 Found 96 hits with Last Name = 'zhou' and Initial = 'bb'
Found 96 hits with Last Name = 'zhou' and Initial = 'bb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cyclin-A2/Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline
| Assay Description
In vitro CDK enzymatic assay using purified CDK mixed with cyclin A, was incubated with substrate, and test compounds in the presence of 1.4 uM ATP/ ... |
J Biol Chem 277: 46609-15 (2002)
Article DOI: 10.1074/jbc.M201233200
BindingDB Entry DOI: 10.7270/Q2D798PF |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline
| Assay Description
In vitro CDK enzymatic assay using purified CDK mixed with cyclin A, was incubated with substrate, and test compounds in the presence of 1.4 uM ATP/ ... |
J Biol Chem 277: 46609-15 (2002)
Article DOI: 10.1074/jbc.M201233200
BindingDB Entry DOI: 10.7270/Q2D798PF |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM17054
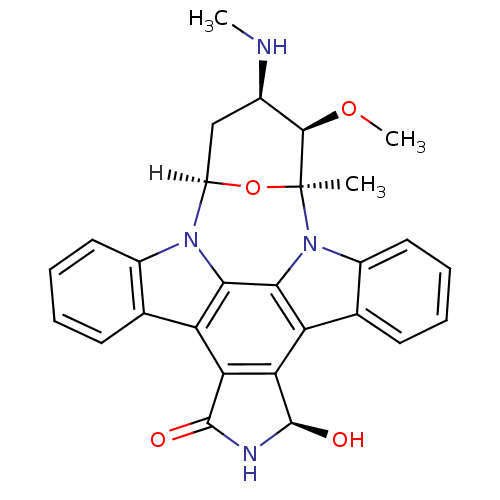
((2S,3R,4R,6R,18R)-18-hydroxy-3-methoxy-2-methyl-4-...)Show SMILES [H][C@]12C[C@@H](NC)[C@@H](OC)[C@](C)(O1)n1c3ccccc3c3c4[C@@H](O)NC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O4/c1-28-25(35-3)15(29-2)12-18(36-28)31-16-10-6-4-8-13(16)19-21-22(27(34)30-26(21)33)20-14-9-5-7-11-17(14)32(28)24(20)23(19)31/h4-11,15,18,25,27,29,34H,12H2,1-3H3,(H,30,33)/t15-,18-,25-,27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.60 | -49.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
GlaxoSmithKline
| Assay Description
In vitro Chk1 enzymatic assay using purified enzyme, was incubated with substrate, and test compounds in the presence of 10 uM ATP/ [gamma-32P] ATP. ... |
J Biol Chem 277: 46609-15 (2002)
Article DOI: 10.1074/jbc.M201233200
BindingDB Entry DOI: 10.7270/Q2D798PF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM17140
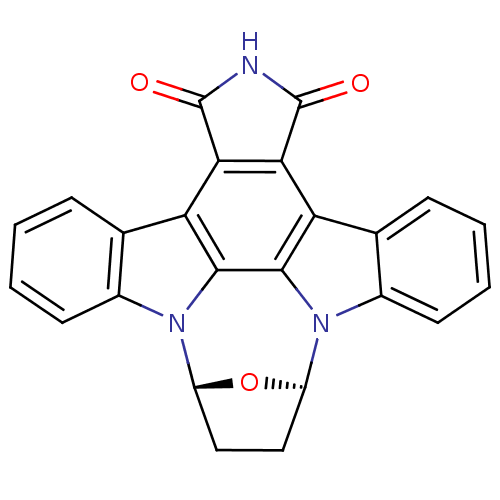
((15R,18R)-28-oxa-4,14,19-triazaoctacyclo[12.11.2.1...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3n3[C@H]4CC[C@@H](O4)n4c5ccccc5c2c4c13 |r| Show InChI InChI=1S/C24H15N3O3/c28-23-19-17-11-5-1-3-7-13(11)26-15-9-10-16(30-15)27-14-8-4-2-6-12(14)18(22(27)21(17)26)20(19)24(29)25-23/h1-8,15-16H,9-10H2,(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 5.60 | -46.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline
| Assay Description
In vitro CDK enzymatic assay using purified CDK mixed with cyclin A, was incubated with substrate, and test compounds in the presence of 1.4 uM ATP/ ... |
J Biol Chem 277: 46609-15 (2002)
Article DOI: 10.1074/jbc.M201233200
BindingDB Entry DOI: 10.7270/Q2D798PF |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 7.80 | -48.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
GlaxoSmithKline
| Assay Description
In vitro Chk1 enzymatic assay using purified enzyme, was incubated with substrate, and test compounds in the presence of 10 uM ATP/ [gamma-32P] ATP. ... |
J Biol Chem 277: 46609-15 (2002)
Article DOI: 10.1074/jbc.M201233200
BindingDB Entry DOI: 10.7270/Q2D798PF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM17140

((15R,18R)-28-oxa-4,14,19-triazaoctacyclo[12.11.2.1...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3n3[C@H]4CC[C@@H](O4)n4c5ccccc5c2c4c13 |r| Show InChI InChI=1S/C24H15N3O3/c28-23-19-17-11-5-1-3-7-13(11)26-15-9-10-16(30-15)27-14-8-4-2-6-12(14)18(22(27)21(17)26)20(19)24(29)25-23/h1-8,15-16H,9-10H2,(H,25,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 15 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
GlaxoSmithKline
| Assay Description
In vitro Chk1 enzymatic assay using purified enzyme, was incubated with substrate, and test compounds in the presence of 10 uM ATP/ [gamma-32P] ATP. ... |
J Biol Chem 277: 46609-15 (2002)
Article DOI: 10.1074/jbc.M201233200
BindingDB Entry DOI: 10.7270/Q2D798PF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM17140

((15R,18R)-28-oxa-4,14,19-triazaoctacyclo[12.11.2.1...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3n3[C@H]4CC[C@@H](O4)n4c5ccccc5c2c4c13 |r| Show InChI InChI=1S/C24H15N3O3/c28-23-19-17-11-5-1-3-7-13(11)26-15-9-10-16(30-15)27-14-8-4-2-6-12(14)18(22(27)21(17)26)20(19)24(29)25-23/h1-8,15-16H,9-10H2,(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 16 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
GlaxoSmithKline
| Assay Description
CDK4 and cyclin D1 were expressed in a baculovirus expression system and subsequently purified. The catalytic activity of the CDK4 protein was assaye... |
J Biol Chem 277: 46609-15 (2002)
Article DOI: 10.1074/jbc.M201233200
BindingDB Entry DOI: 10.7270/Q2D798PF |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM17140

((15R,18R)-28-oxa-4,14,19-triazaoctacyclo[12.11.2.1...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3n3[C@H]4CC[C@@H](O4)n4c5ccccc5c2c4c13 |r| Show InChI InChI=1S/C24H15N3O3/c28-23-19-17-11-5-1-3-7-13(11)26-15-9-10-16(30-15)27-14-8-4-2-6-12(14)18(22(27)21(17)26)20(19)24(29)25-23/h1-8,15-16H,9-10H2,(H,25,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 23 | -43.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline
| Assay Description
In vitro CDK enzymatic assay using purified CDK mixed with cyclin A, was incubated with substrate, and test compounds in the presence of 1.4 uM ATP/ ... |
J Biol Chem 277: 46609-15 (2002)
Article DOI: 10.1074/jbc.M201233200
BindingDB Entry DOI: 10.7270/Q2D798PF |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM17054

((2S,3R,4R,6R,18R)-18-hydroxy-3-methoxy-2-methyl-4-...)Show SMILES [H][C@]12C[C@@H](NC)[C@@H](OC)[C@](C)(O1)n1c3ccccc3c3c4[C@@H](O)NC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O4/c1-28-25(35-3)15(29-2)12-18(36-28)31-16-10-6-4-8-13(16)19-21-22(27(34)30-26(21)33)20-14-9-5-7-11-17(14)32(28)24(20)23(19)31/h4-11,15,18,25,27,29,34H,12H2,1-3H3,(H,30,33)/t15-,18-,25-,27-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 30 | -42.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline
| Assay Description
In vitro CDK enzymatic assay using purified CDK mixed with cyclin A, was incubated with substrate, and test compounds in the presence of 1.4 uM ATP/ ... |
J Biol Chem 277: 46609-15 (2002)
Article DOI: 10.1074/jbc.M201233200
BindingDB Entry DOI: 10.7270/Q2D798PF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 41 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
GlaxoSmithKline
| Assay Description
CDK4 and cyclin D1 were expressed in a baculovirus expression system and subsequently purified. The catalytic activity of the CDK4 protein was assaye... |
J Biol Chem 277: 46609-15 (2002)
Article DOI: 10.1074/jbc.M201233200
BindingDB Entry DOI: 10.7270/Q2D798PF |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM17054

((2S,3R,4R,6R,18R)-18-hydroxy-3-methoxy-2-methyl-4-...)Show SMILES [H][C@]12C[C@@H](NC)[C@@H](OC)[C@](C)(O1)n1c3ccccc3c3c4[C@@H](O)NC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O4/c1-28-25(35-3)15(29-2)12-18(36-28)31-16-10-6-4-8-13(16)19-21-22(27(34)30-26(21)33)20-14-9-5-7-11-17(14)32(28)24(20)23(19)31/h4-11,15,18,25,27,29,34H,12H2,1-3H3,(H,30,33)/t15-,18-,25-,27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 95 | -39.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline
| Assay Description
In vitro CDK enzymatic assay using purified CDK mixed with cyclin A, was incubated with substrate, and test compounds in the presence of 1.4 uM ATP/ ... |
J Biol Chem 277: 46609-15 (2002)
Article DOI: 10.1074/jbc.M201233200
BindingDB Entry DOI: 10.7270/Q2D798PF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM17054

((2S,3R,4R,6R,18R)-18-hydroxy-3-methoxy-2-methyl-4-...)Show SMILES [H][C@]12C[C@@H](NC)[C@@H](OC)[C@](C)(O1)n1c3ccccc3c3c4[C@@H](O)NC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O4/c1-28-25(35-3)15(29-2)12-18(36-28)31-16-10-6-4-8-13(16)19-21-22(27(34)30-26(21)33)20-14-9-5-7-11-17(14)32(28)24(20)23(19)31/h4-11,15,18,25,27,29,34H,12H2,1-3H3,(H,30,33)/t15-,18-,25-,27-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.60E+3 | -31.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
GlaxoSmithKline
| Assay Description
CDK4 and cyclin D1 were expressed in a baculovirus expression system and subsequently purified. The catalytic activity of the CDK4 protein was assaye... |
J Biol Chem 277: 46609-15 (2002)
Article DOI: 10.1074/jbc.M201233200
BindingDB Entry DOI: 10.7270/Q2D798PF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50203526
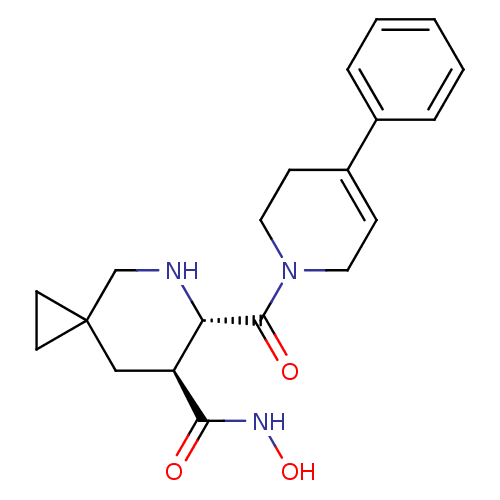
((6S,7S)-6-(4-phenyl-3,6-dihydro-2H-pyridine-1-carb...)Show SMILES ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CCC(=CC1)c1ccccc1 |c:19| Show InChI InChI=1S/C20H25N3O3/c24-18(22-26)16-12-20(8-9-20)13-21-17(16)19(25)23-10-6-15(7-11-23)14-4-2-1-3-5-14/h1-6,16-17,21,26H,7-13H2,(H,22,24)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50203538
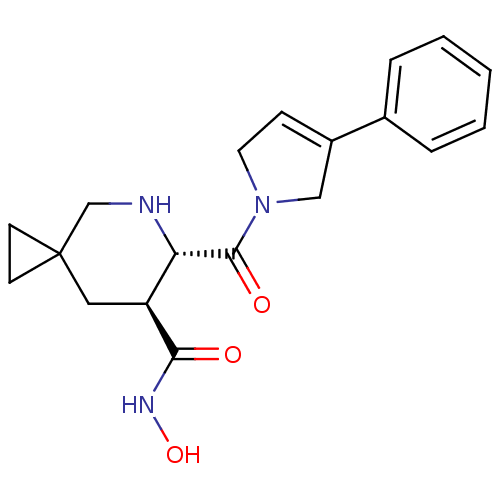
((6S,7S)-6-(3-phenyl-2,5-dihydro-pyrrole-1-carbonyl...)Show SMILES ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CC=C(C1)c1ccccc1 |c:18| Show InChI InChI=1S/C19H23N3O3/c23-17(21-25)15-10-19(7-8-19)12-20-16(15)18(24)22-9-6-14(11-22)13-4-2-1-3-5-13/h1-6,15-16,20,25H,7-12H2,(H,21,23)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50203538

((6S,7S)-6-(3-phenyl-2,5-dihydro-pyrrole-1-carbonyl...)Show SMILES ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CC=C(C1)c1ccccc1 |c:18| Show InChI InChI=1S/C19H23N3O3/c23-17(21-25)15-10-19(7-8-19)12-20-16(15)18(24)22-9-6-14(11-22)13-4-2-1-3-5-13/h1-6,15-16,20,25H,7-12H2,(H,21,23)/t15-,16-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Her2 sheddase activity in BT474 cell line |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50203526

((6S,7S)-6-(4-phenyl-3,6-dihydro-2H-pyridine-1-carb...)Show SMILES ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CCC(=CC1)c1ccccc1 |c:19| Show InChI InChI=1S/C20H25N3O3/c24-18(22-26)16-12-20(8-9-20)13-21-17(16)19(25)23-10-6-15(7-11-23)14-4-2-1-3-5-14/h1-6,16-17,21,26H,7-13H2,(H,22,24)/t16-,17-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Her2 sheddase activity in BT474 cell line |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 10
(Homo sapiens (Human)) | BDBM50203538

((6S,7S)-6-(3-phenyl-2,5-dihydro-pyrrole-1-carbonyl...)Show SMILES ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CC=C(C1)c1ccccc1 |c:18| Show InChI InChI=1S/C19H23N3O3/c23-17(21-25)15-10-19(7-8-19)12-20-16(15)18(24)22-9-6-14(11-22)13-4-2-1-3-5-13/h1-6,15-16,20,25H,7-12H2,(H,21,23)/t15-,16-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of ADAM10 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 10
(Homo sapiens (Human)) | BDBM50203526

((6S,7S)-6-(4-phenyl-3,6-dihydro-2H-pyridine-1-carb...)Show SMILES ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CCC(=CC1)c1ccccc1 |c:19| Show InChI InChI=1S/C20H25N3O3/c24-18(22-26)16-12-20(8-9-20)13-21-17(16)19(25)23-10-6-15(7-11-23)14-4-2-1-3-5-14/h1-6,16-17,21,26H,7-13H2,(H,22,24)/t16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of ADAM10 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50203527
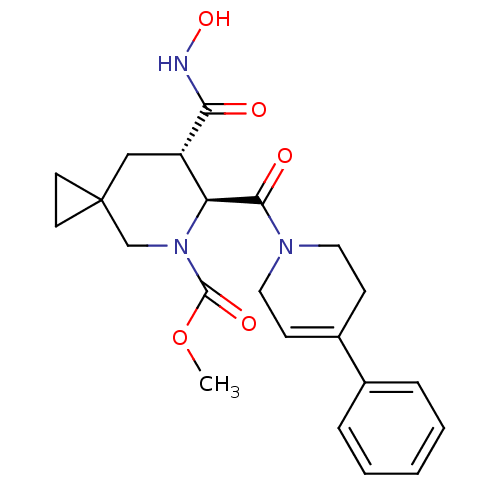
((6S,7S)-methyl 7-(hydroxycarbamoyl)-6-(4-phenyl-1,...)Show SMILES COC(=O)N1CC2(CC2)C[C@@H]([C@H]1C(=O)N1CCC(=CC1)c1ccccc1)C(=O)NO |r,c:19| Show InChI InChI=1S/C22H27N3O5/c1-30-21(28)25-14-22(9-10-22)13-17(19(26)23-29)18(25)20(27)24-11-7-16(8-12-24)15-5-3-2-4-6-15/h2-7,17-18,29H,8-14H2,1H3,(H,23,26)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50203529
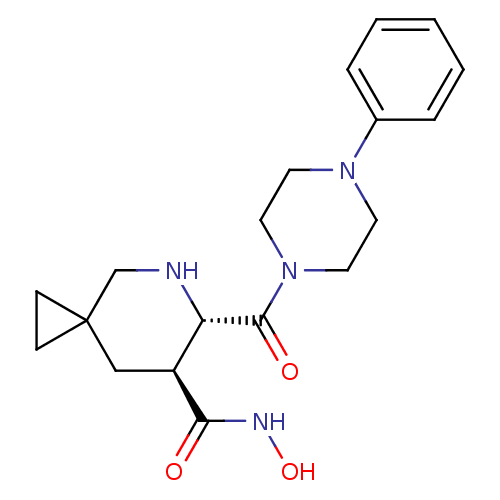
((6S,7S)-6-(4-phenyl-piperazine-1-carbonyl)-5-aza-s...)Show SMILES ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CCN(CC1)c1ccccc1 |r| Show InChI InChI=1S/C19H26N4O3/c24-17(21-26)15-12-19(6-7-19)13-20-16(15)18(25)23-10-8-22(9-11-23)14-4-2-1-3-5-14/h1-5,15-16,20,26H,6-13H2,(H,21,24)/t15-,16-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Her2 sheddase activity in BT474 cell line |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 10
(Homo sapiens (Human)) | BDBM50203527

((6S,7S)-methyl 7-(hydroxycarbamoyl)-6-(4-phenyl-1,...)Show SMILES COC(=O)N1CC2(CC2)C[C@@H]([C@H]1C(=O)N1CCC(=CC1)c1ccccc1)C(=O)NO |r,c:19| Show InChI InChI=1S/C22H27N3O5/c1-30-21(28)25-14-22(9-10-22)13-17(19(26)23-29)18(25)20(27)24-11-7-16(8-12-24)15-5-3-2-4-6-15/h2-7,17-18,29H,8-14H2,1H3,(H,23,26)/t17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of ADAM10 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50203528
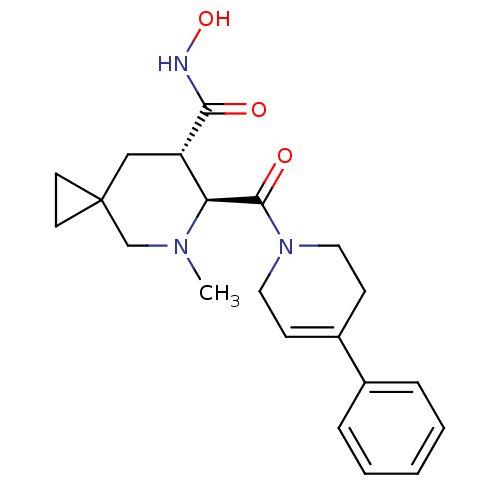
((6S,7S)-N-hydroxy-5-methyl-6-[(4-phenyl-3,6-dihydr...)Show SMILES CN1CC2(CC2)C[C@@H]([C@H]1C(=O)N1CCC(=CC1)c1ccccc1)C(=O)NO |c:16| Show InChI InChI=1S/C21H27N3O3/c1-23-14-21(9-10-21)13-17(19(25)22-27)18(23)20(26)24-11-7-16(8-12-24)15-5-3-2-4-6-15/h2-7,17-18,27H,8-14H2,1H3,(H,22,25)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50203527

((6S,7S)-methyl 7-(hydroxycarbamoyl)-6-(4-phenyl-1,...)Show SMILES COC(=O)N1CC2(CC2)C[C@@H]([C@H]1C(=O)N1CCC(=CC1)c1ccccc1)C(=O)NO |r,c:19| Show InChI InChI=1S/C22H27N3O5/c1-30-21(28)25-14-22(9-10-22)13-17(19(26)23-29)18(25)20(27)24-11-7-16(8-12-24)15-5-3-2-4-6-15/h2-7,17-18,29H,8-14H2,1H3,(H,23,26)/t17-,18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Her2 sheddase activity in BT474 cell line |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50203538

((6S,7S)-6-(3-phenyl-2,5-dihydro-pyrrole-1-carbonyl...)Show SMILES ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CC=C(C1)c1ccccc1 |c:18| Show InChI InChI=1S/C19H23N3O3/c23-17(21-25)15-10-19(7-8-19)12-20-16(15)18(24)22-9-6-14(11-22)13-4-2-1-3-5-13/h1-6,15-16,20,25H,7-12H2,(H,21,23)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 10
(Homo sapiens (Human)) | BDBM50203528

((6S,7S)-N-hydroxy-5-methyl-6-[(4-phenyl-3,6-dihydr...)Show SMILES CN1CC2(CC2)C[C@@H]([C@H]1C(=O)N1CCC(=CC1)c1ccccc1)C(=O)NO |c:16| Show InChI InChI=1S/C21H27N3O3/c1-23-14-21(9-10-21)13-17(19(25)22-27)18(23)20(26)24-11-7-16(8-12-24)15-5-3-2-4-6-15/h2-7,17-18,27H,8-14H2,1H3,(H,22,25)/t17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of ADAM10 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50203533
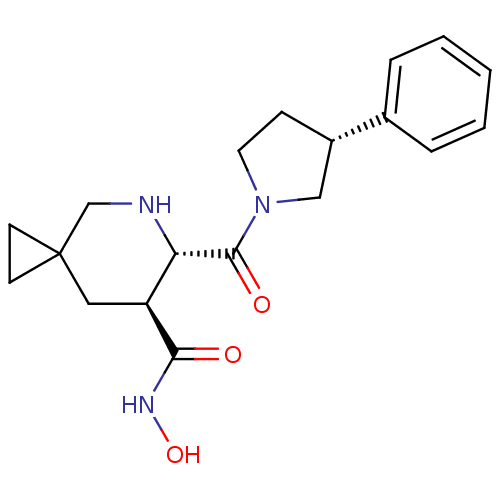
((6S,7S)-N-hydroxy-6-((R)-3-phenylpyrrolidine-1-car...)Show SMILES ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CC[C@@H](C1)c1ccccc1 Show InChI InChI=1S/C19H25N3O3/c23-17(21-25)15-10-19(7-8-19)12-20-16(15)18(24)22-9-6-14(11-22)13-4-2-1-3-5-13/h1-5,14-16,20,25H,6-12H2,(H,21,23)/t14-,15-,16-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Her2 sheddase activity in BT474 cell line |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 10
(Homo sapiens (Human)) | BDBM50203533

((6S,7S)-N-hydroxy-6-((R)-3-phenylpyrrolidine-1-car...)Show SMILES ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CC[C@@H](C1)c1ccccc1 Show InChI InChI=1S/C19H25N3O3/c23-17(21-25)15-10-19(7-8-19)12-20-16(15)18(24)22-9-6-14(11-22)13-4-2-1-3-5-13/h1-5,14-16,20,25H,6-12H2,(H,21,23)/t14-,15-,16-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of ADAM10 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 10
(Homo sapiens (Human)) | BDBM50203530
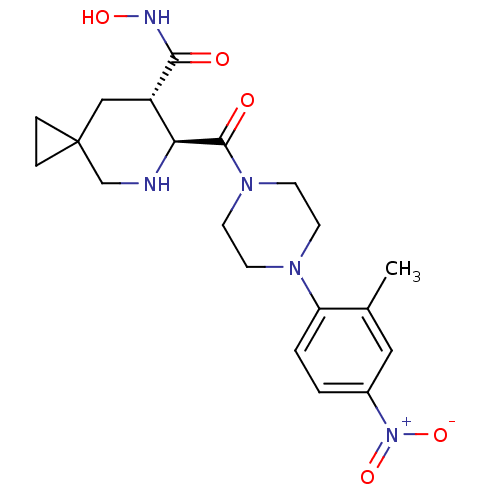
((6S,7S)-N-hydroxy-6-{[4-(2-methyl-4-nitrophenyl)pi...)Show SMILES Cc1cc(ccc1N1CCN(CC1)C(=O)[C@H]1NCC2(CC2)C[C@@H]1C(=O)NO)[N+]([O-])=O Show InChI InChI=1S/C20H27N5O5/c1-13-10-14(25(29)30)2-3-16(13)23-6-8-24(9-7-23)19(27)17-15(18(26)22-28)11-20(4-5-20)12-21-17/h2-3,10,15,17,21,28H,4-9,11-12H2,1H3,(H,22,26)/t15-,17-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of ADAM10 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50203526

((6S,7S)-6-(4-phenyl-3,6-dihydro-2H-pyridine-1-carb...)Show SMILES ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CCC(=CC1)c1ccccc1 |c:19| Show InChI InChI=1S/C20H25N3O3/c24-18(22-26)16-12-20(8-9-20)13-21-17(16)19(25)23-10-6-15(7-11-23)14-4-2-1-3-5-14/h1-6,16-17,21,26H,7-13H2,(H,22,24)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 10
(Homo sapiens (Human)) | BDBM50203529

((6S,7S)-6-(4-phenyl-piperazine-1-carbonyl)-5-aza-s...)Show SMILES ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CCN(CC1)c1ccccc1 |r| Show InChI InChI=1S/C19H26N4O3/c24-17(21-26)15-12-19(6-7-19)13-20-16(15)18(25)23-10-8-22(9-11-23)14-4-2-1-3-5-14/h1-5,15-16,20,26H,6-13H2,(H,21,24)/t15-,16-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of ADAM10 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 10
(Homo sapiens (Human)) | BDBM50203531

((6S,7S)-N-hydroxy-6-{[4-(2-methyl-4-nitrophenyl)-3...)Show SMILES Cc1cc(ccc1C1=CCN(CC1)C(=O)[C@H]1NCC2(CC2)C[C@@H]1C(=O)NO)[N+]([O-])=O |t:8| Show InChI InChI=1S/C21H26N4O5/c1-13-10-15(25(29)30)2-3-16(13)14-4-8-24(9-5-14)20(27)18-17(19(26)23-28)11-21(6-7-21)12-22-18/h2-4,10,17-18,22,28H,5-9,11-12H2,1H3,(H,23,26)/t17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of ADAM10 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50203537

((6S,7S)-6-(4-phenyl-piperidine-1-carbonyl)-5-aza-s...)Show SMILES ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CCC(CC1)c1ccccc1 Show InChI InChI=1S/C20H27N3O3/c24-18(22-26)16-12-20(8-9-20)13-21-17(16)19(25)23-10-6-15(7-11-23)14-4-2-1-3-5-14/h1-5,15-17,21,26H,6-13H2,(H,22,24)/t16-,17-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Her2 sheddase activity in BT474 cell line |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50203534
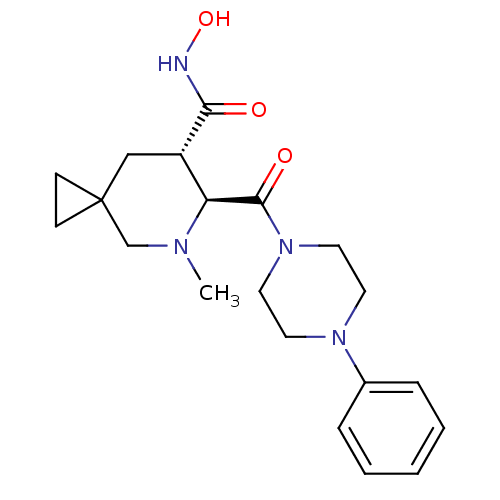
((6S,7S)-N-hydroxy-5-methyl-6-(4-phenylpiperazine-1...)Show SMILES CN1CC2(CC2)C[C@@H]([C@H]1C(=O)N1CCN(CC1)c1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C20H28N4O3/c1-22-14-20(7-8-20)13-16(18(25)21-27)17(22)19(26)24-11-9-23(10-12-24)15-5-3-2-4-6-15/h2-6,16-17,27H,7-14H2,1H3,(H,21,25)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50203530

((6S,7S)-N-hydroxy-6-{[4-(2-methyl-4-nitrophenyl)pi...)Show SMILES Cc1cc(ccc1N1CCN(CC1)C(=O)[C@H]1NCC2(CC2)C[C@@H]1C(=O)NO)[N+]([O-])=O Show InChI InChI=1S/C20H27N5O5/c1-13-10-14(25(29)30)2-3-16(13)23-6-8-24(9-7-23)19(27)17-15(18(26)22-28)11-20(4-5-20)12-21-17/h2-3,10,15,17,21,28H,4-9,11-12H2,1H3,(H,22,26)/t15-,17-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Her2 sheddase activity in BT474 cell line |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50203528

((6S,7S)-N-hydroxy-5-methyl-6-[(4-phenyl-3,6-dihydr...)Show SMILES CN1CC2(CC2)C[C@@H]([C@H]1C(=O)N1CCC(=CC1)c1ccccc1)C(=O)NO |c:16| Show InChI InChI=1S/C21H27N3O3/c1-23-14-21(9-10-21)13-17(19(25)22-27)18(23)20(26)24-11-7-16(8-12-24)15-5-3-2-4-6-15/h2-7,17-18,27H,8-14H2,1H3,(H,22,25)/t17-,18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Her2 sheddase activity in BT474 cell line |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50203529

((6S,7S)-6-(4-phenyl-piperazine-1-carbonyl)-5-aza-s...)Show SMILES ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CCN(CC1)c1ccccc1 |r| Show InChI InChI=1S/C19H26N4O3/c24-17(21-26)15-12-19(6-7-19)13-20-16(15)18(25)23-10-8-22(9-11-23)14-4-2-1-3-5-14/h1-5,15-16,20,26H,6-13H2,(H,21,24)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50203534

((6S,7S)-N-hydroxy-5-methyl-6-(4-phenylpiperazine-1...)Show SMILES CN1CC2(CC2)C[C@@H]([C@H]1C(=O)N1CCN(CC1)c1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C20H28N4O3/c1-22-14-20(7-8-20)13-16(18(25)21-27)17(22)19(26)24-11-9-23(10-12-24)15-5-3-2-4-6-15/h2-6,16-17,27H,7-14H2,1H3,(H,21,25)/t16-,17-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Her2 sheddase activity in BT474 cell line |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50203535

((6S,7S)-N-hydroxy-6-(3,4,10,10a-tetrahydropyrazino...)Show SMILES ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CCN2C(Cc3ccccc23)C1 Show InChI InChI=1S/C20H26N4O3/c25-18(22-27)15-10-20(5-6-20)12-21-17(15)19(26)23-7-8-24-14(11-23)9-13-3-1-2-4-16(13)24/h1-4,14-15,17,21,27H,5-12H2,(H,22,25)/t14?,15-,17-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Her2 sheddase activity in BT474 cell line |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50203531

((6S,7S)-N-hydroxy-6-{[4-(2-methyl-4-nitrophenyl)-3...)Show SMILES Cc1cc(ccc1C1=CCN(CC1)C(=O)[C@H]1NCC2(CC2)C[C@@H]1C(=O)NO)[N+]([O-])=O |t:8| Show InChI InChI=1S/C21H26N4O5/c1-13-10-15(25(29)30)2-3-16(13)14-4-8-24(9-5-14)20(27)18-17(19(26)23-28)11-21(6-7-21)12-22-18/h2-4,10,17-18,22,28H,5-9,11-12H2,1H3,(H,23,26)/t17-,18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Her2 sheddase activity in BT474 cell line |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 10
(Homo sapiens (Human)) | BDBM50203534

((6S,7S)-N-hydroxy-5-methyl-6-(4-phenylpiperazine-1...)Show SMILES CN1CC2(CC2)C[C@@H]([C@H]1C(=O)N1CCN(CC1)c1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C20H28N4O3/c1-22-14-20(7-8-20)13-16(18(25)21-27)17(22)19(26)24-11-9-23(10-12-24)15-5-3-2-4-6-15/h2-6,16-17,27H,7-14H2,1H3,(H,21,25)/t16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of ADAM10 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 10
(Homo sapiens (Human)) | BDBM50203537

((6S,7S)-6-(4-phenyl-piperidine-1-carbonyl)-5-aza-s...)Show SMILES ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CCC(CC1)c1ccccc1 Show InChI InChI=1S/C20H27N3O3/c24-18(22-26)16-12-20(8-9-20)13-21-17(16)19(25)23-10-6-15(7-11-23)14-4-2-1-3-5-14/h1-5,15-17,21,26H,6-13H2,(H,22,24)/t16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of ADAM10 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 10
(Homo sapiens (Human)) | BDBM50203535

((6S,7S)-N-hydroxy-6-(3,4,10,10a-tetrahydropyrazino...)Show SMILES ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CCN2C(Cc3ccccc23)C1 Show InChI InChI=1S/C20H26N4O3/c25-18(22-27)15-10-20(5-6-20)12-21-17(15)19(26)23-7-8-24-14(11-23)9-13-3-1-2-4-16(13)24/h1-4,14-15,17,21,27H,5-12H2,(H,22,25)/t14?,15-,17-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of ADAM10 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50203535

((6S,7S)-N-hydroxy-6-(3,4,10,10a-tetrahydropyrazino...)Show SMILES ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CCN2C(Cc3ccccc23)C1 Show InChI InChI=1S/C20H26N4O3/c25-18(22-27)15-10-20(5-6-20)12-21-17(15)19(26)23-7-8-24-14(11-23)9-13-3-1-2-4-16(13)24/h1-4,14-15,17,21,27H,5-12H2,(H,22,25)/t14?,15-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50203527

((6S,7S)-methyl 7-(hydroxycarbamoyl)-6-(4-phenyl-1,...)Show SMILES COC(=O)N1CC2(CC2)C[C@@H]([C@H]1C(=O)N1CCC(=CC1)c1ccccc1)C(=O)NO |r,c:19| Show InChI InChI=1S/C22H27N3O5/c1-30-21(28)25-14-22(9-10-22)13-17(19(26)23-29)18(25)20(27)24-11-7-16(8-12-24)15-5-3-2-4-6-15/h2-7,17-18,29H,8-14H2,1H3,(H,23,26)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 428 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50203537

((6S,7S)-6-(4-phenyl-piperidine-1-carbonyl)-5-aza-s...)Show SMILES ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CCC(CC1)c1ccccc1 Show InChI InChI=1S/C20H27N3O3/c24-18(22-26)16-12-20(8-9-20)13-21-17(16)19(25)23-10-6-15(7-11-23)14-4-2-1-3-5-14/h1-5,15-17,21,26H,6-13H2,(H,22,24)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50203538

((6S,7S)-6-(3-phenyl-2,5-dihydro-pyrrole-1-carbonyl...)Show SMILES ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CC=C(C1)c1ccccc1 |c:18| Show InChI InChI=1S/C19H23N3O3/c23-17(21-25)15-10-19(7-8-19)12-20-16(15)18(24)22-9-6-14(11-22)13-4-2-1-3-5-13/h1-6,15-16,20,25H,7-12H2,(H,21,23)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 10
(Homo sapiens (Human)) | BDBM50203536
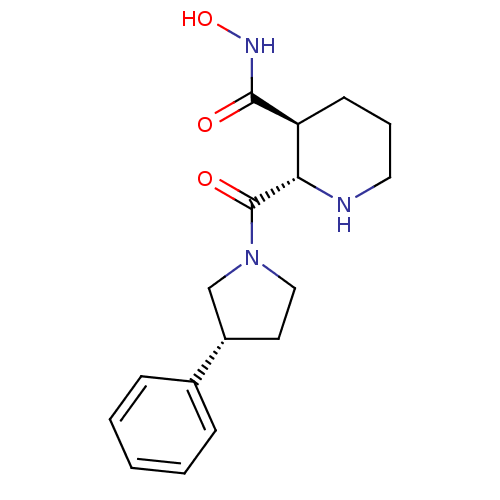
((2S,3S)-N-hydroxy-2-((R)-3-phenylpyrrolidine-1-car...)Show SMILES ONC(=O)[C@H]1CCCN[C@@H]1C(=O)N1CC[C@@H](C1)c1ccccc1 Show InChI InChI=1S/C17H23N3O3/c21-16(19-23)14-7-4-9-18-15(14)17(22)20-10-8-13(11-20)12-5-2-1-3-6-12/h1-3,5-6,13-15,18,23H,4,7-11H2,(H,19,21)/t13-,14-,15-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of ADAM10 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50203536

((2S,3S)-N-hydroxy-2-((R)-3-phenylpyrrolidine-1-car...)Show SMILES ONC(=O)[C@H]1CCCN[C@@H]1C(=O)N1CC[C@@H](C1)c1ccccc1 Show InChI InChI=1S/C17H23N3O3/c21-16(19-23)14-7-4-9-18-15(14)17(22)20-10-8-13(11-20)12-5-2-1-3-6-12/h1-3,5-6,13-15,18,23H,4,7-11H2,(H,19,21)/t13-,14-,15-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Her2 sheddase activity in BT474 cell line |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50203529

((6S,7S)-6-(4-phenyl-piperazine-1-carbonyl)-5-aza-s...)Show SMILES ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CCN(CC1)c1ccccc1 |r| Show InChI InChI=1S/C19H26N4O3/c24-17(21-26)15-12-19(6-7-19)13-20-16(15)18(25)23-10-8-22(9-11-23)14-4-2-1-3-5-14/h1-5,15-16,20,26H,6-13H2,(H,21,24)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50082539

(1-(4-Methoxy-benzenesulfonyl)-piperidine-2-carboxy...)Show InChI InChI=1S/C13H18N2O5S/c1-20-10-5-7-11(8-6-10)21(18,19)15-9-3-2-4-12(15)13(16)14-17/h5-8,12,17H,2-4,9H2,1H3,(H,14,16) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Her2 sheddase activity in BT474 cell line |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data