

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50184825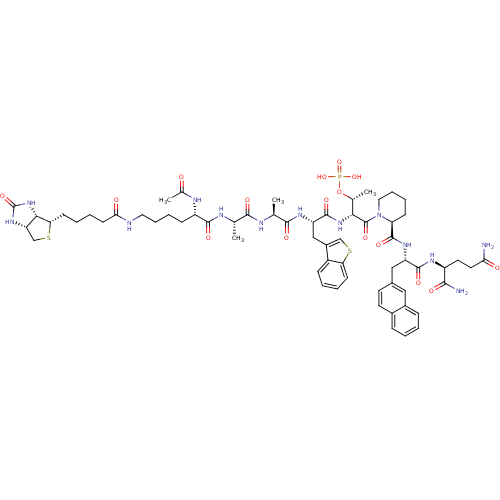 (Ac-Lys(N-epsilon-biotinoyl)-Ala-Ala-Bth-D-Thr(PO3H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of human Pin1 PPIase Activity by protease free PPIase assay | J Med Chem 49: 2147-50 (2006) Article DOI: 10.1021/jm060036n BindingDB Entry DOI: 10.7270/Q2S46RJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50184823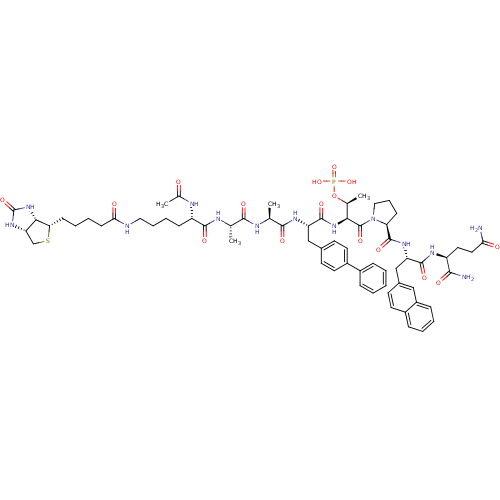 (Ac-Lys(N-epsilon-biotinoyl)-Ala-Ala-Bip-Thr(PO3H2)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of human Pin1 PPIase Activity by protease free PPIase assay | J Med Chem 49: 2147-50 (2006) Article DOI: 10.1021/jm060036n BindingDB Entry DOI: 10.7270/Q2S46RJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343354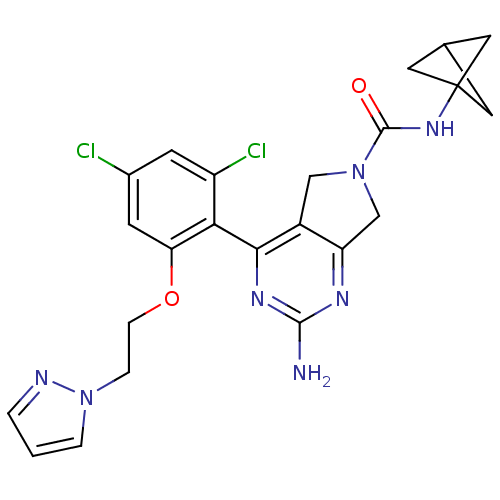 (2-Amino-4-[2,4-dichloro-6-(2-pyrazol-1-ylethoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343380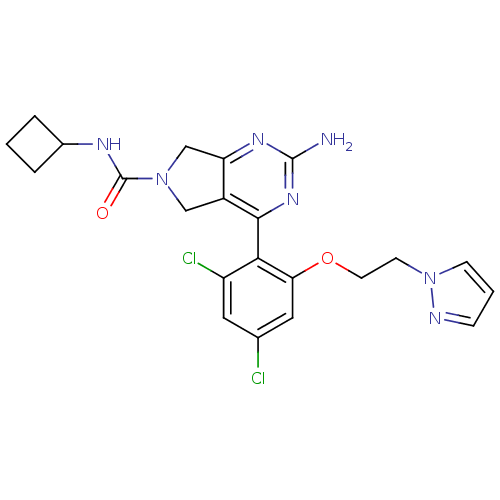 (2-Amino-4-[2,4-dichloro-6-(2-pyrazol-1-ylethoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343355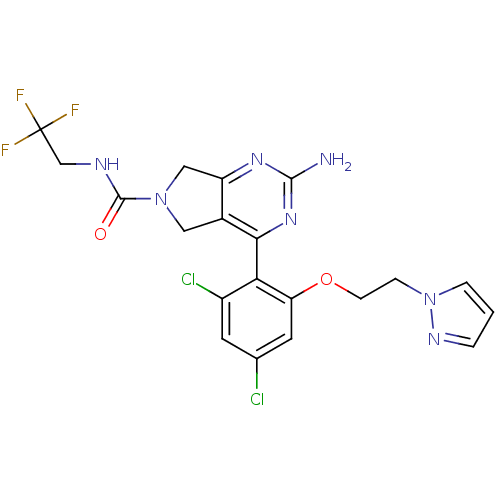 (2-Amino-4-[2,4-dichloro-6-(2-pyrazol-1-ylethoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343372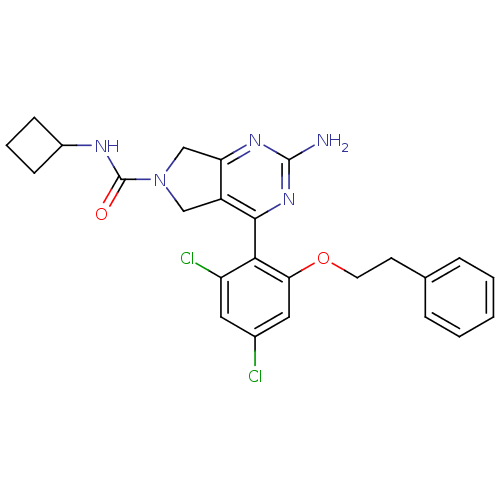 (Amino-4-(2,4-dichloro-6-phenethyloxyphenyl)-5,7-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343370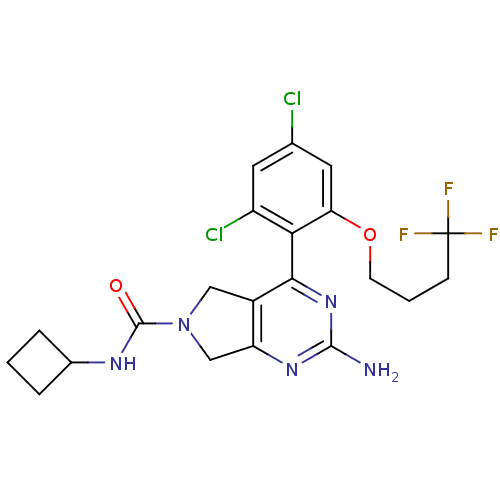 (2-Amino-4-[2,4-dichloro-6-(4,4,4-trifluorobutoxy)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50206172 (1-(benzo[d][1,3]dioxol-5-ylmethyl)-2,3,4,9-tetrahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Displacement of [3H]5CT from 5HT7 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2649-55 (2007) Article DOI: 10.1016/j.bmcl.2007.01.093 BindingDB Entry DOI: 10.7270/Q2WQ03F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343385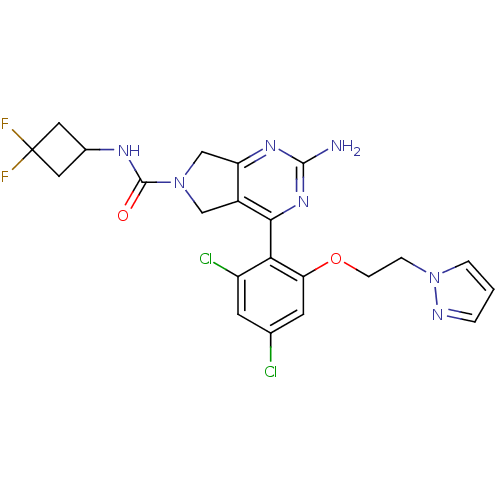 (2-Amino-4-[2,4-dichloro-6-(2-pyrazol-1-ylethoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343386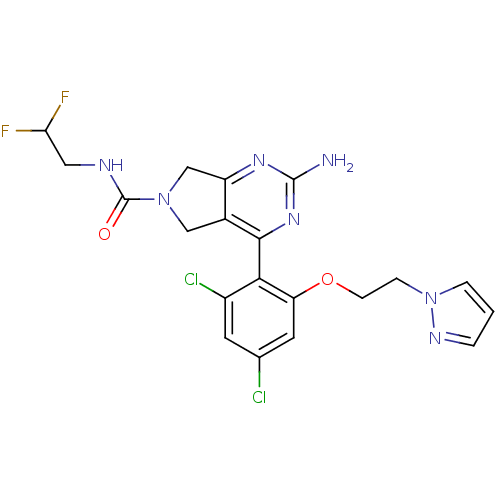 (2-Amino-4-[2,4-dichloro-6-(2-pyrazol-1-ylethoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343356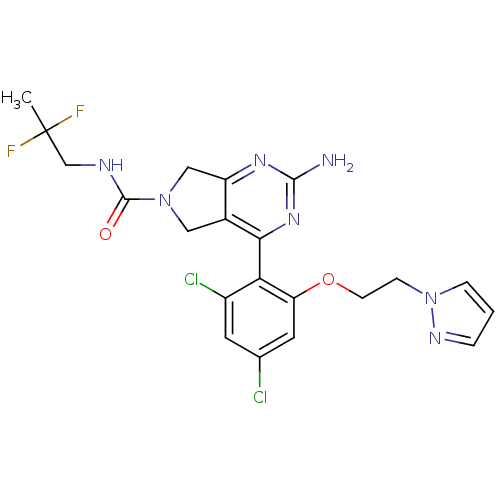 (2-Amino-4-[2,4-dichloro-6-(2-pyrazol-1-ylethoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343384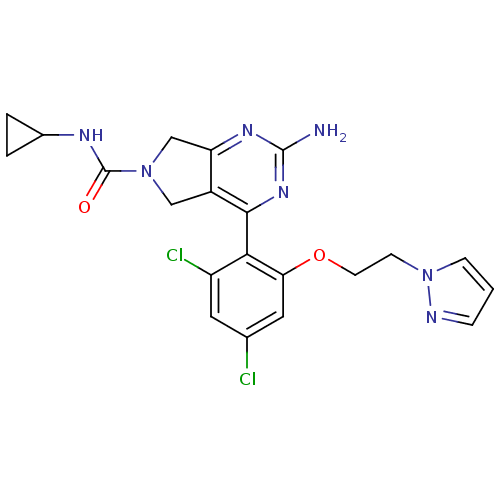 (4-(2-(2-(1H-pyrazol-1-yl)ethoxy)-4,6-dichloropheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343383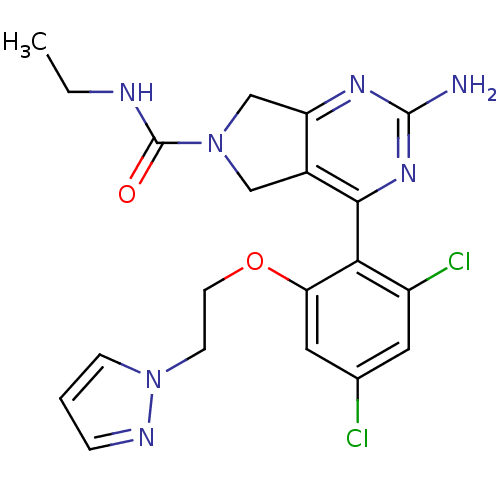 (2-Amino-4-[2,4-dichloro-6-(2-pyrazol-1-ylethoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343378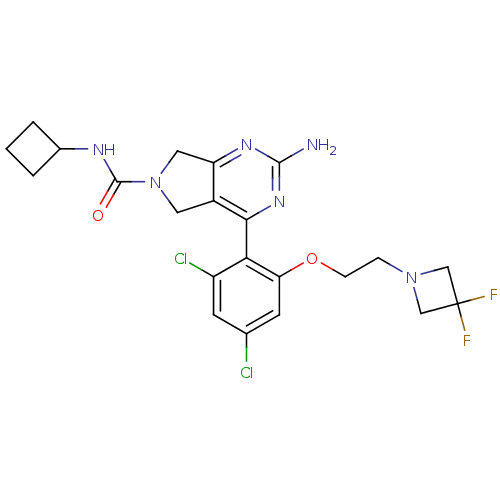 (2-Amino-4-{2,4-dichloro-6-[2-(3,3-difluoro-azetidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343369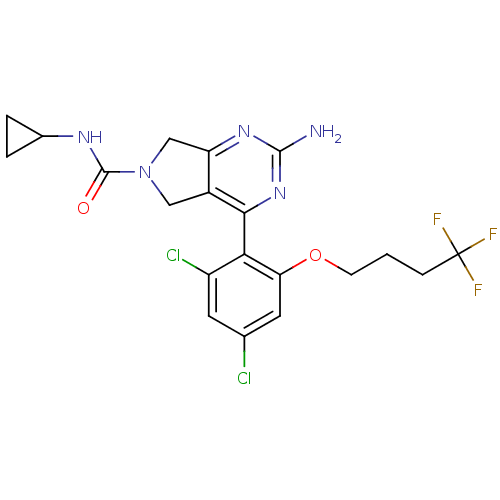 (2-Amino-4-[2,4-dichloro-6-(4,4,4-trifluorobutoxy)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50184841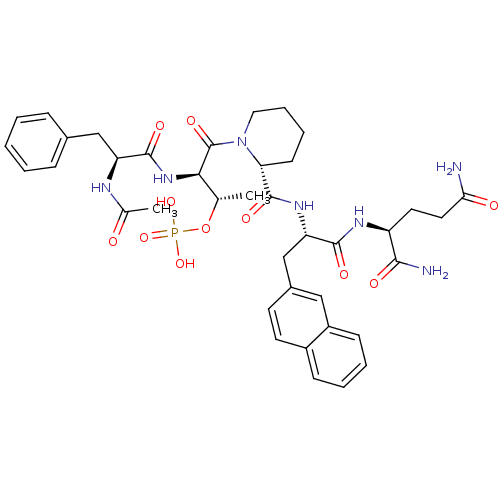 (Ac-Phe-D-Thr(PO3H2)-Pip-Nal-Gln-NH2 | CHEMBL436759) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of human Pin1 PPIase Activity by protease free PPIase assay | J Med Chem 49: 2147-50 (2006) Article DOI: 10.1021/jm060036n BindingDB Entry DOI: 10.7270/Q2S46RJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343382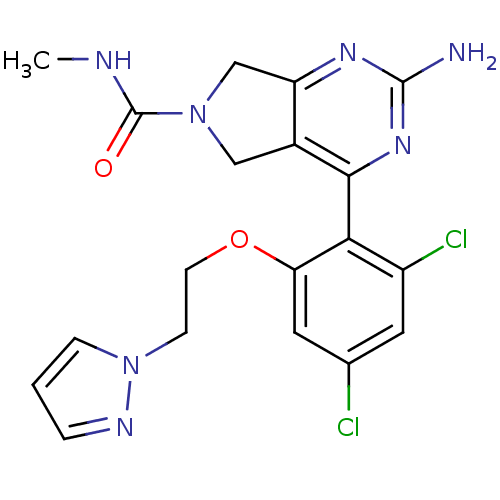 (2-Amino-4-[2,4-dichloro-6-(2-pyrazol-1-ylethoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50206189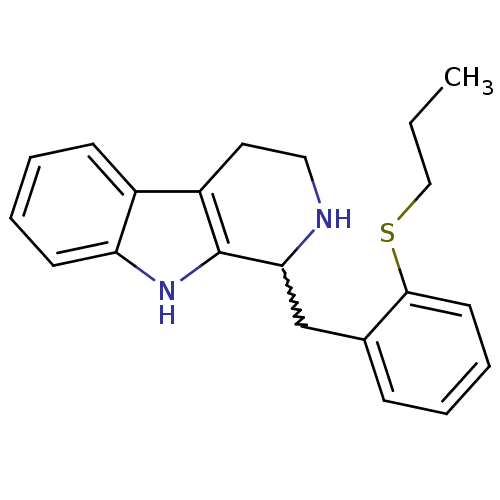 (1-(2-(propylthio)benzyl)-2,3,4,9-tetrahydro-1H-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Displacement of [3H]5CT from 5HT7 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2649-55 (2007) Article DOI: 10.1016/j.bmcl.2007.01.093 BindingDB Entry DOI: 10.7270/Q2WQ03F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343368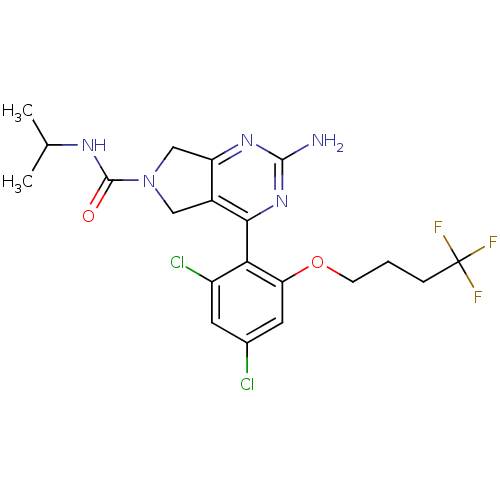 (2-Amino-4-[2,4-dichloro-6-(4,4,4-trifluorobutoxy)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50206163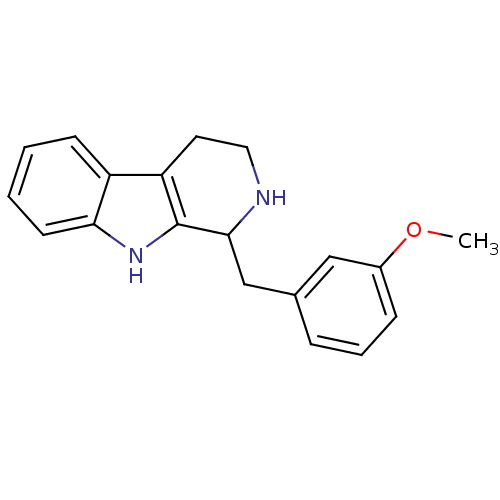 (1-(3-Methoxy-benzyl)-2,3,4,9-tetrahydro-1H-beta-ca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Displacement of [3H]5CT from 5HT7 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2649-55 (2007) Article DOI: 10.1016/j.bmcl.2007.01.093 BindingDB Entry DOI: 10.7270/Q2WQ03F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343366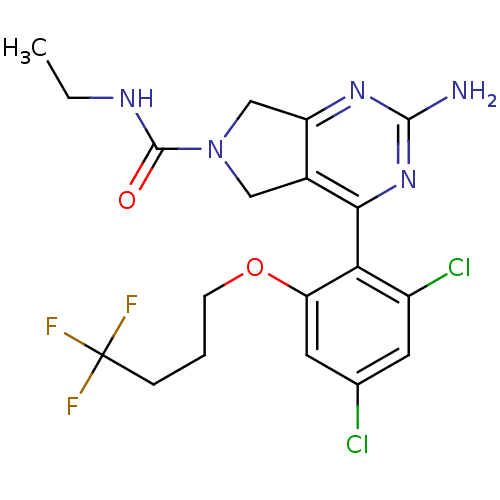 (2-Amino-4-[2,4-dichloro-6-(4,4,4-trifluorobutoxy)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343373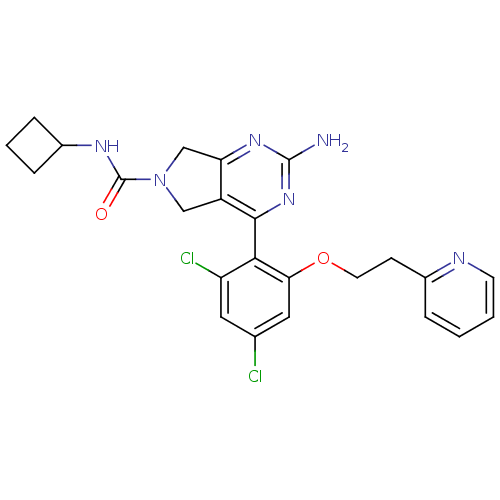 (2-Amino-4-[2,4-dichloro-6-(2-pyridin-2-ylethoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343381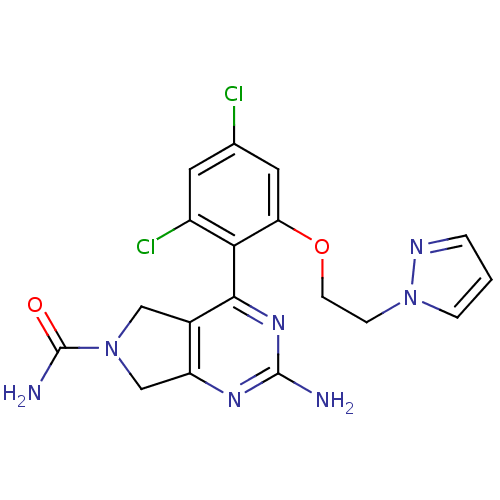 (2-Amino-4-[2,4-dichloro-6-(2-pyrazol-1-ylethoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50206164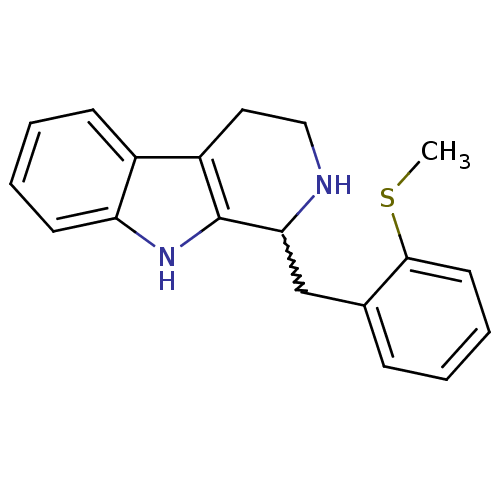 (1-(2-(methylthio)benzyl)-2,3,4,9-tetrahydro-1H-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Displacement of [3H]5CT from 5HT7 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2649-55 (2007) Article DOI: 10.1016/j.bmcl.2007.01.093 BindingDB Entry DOI: 10.7270/Q2WQ03F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50206169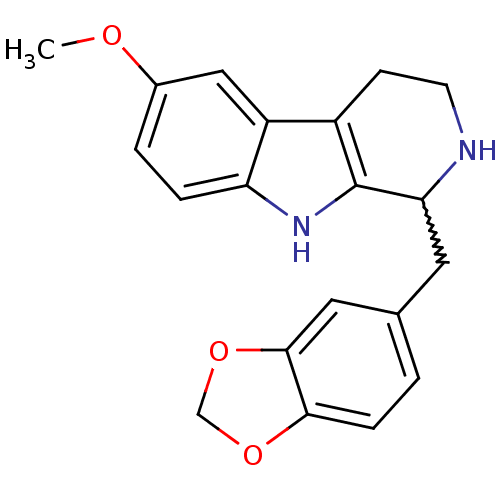 (1-(benzo[d][1,3]dioxol-5-ylmethyl)-6-methoxy-2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Displacement of [3H]5CT from 5HT7 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2649-55 (2007) Article DOI: 10.1016/j.bmcl.2007.01.093 BindingDB Entry DOI: 10.7270/Q2WQ03F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343367 (2-Amino-4-[2,4-dichloro-6-(4,4,4-trifluorobutoxy)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343374 (2-amino-N-cyclobutyl-4-(2,4-dichloro-6-(3-cyanopro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50099934 (4-[4-(4-Hydroxy-3-methyl-phenyl)-thiazol-2-yl]-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 1379-82 (2001) BindingDB Entry DOI: 10.7270/Q24M93S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50099928 (CHEMBL28952 | {3-[2-(5-Carbamimidoyl-2-methylsulfa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 1379-82 (2001) BindingDB Entry DOI: 10.7270/Q24M93S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50206175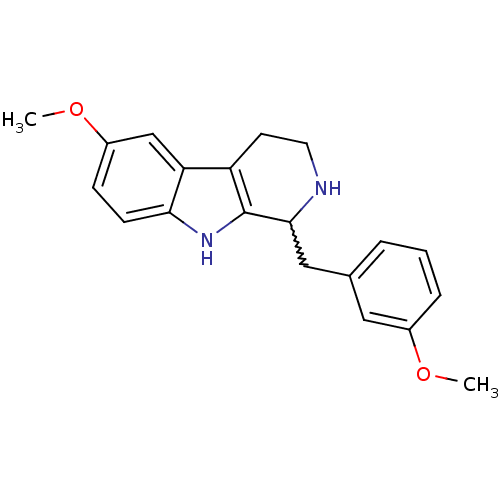 (1-(3-methoxybenzyl)-6-methoxy-2,3,4,9-tetrahydro-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Displacement of [3H]5CT from 5HT7 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2649-55 (2007) Article DOI: 10.1016/j.bmcl.2007.01.093 BindingDB Entry DOI: 10.7270/Q2WQ03F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50206186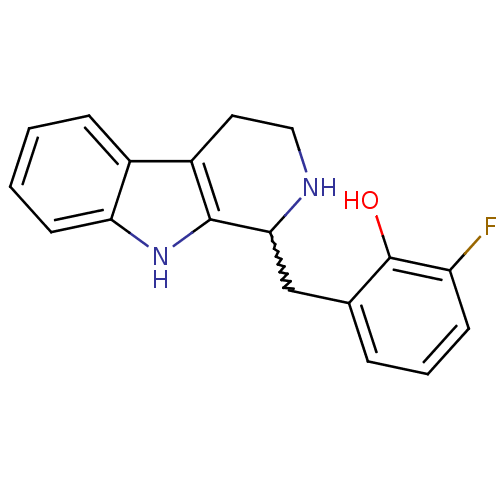 (2-fluoro-6-((2,3,4,9-tetrahydro-1H-pyrido[3,4-b]in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Displacement of [3H]5CT from 5HT7 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2649-55 (2007) Article DOI: 10.1016/j.bmcl.2007.01.093 BindingDB Entry DOI: 10.7270/Q2WQ03F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50099923 (CHEMBL29037 | N-{3-[2-(5-Carbamimidoyl-2-methylsul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 1379-82 (2001) BindingDB Entry DOI: 10.7270/Q24M93S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50343365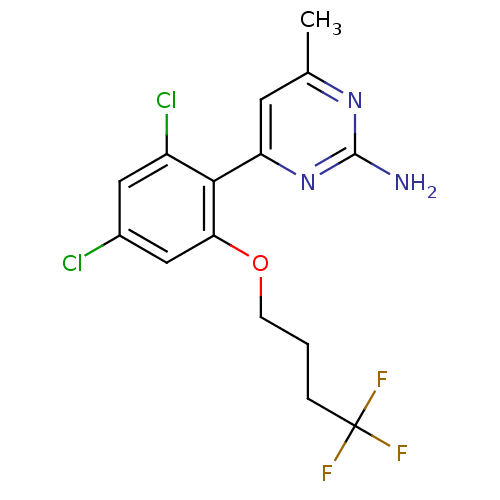 (4-[2,4-Dichloro-6-(4,4,4-trifluoro-butoxy)-phenyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin... | J Med Chem 54: 3368-85 (2011) Article DOI: 10.1021/jm200128m BindingDB Entry DOI: 10.7270/Q2Z320M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50109377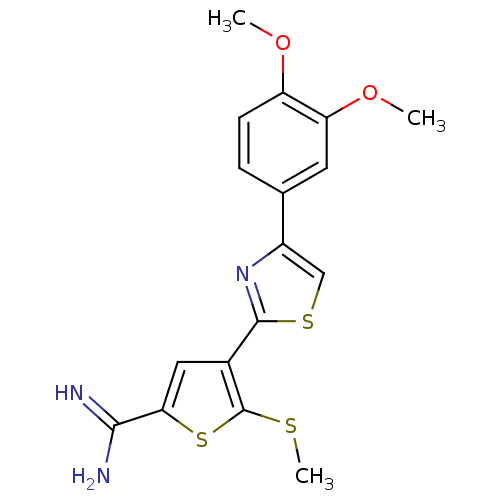 (4-[4-(3,4-Dimethoxy-phenyl)-thiazol-2-yl]-5-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Human kidney cell urokinase | Bioorg Med Chem Lett 12: 491-5 (2002) BindingDB Entry DOI: 10.7270/Q2H994H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098163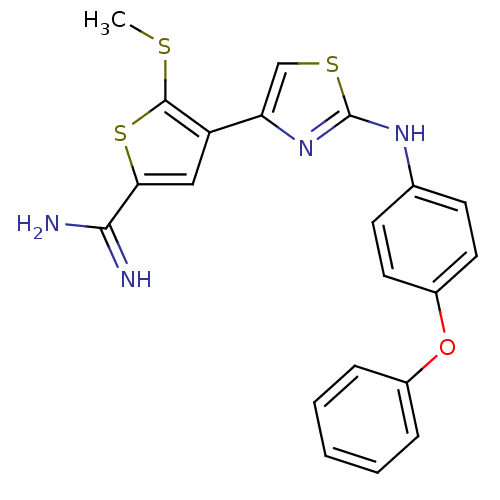 (5-Methylsulfanyl-4-[2-(4-phenoxy-phenylamino)-thia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098169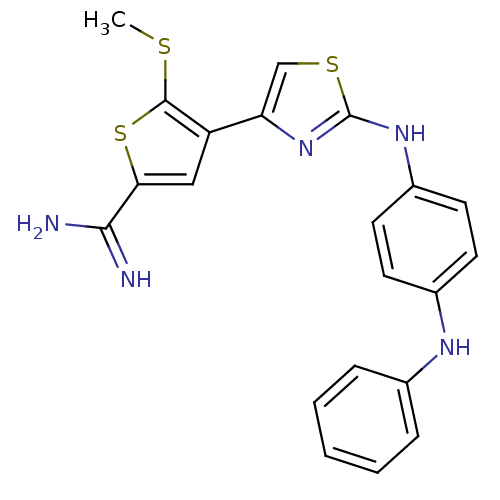 (5-Methylsulfanyl-4-[2-(4-phenylamino-phenylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50206168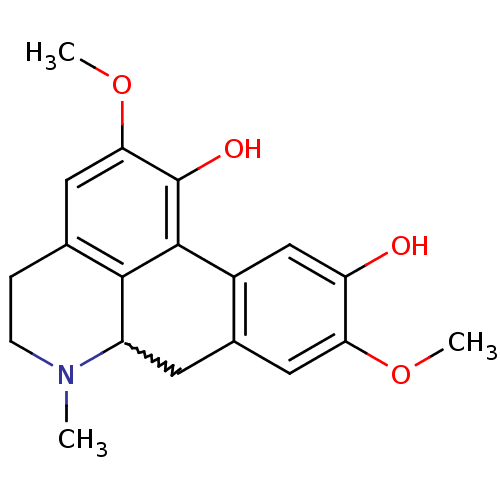 (2,9-dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-dibe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Displacement of [3H]5CT from 5HT7 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2649-55 (2007) Article DOI: 10.1016/j.bmcl.2007.01.093 BindingDB Entry DOI: 10.7270/Q2WQ03F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098144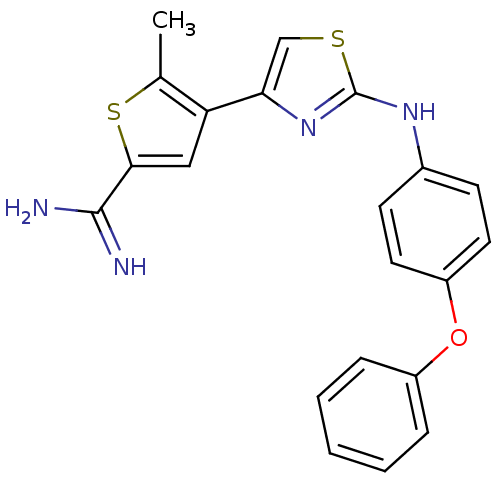 (5-Methyl-4-[2-(4-phenoxy-phenylamino)-thiazol-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50099902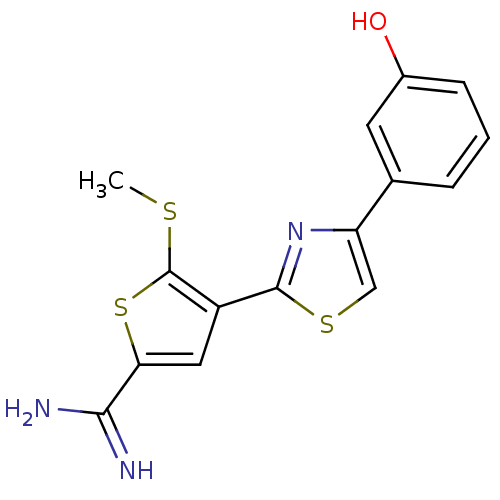 (4-[4-(3-Hydroxy-phenyl)-thiazol-2-yl]-5-methylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 1379-82 (2001) BindingDB Entry DOI: 10.7270/Q24M93S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50099933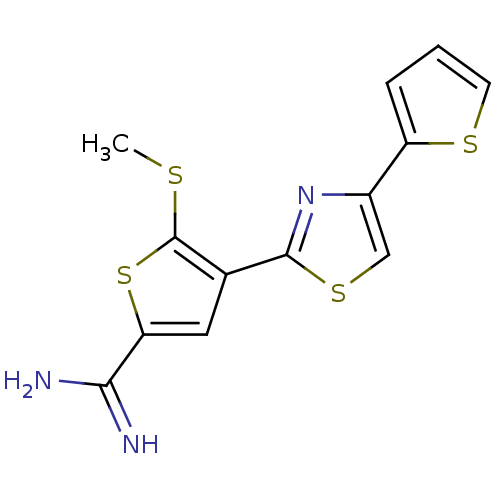 (5-Methylsulfanyl-4-(4-thiophen-2-yl-thiazol-2-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 1379-82 (2001) BindingDB Entry DOI: 10.7270/Q24M93S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50099921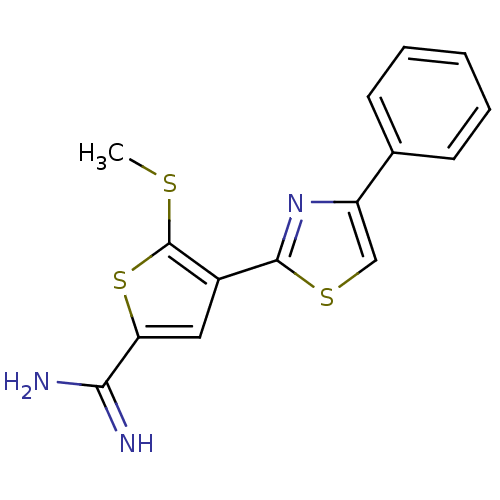 (5-Methylsulfanyl-4-(4-phenyl-thiazol-2-yl)-thiophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 1379-82 (2001) BindingDB Entry DOI: 10.7270/Q24M93S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50099911 (4-(4-Benzo[1,3]dioxol-5-yl-thiazol-2-yl)-5-methyls...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 1379-82 (2001) BindingDB Entry DOI: 10.7270/Q24M93S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50099897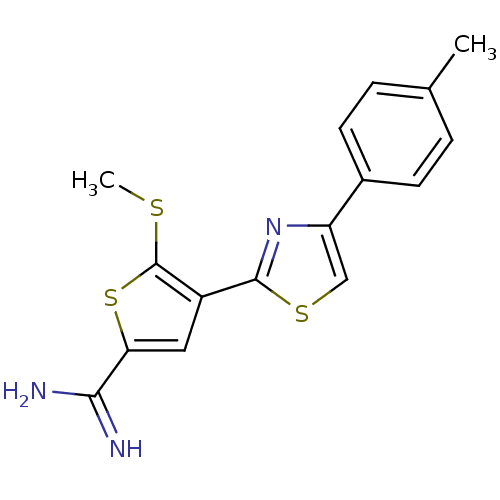 (5-Methylsulfanyl-4-(4-p-tolyl-thiazol-2-yl)-thioph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 1379-82 (2001) BindingDB Entry DOI: 10.7270/Q24M93S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50099901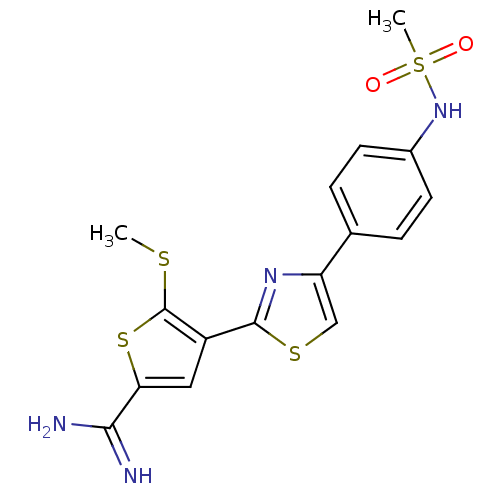 (4-[4-(4-Methanesulfonylamino-phenyl)-thiazol-2-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 1379-82 (2001) BindingDB Entry DOI: 10.7270/Q24M93S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50099900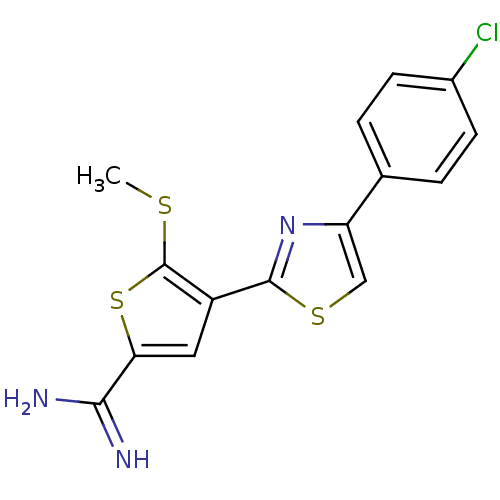 (4-[4-(4-Chloro-phenyl)-thiazol-2-yl]-5-methylsulfa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 1379-82 (2001) BindingDB Entry DOI: 10.7270/Q24M93S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50206178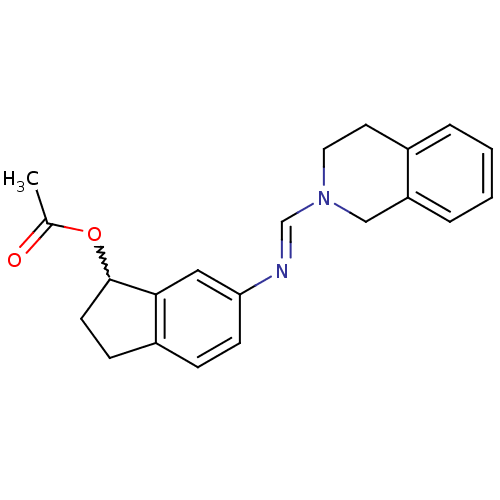 ((E)-6-((3,4-dihydroisoquinolin-2(1H)-yl)methylenea...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Displacement of [3H]5CT from 5HT7 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2649-55 (2007) Article DOI: 10.1016/j.bmcl.2007.01.093 BindingDB Entry DOI: 10.7270/Q2WQ03F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50206183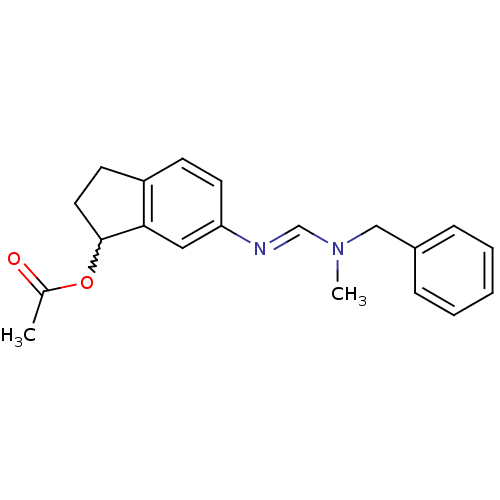 ((E)-6-((benzyl(methyl)amino)methyleneamino)-2,3-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Displacement of [3H]5CT from 5HT7 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2649-55 (2007) Article DOI: 10.1016/j.bmcl.2007.01.093 BindingDB Entry DOI: 10.7270/Q2WQ03F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50099915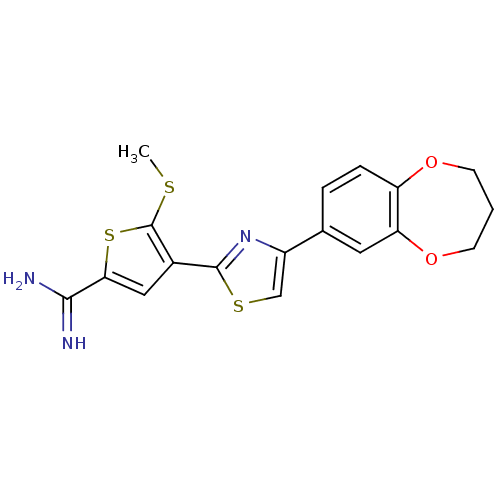 (4-[4-(3,4-Dihydro-2H-benzo[b][1,4]dioxepin-7-yl)-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 1379-82 (2001) BindingDB Entry DOI: 10.7270/Q24M93S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50099921 (5-Methylsulfanyl-4-(4-phenyl-thiazol-2-yl)-thiophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Human kidney cell urokinase | Bioorg Med Chem Lett 12: 491-5 (2002) BindingDB Entry DOI: 10.7270/Q2H994H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50099900 (4-[4-(4-Chloro-phenyl)-thiazol-2-yl]-5-methylsulfa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Human kidney cell urokinase | Bioorg Med Chem Lett 12: 491-5 (2002) BindingDB Entry DOI: 10.7270/Q2H994H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2852 total ) | Next | Last >> |