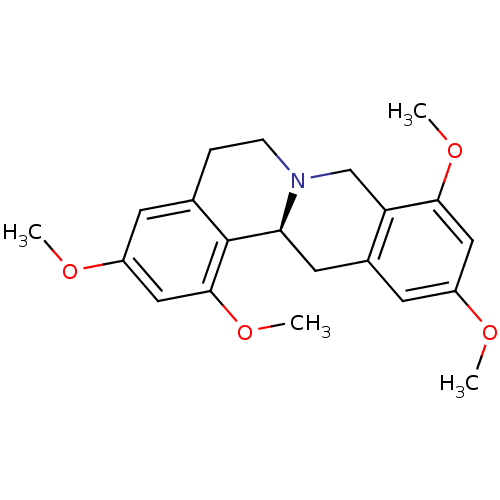 (CHEMBL2057445) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human dopamine D1 receptor expressed in HEK293 cells | Bioorg Med Chem 20: 4862-71 (2012) Article DOI: 10.1016/j.bmc.2012.05.057 BindingDB Entry DOI: 10.7270/Q28P61K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50116857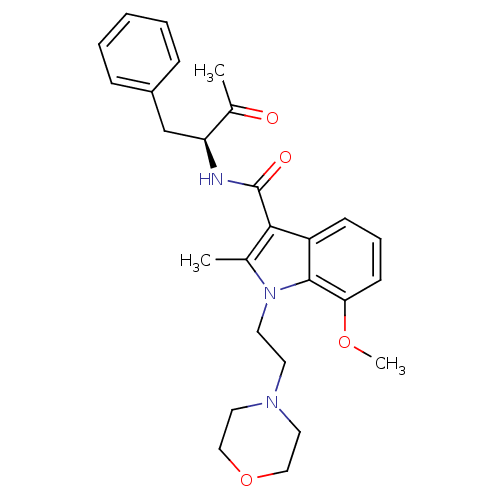 (7-Methoxy-2-methyl-1-(2-morpholin-4-yl-ethyl)-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity against human CB2 receptor expressed in CHO cells by using WIN55,2122 Mesylate [5,73H] as Radioactive tracer | Bioorg Med Chem Lett 12: 2399-402 (2002) BindingDB Entry DOI: 10.7270/Q2TM79FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50116851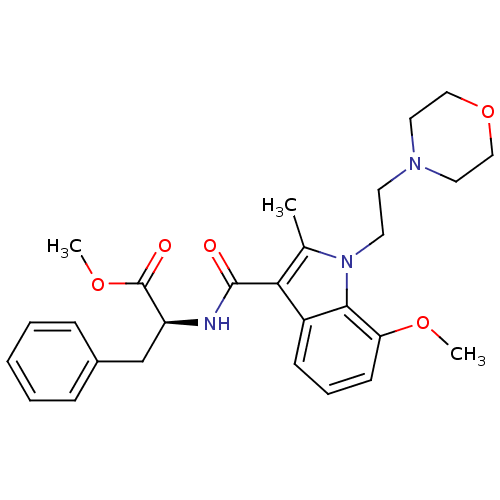 ((S)-2-{[7-Methoxy-2-methyl-1-(2-morpholin-4-yl-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity against human Cannabinoid receptor 2 expressed in CHO cells by using WIN 55,2122Mesylate [5,73H] as Radioactive tracer | Bioorg Med Chem Lett 12: 2399-402 (2002) BindingDB Entry DOI: 10.7270/Q2TM79FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50235306 (CHEMBL4084621) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [125I]alpha-bungarotoxin from human alpha7 nAChR expressed in HEK293 cell membranes incubated for 2 hrs and measured by gamma countin... | ACS Med Chem Lett 8: 366-371 (2017) Article DOI: 10.1021/acsmedchemlett.7b00032 BindingDB Entry DOI: 10.7270/Q2S46V8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50077581 ((R)-2-(Benzylamino-methyl)-3,4,7,9-tetrahydro-2H-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity for the Dopamine receptor D2S was determined using membranes from CHO cells labeled with [3H]spiperone | Bioorg Med Chem Lett 12: 271-4 (2002) BindingDB Entry DOI: 10.7270/Q22J6CDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Escherichia coli (strain K12)) | BDBM50385299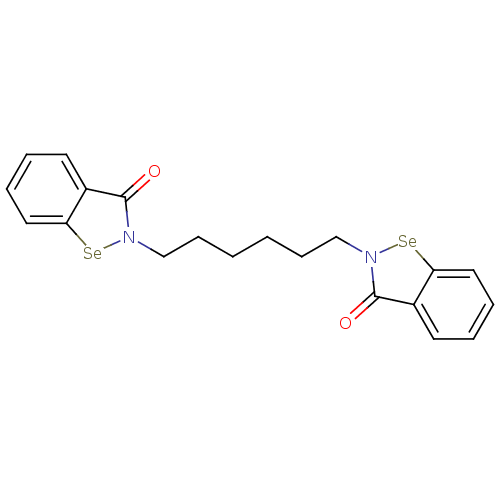 (CHEMBL2035464 | US8592468, EbSe16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 10 | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB US Patent | Assay Description All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one derivatives were tested as potential E. coli TrxR inhibitors by standard DTNB ... | US Patent US8592468 (2013) BindingDB Entry DOI: 10.7270/Q29P3081 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50061669 ((R)-2-(Benzylamino-methyl)-chroman-7-ol; oxalic ac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity for the Dopamine receptor D2S was determined using membranes from CHO cells labeled with [3H]spiperone | Bioorg Med Chem Lett 12: 271-4 (2002) BindingDB Entry DOI: 10.7270/Q22J6CDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50109020 (8-(Benzylamino-methyl)-3,6,7,8-tetrahydro-1H-chrom...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity for the Human Dopamine receptor D4 was determined using membranes from CHO cells labeled with [3H]spiperone | Bioorg Med Chem Lett 12: 271-4 (2002) BindingDB Entry DOI: 10.7270/Q22J6CDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50116855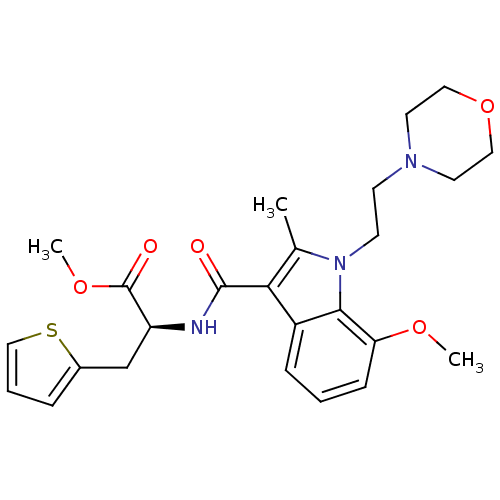 ((S)-2-{[7-Methoxy-2-methyl-1-(2-morpholin-4-yl-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity against human CB2 receptor expressed in CHO cells by using WIN55,2122 Mesylate [5,73H] as Radioactive tracer | Bioorg Med Chem Lett 12: 2399-402 (2002) BindingDB Entry DOI: 10.7270/Q2TM79FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50116849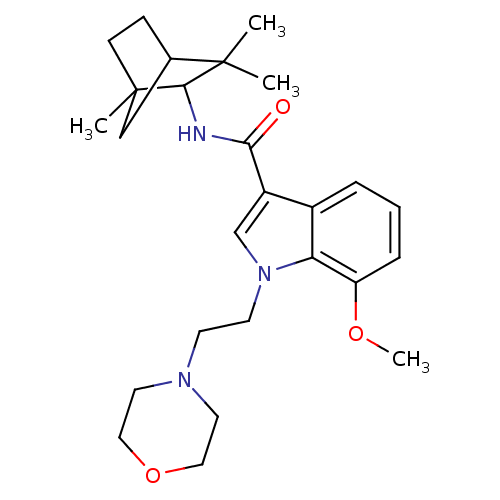 (7-Methoxy-1-(2-morpholin-4-yl-ethyl)-1H-indole-3-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity against human CB2 receptor expressed in CHO cells by using WIN55,2122 Mesylate [5,73H] as Radioactive tracer | Bioorg Med Chem Lett 12: 2399-402 (2002) BindingDB Entry DOI: 10.7270/Q2TM79FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50451436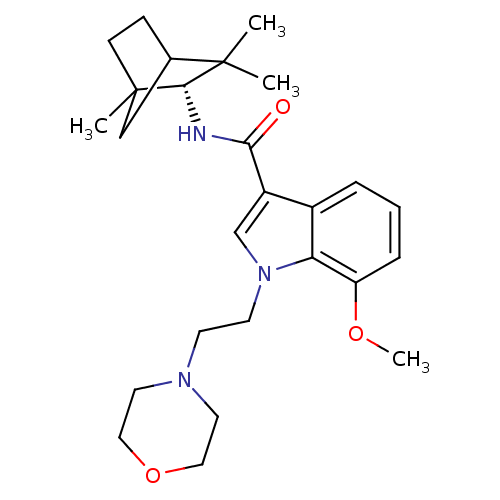 (CHEMBL2112287) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity against human CB2 receptor expressed in CHO cells by using WIN55,2122 Mesylate [5,73H] as Radioactive tracer | Bioorg Med Chem Lett 12: 2399-402 (2002) BindingDB Entry DOI: 10.7270/Q2TM79FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50387558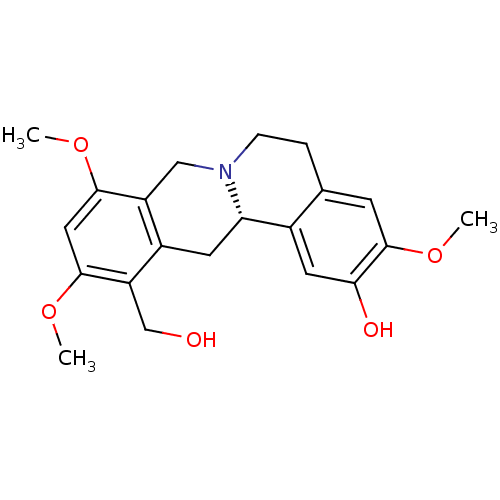 (CHEMBL2057456) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human dopamine D1 receptor expressed in HEK293 cells | Bioorg Med Chem 20: 4862-71 (2012) Article DOI: 10.1016/j.bmc.2012.05.057 BindingDB Entry DOI: 10.7270/Q28P61K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50451431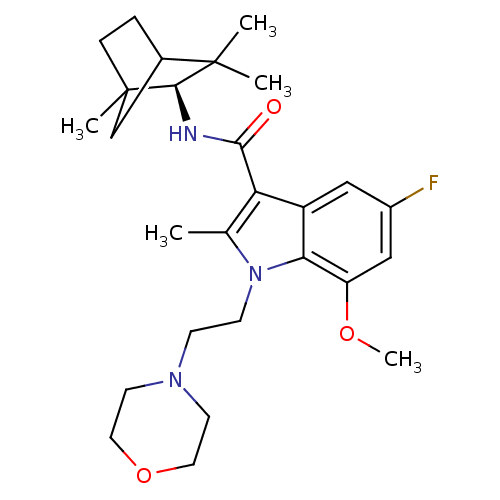 (CHEMBL2112293) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity against human CB2 receptor expressed in CHO cells by using WIN55,2122 Mesylate [5,73H] as Radioactive tracer | Bioorg Med Chem Lett 12: 2399-402 (2002) BindingDB Entry DOI: 10.7270/Q2TM79FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50116837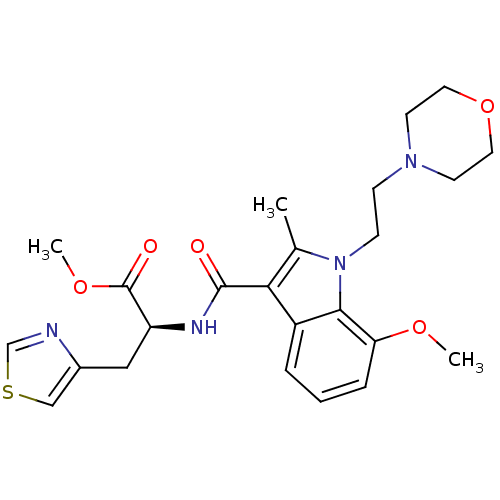 ((S)-2-{[7-Methoxy-2-methyl-1-(2-morpholin-4-yl-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity against human CB2 receptor expressed in CHO cells by using WIN55,2122 Mesylate [5,73H] as Radioactive tracer | Bioorg Med Chem Lett 12: 2399-402 (2002) BindingDB Entry DOI: 10.7270/Q2TM79FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50116859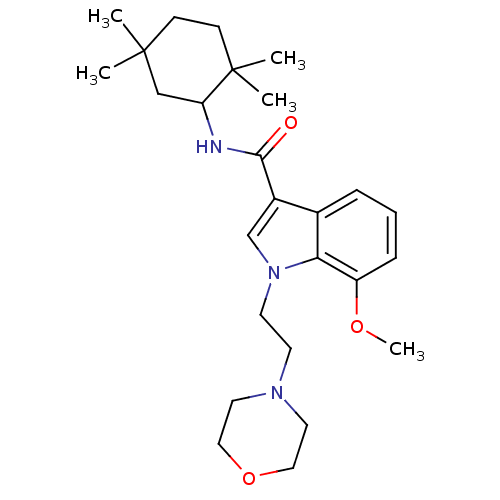 (7-Methoxy-1-(2-morpholin-4-yl-ethyl)-1H-indole-3-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity against human CB2 receptor expressed in CHO cells by using WIN55,2122 Mesylate [5,73H] as Radioactive tracer | Bioorg Med Chem Lett 12: 2399-402 (2002) BindingDB Entry DOI: 10.7270/Q2TM79FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50387563 (CHEMBL2057446) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human dopamine D1 receptor expressed in HEK293 cells | Bioorg Med Chem 20: 4862-71 (2012) Article DOI: 10.1016/j.bmc.2012.05.057 BindingDB Entry DOI: 10.7270/Q28P61K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50116847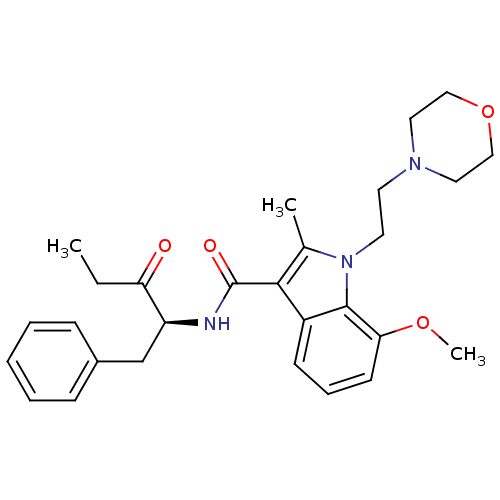 (7-Methoxy-2-methyl-1-(2-morpholin-4-yl-ethyl)-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity against human CB2 receptor expressed in CHO cells by using WIN55,2122 Mesylate [5,73H] as Radioactive tracer | Bioorg Med Chem Lett 12: 2399-402 (2002) BindingDB Entry DOI: 10.7270/Q2TM79FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50451434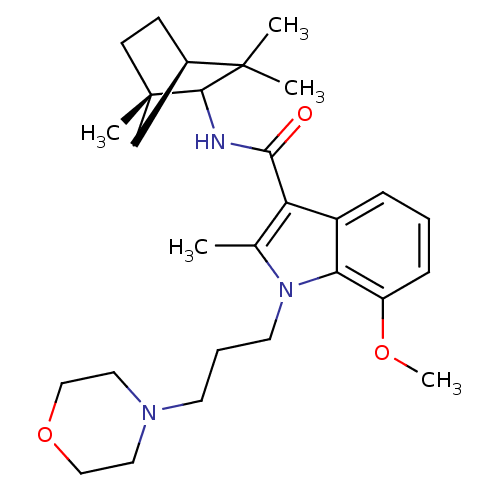 (CHEMBL2112290) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity against human CB2 receptor expressed in CHO cells by using WIN55,2122 Mesylate [5,73H] as Radioactive tracer | Bioorg Med Chem Lett 12: 2399-402 (2002) BindingDB Entry DOI: 10.7270/Q2TM79FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50387557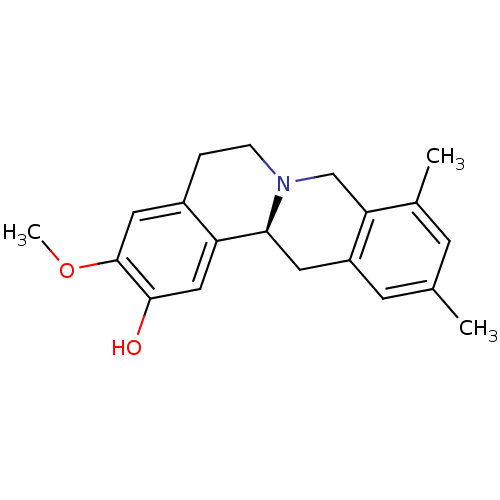 (CHEMBL2057457 | US9359372, DC037031) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human dopamine D1 receptor expressed in HEK293 cells | Bioorg Med Chem 20: 4862-71 (2012) Article DOI: 10.1016/j.bmc.2012.05.057 BindingDB Entry DOI: 10.7270/Q28P61K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50116860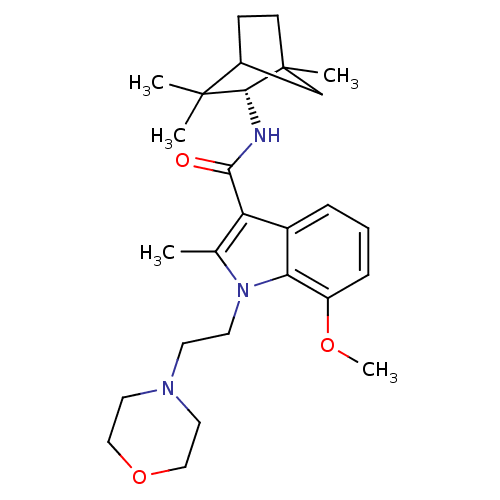 (7-Methoxy-2-methyl-1-(2-morpholin-4-yl-ethyl)-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity against human CB2 receptor expressed in CHO cells by using WIN55,2122 Mesylate [5,73H] as Radioactive tracer | Bioorg Med Chem Lett 12: 2399-402 (2002) BindingDB Entry DOI: 10.7270/Q2TM79FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1038 total ) | Next | Last >> |