

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50183266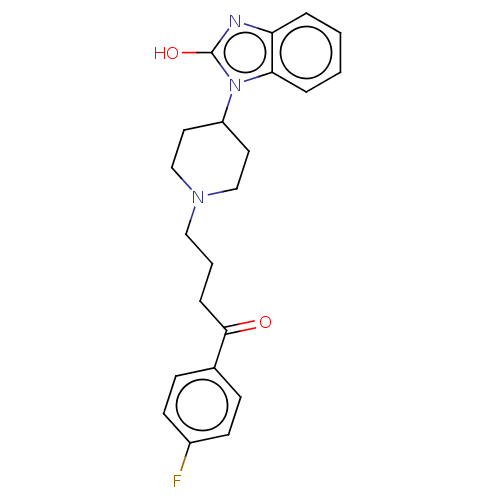 (Anquil | Benperidol | Benquil | MCN-JR-4584 | R-45...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Binding affinity to human dopamine D2 receptor by radioligand displacement assay | Bioorg Med Chem 24: 3671-9 (2016) Article DOI: 10.1016/j.bmc.2016.06.011 BindingDB Entry DOI: 10.7270/Q2W66NPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50183266 (Anquil | Benperidol | Benquil | MCN-JR-4584 | R-45...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Binding affinity to human dopamine D4 receptor by radioligand displacement assay | Bioorg Med Chem 24: 3671-9 (2016) Article DOI: 10.1016/j.bmc.2016.06.011 BindingDB Entry DOI: 10.7270/Q2W66NPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50264895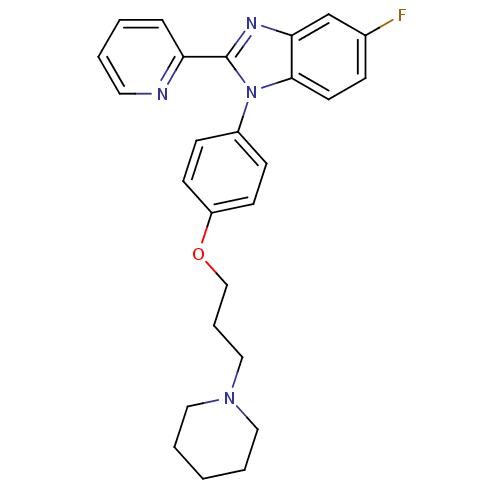 (5-fluoro-1-(4-(3-(piperidin-1-yl)propoxy)phenyl)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in HEK293 cells assessed as reversal of N-alpha-methylhistamine-induced inhibition of fo... | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM472930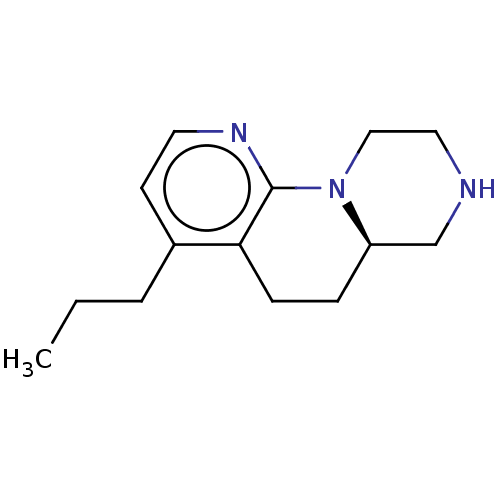 ((R)-4-propyl-6,6a,7,8,9,10-hexahydro-5H- pyrazino[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-DOI from recombinant human 5HT2CR transfected in human HEK293 cells incubated for 45 mins by radioligand binding assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127872 BindingDB Entry DOI: 10.7270/Q2SF30VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50596723 (CHEMBL5205903 | US20230348421, Compound 59) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114246 BindingDB Entry DOI: 10.7270/Q20P142F | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303248 (2-amino-N-[(1-ethyl-2- oxo-3-pyridyl)methyl]-8- me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139771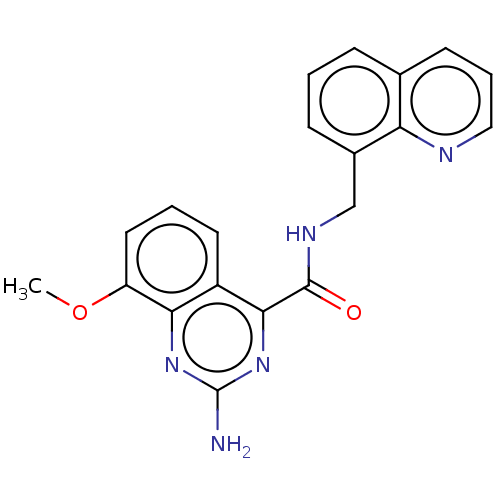 (CHEMBL3765580 | US10138212, Example 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303246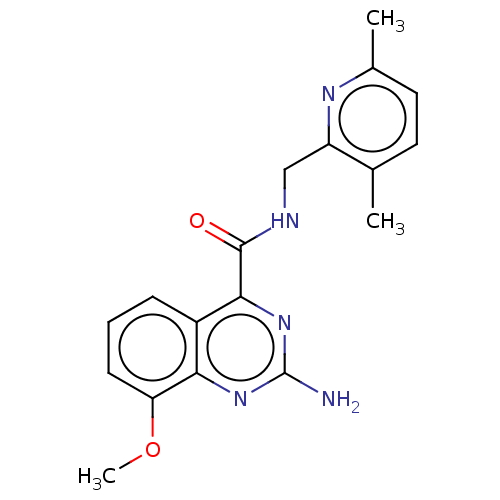 (2-amino-N-[(3,6- dimethyl-2- pyridyl)methyl]-8- me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50200981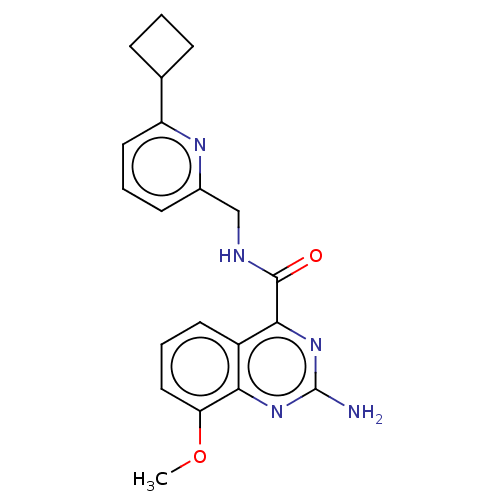 (CHEMBL3960148 | US10138212, Example 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50201006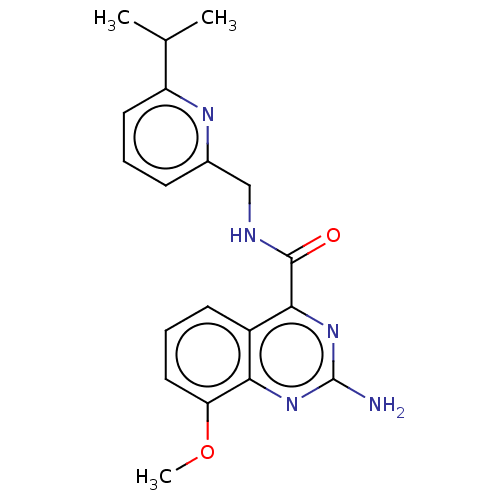 (CHEMBL3923709 | US10138212, Example 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50201019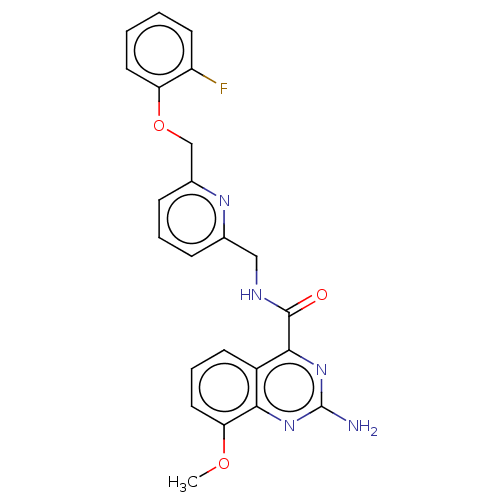 (CHEMBL3973920 | US10138212, Example 44) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139773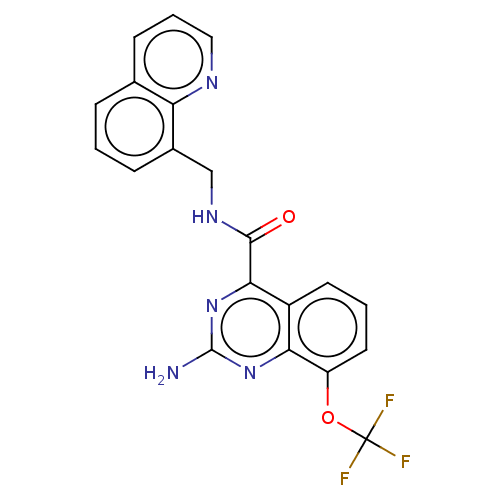 (CHEMBL3765379 | US10138212, Example 101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50459718 (CHEMBL4213379 | US10836764, Compound 106) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-DOI from recombinant human 5HT2CR transfected in human HEK293 cells incubated for 45 mins by radioligand binding assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127872 BindingDB Entry DOI: 10.7270/Q2SF30VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50183266 (Anquil | Benperidol | Benquil | MCN-JR-4584 | R-45...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [125I]-IABN from human recombinant dopamine D3 receptor expressed in HEK293 cell membrane incubated for 60 mins filtration binding as... | Bioorg Med Chem 24: 3671-9 (2016) Article DOI: 10.1016/j.bmc.2016.06.011 BindingDB Entry DOI: 10.7270/Q2W66NPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Binding affinity to human cloned 5HT2A receptor | Bioorg Med Chem 16: 7291-301 (2008) Article DOI: 10.1016/j.bmc.2008.06.030 BindingDB Entry DOI: 10.7270/Q2ZP45XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50151982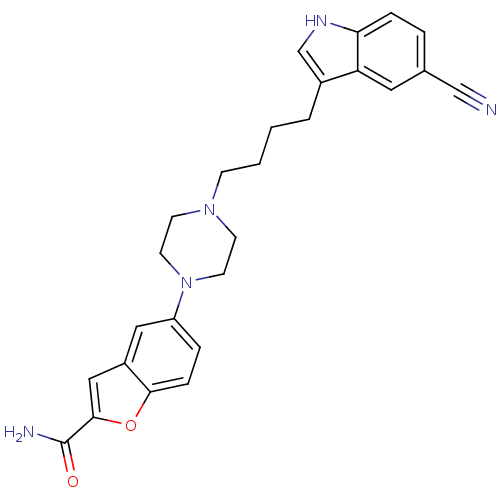 (5-{4-[4-(5-Cyano-1H-indol-3-yl)-butyl]-piperazin-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor by liquid scintillation counting | Eur J Med Chem 53: 124-32 (2012) Article DOI: 10.1016/j.ejmech.2012.03.042 BindingDB Entry DOI: 10.7270/Q2PN96NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303251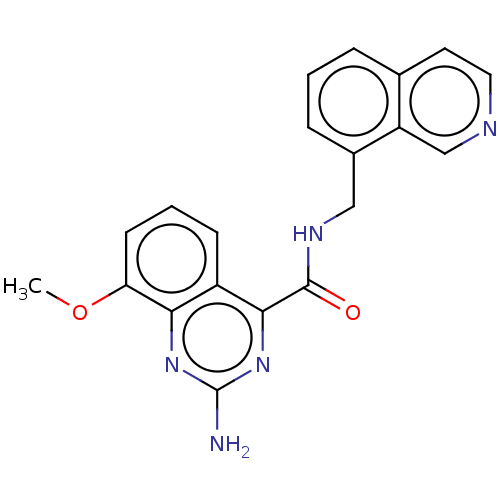 (2-amino-N-(8- isoquinolylmethyl)-8- methoxy-quinaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303181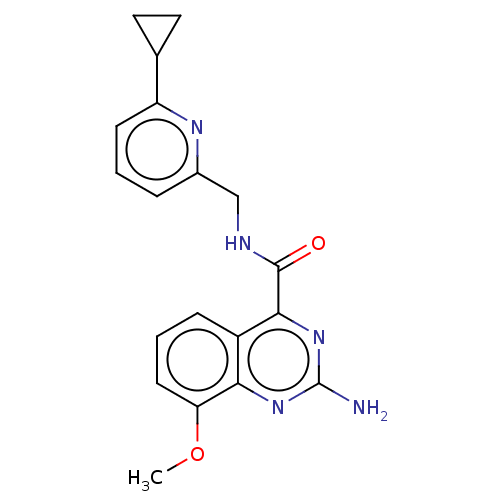 (2-amino-N-[(6- cyclopropyl-2- pyridyl)methyl]-8- m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303280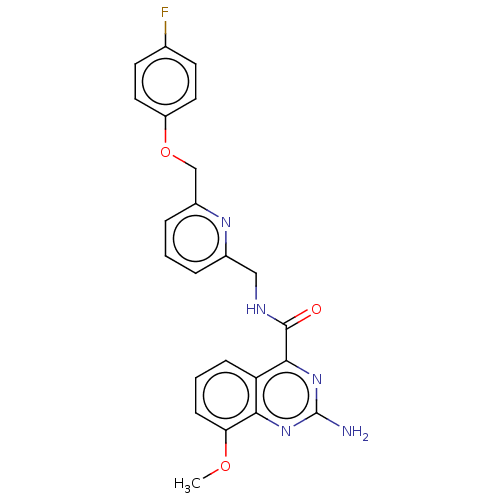 (2-amino-N-[[6-[(4- fluorophenoxy)methyl]- 2-pyridy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50185473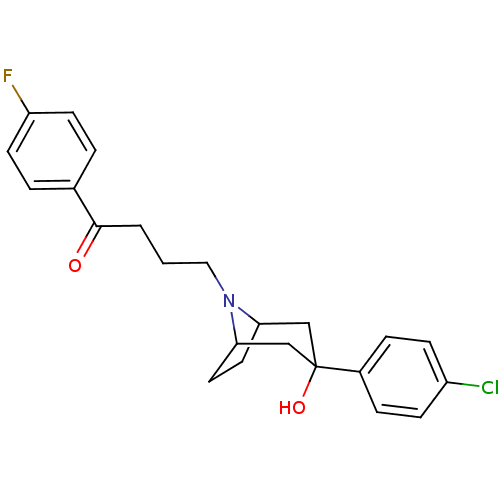 (4-(3-(4-chlorophenyl)-3-hydroxy-8-aza-bicyclo[3.2....) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D2 receptor by liquid scintillation counting | Bioorg Med Chem Lett 24: 4294-7 (2014) Article DOI: 10.1016/j.bmcl.2014.07.018 BindingDB Entry DOI: 10.7270/Q2222WF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50557505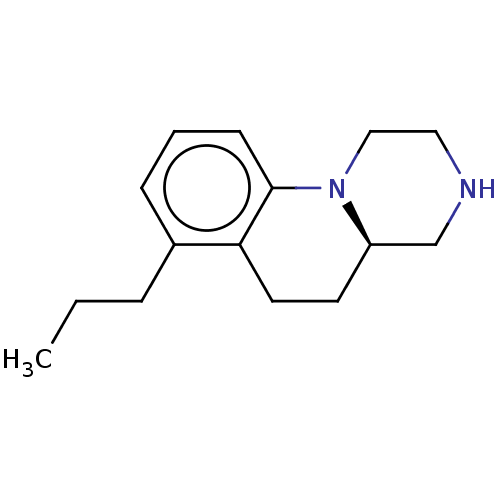 (CHEMBL4776557) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-DOI from recombinant human 5HT2CR transfected in human HEK293 cells incubated for 45 mins by radioligand binding assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127872 BindingDB Entry DOI: 10.7270/Q2SF30VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Binding affinity to dopamine D3 receptor (unknown origin) | Bioorg Med Chem 24: 3671-9 (2016) Article DOI: 10.1016/j.bmc.2016.06.011 BindingDB Entry DOI: 10.7270/Q2W66NPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT2B receptor expressed in cell membranes after 1 hr by liquid scintillation counting | Bioorg Med Chem 24: 3464-71 (2016) Article DOI: 10.1016/j.bmc.2016.05.053 BindingDB Entry DOI: 10.7270/Q2X63PVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50048803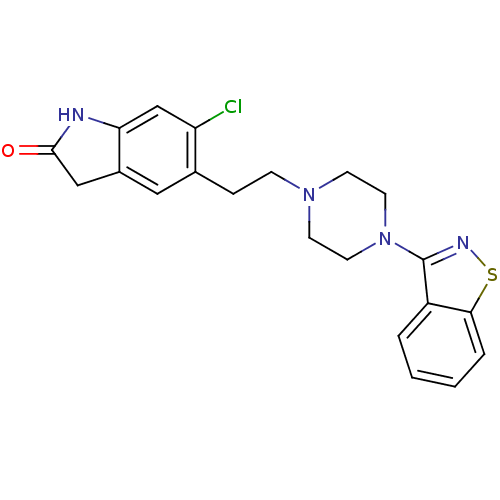 (5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Binding affinity to human cloned 5HT2A receptor | Bioorg Med Chem 16: 7291-301 (2008) Article DOI: 10.1016/j.bmc.2008.06.030 BindingDB Entry DOI: 10.7270/Q2ZP45XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303252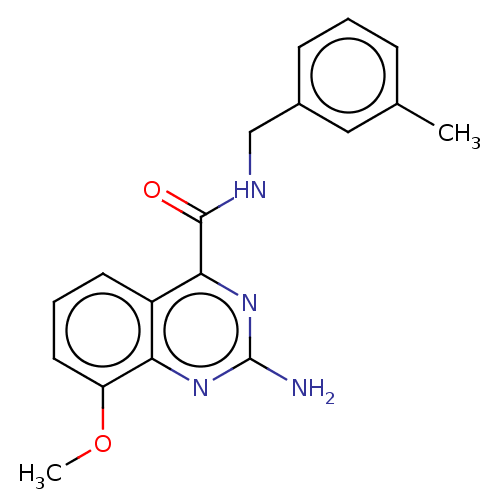 (2-amino-8-methoxy-N- (m- tolylmethyl)quinazoline- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50200989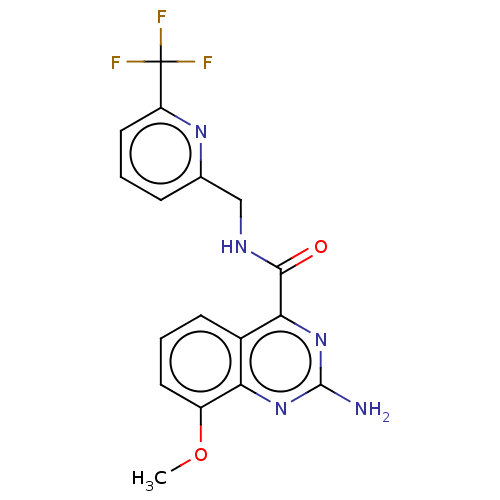 (CHEMBL3906827 | US10138212, Example 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50200984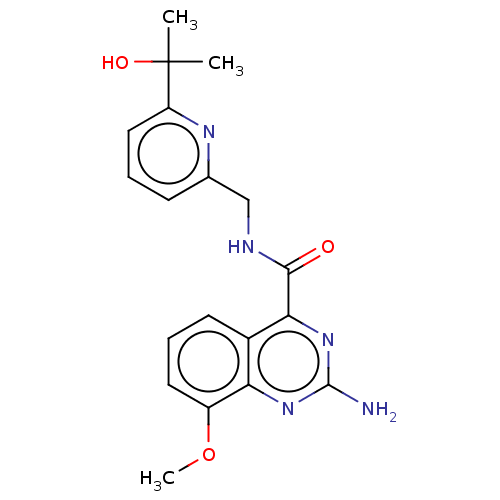 (CHEMBL3932655 | US10138212, Example 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM85093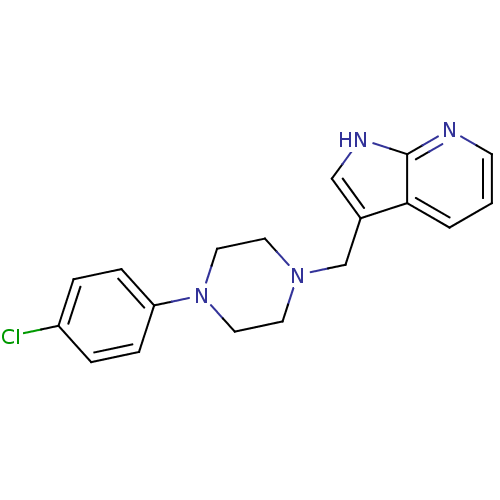 (CAS_3853 | CHEMBL267014 | CHEMBL555670 | L 745,870...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [125I]DOI from human dopamine D4 receptor by liquid scintillation counting | Bioorg Med Chem 17: 1716-23 (2009) Article DOI: 10.1016/j.bmc.2008.12.054 BindingDB Entry DOI: 10.7270/Q2NG4QGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (RAT) | BDBM85093 (CAS_3853 | CHEMBL267014 | CHEMBL555670 | L 745,870...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from rat dopamine D4 receptor by PDSP assay | Bioorg Med Chem 22: 3105-14 (2014) Article DOI: 10.1016/j.bmc.2014.04.026 BindingDB Entry DOI: 10.7270/Q25140RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50199141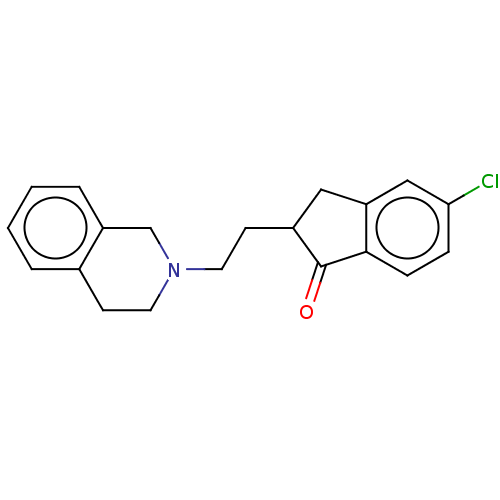 (CHEMBL3898250) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.468 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human 5-HT7 receptor by liquid scintillation counting method | Bioorg Med Chem 24: 5730-5740 (2016) Article DOI: 10.1016/j.bmc.2016.09.019 BindingDB Entry DOI: 10.7270/Q2639RQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303284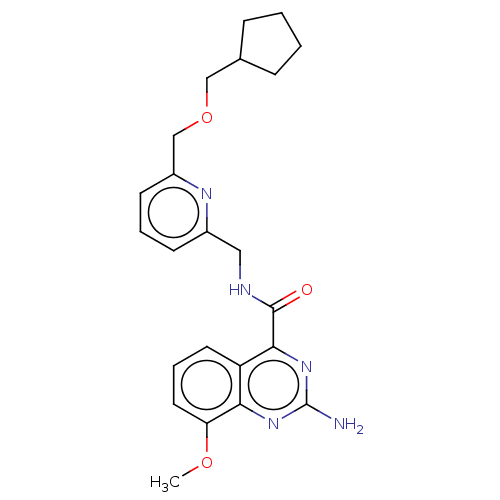 (2-amino-N-[[6- (cyclopentylmethoxy- methyl)-2-pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303145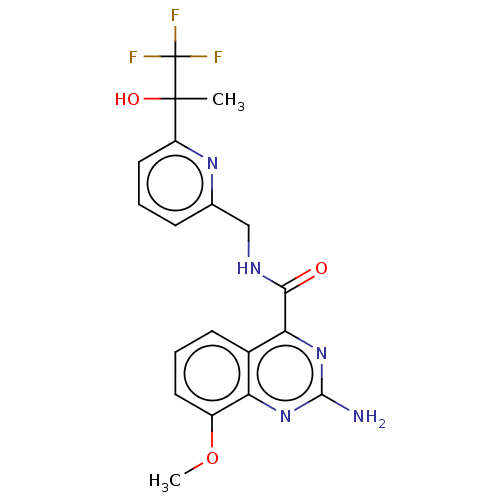 (2-amino-8-methoxy-N- [[6-(2,2,2-trifluoro-1- hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303255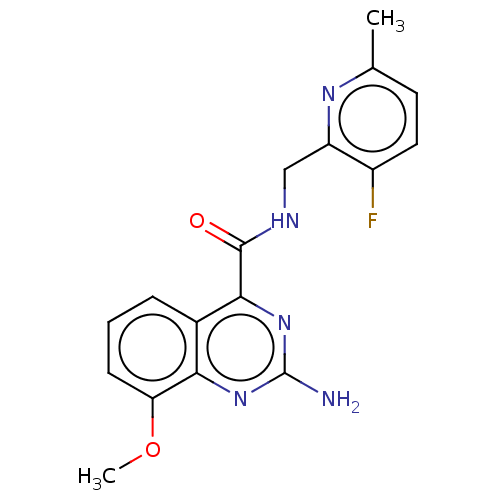 (2-amino-N-[(3-fluoro-6- methyl-2- pyridyl)methyl]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50200986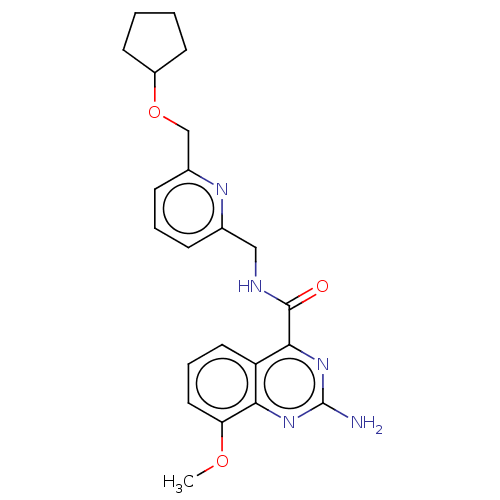 (CHEMBL3902955 | US10138212, Example 48) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303298 (2-amino-8-fluoro-N-[(2- pyrazol-1- ylphenyl)methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50151982 (5-{4-[4-(5-Cyano-1H-indol-3-yl)-butyl]-piperazin-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]Citalopram from human SERT by liquid scintillation counting | Eur J Med Chem 53: 124-32 (2012) Article DOI: 10.1016/j.ejmech.2012.03.042 BindingDB Entry DOI: 10.7270/Q2PN96NS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50199141 (CHEMBL3898250) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human 5-HT7 receptor by liquid scintillation counting method | Bioorg Med Chem 24: 5730-5740 (2016) Article DOI: 10.1016/j.bmc.2016.09.019 BindingDB Entry DOI: 10.7270/Q2639RQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303313 (2-amino-8-fluoro-N-[(3- fluoro-6-methyl-2- pyridyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50201017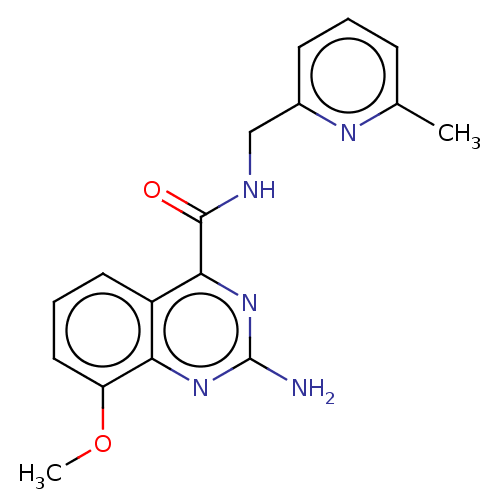 (CHEMBL3941632 | US10138212, Example 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50459713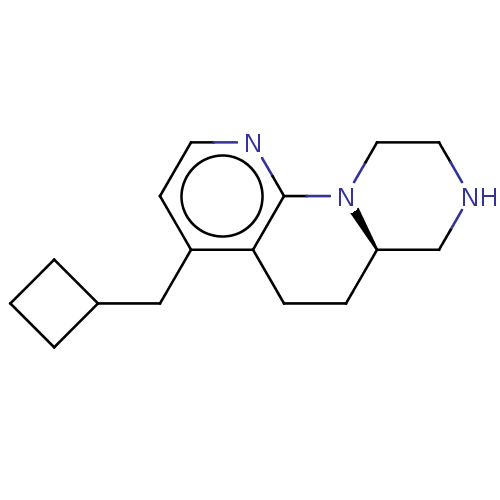 (CHEMBL4213447 | US10836764, Compound 176) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-DOI from recombinant human 5HT2CR transfected in human HEK293 cells incubated for 45 mins by radioligand binding assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127872 BindingDB Entry DOI: 10.7270/Q2SF30VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM473021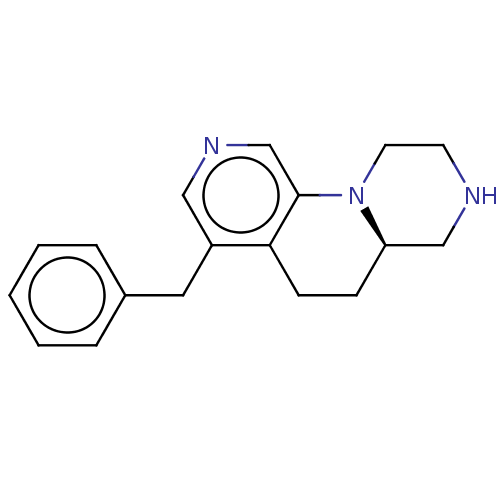 ((R)-4-benzyl-6,6a,7,8,9,10-hexahydro-5H- pyrazino[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-DOI from recombinant human 5HT2CR transfected in human HEK293 cells incubated for 45 mins by radioligand binding assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127872 BindingDB Entry DOI: 10.7270/Q2SF30VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome b (Sus scrofa) | BDBM50487138 (CHEMBL2251795) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Sus scrofa (pig) heart cytochrome bc1 complex using DBH2 as substrate by spectrophotometric analysis | J Am Chem Soc 132: 185-94 (2010) Article DOI: 10.1021/ja905756c BindingDB Entry DOI: 10.7270/Q27W6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303150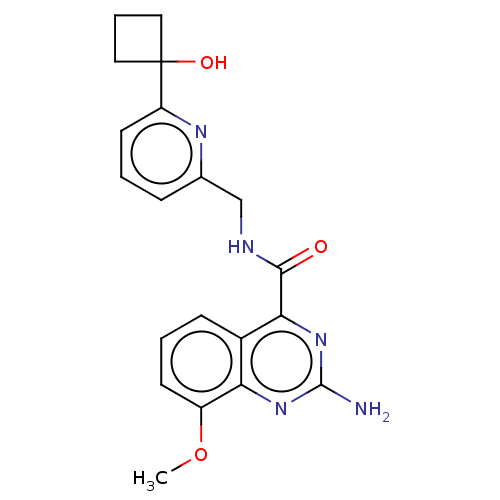 (2-amino-N-[[6-(1- hydroxycyclobutyl)-2- pyridyl]me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50541196 (CHEMBL4639090) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from 5HT2C receptor (unknown origin) | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126929 BindingDB Entry DOI: 10.7270/Q28W3HT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303319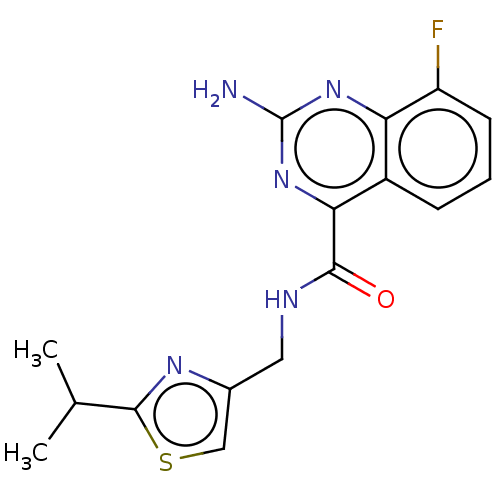 (2-amino-8-fluoro-N-[(2- isopropylthiazol-4- yl)met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50201020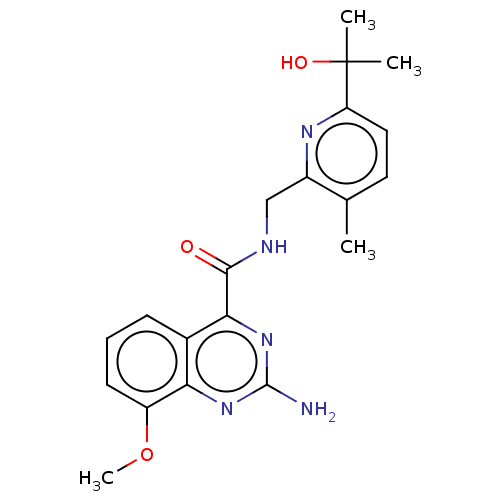 (CHEMBL3951425 | US10138212, Example 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM472931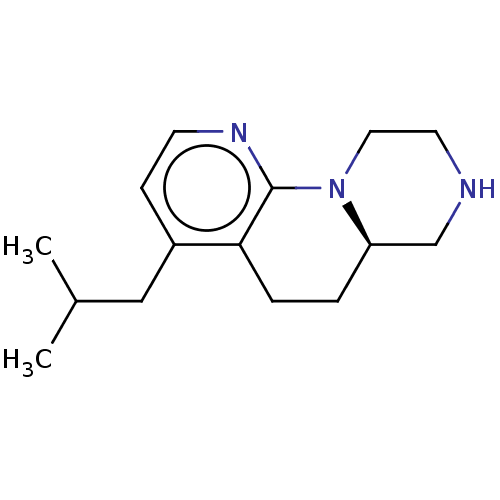 ((R)-4-isobutyl-6,6a,7,8,9,10-hexahydro-5H- pyrazin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-DOI from recombinant human 5HT2CR transfected in human HEK293 cells incubated for 45 mins by radioligand binding assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127872 BindingDB Entry DOI: 10.7270/Q2SF30VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139765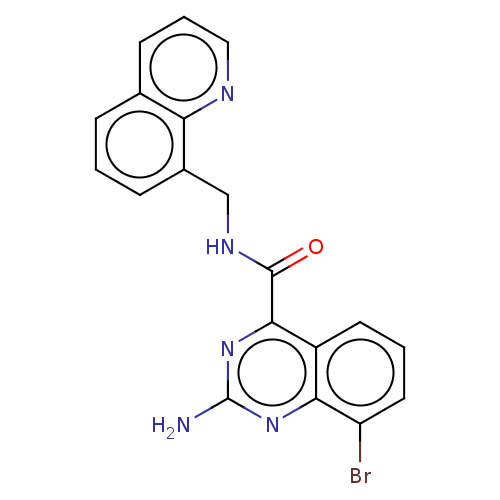 (CHEMBL3763830 | US10138212, Example 96) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50055521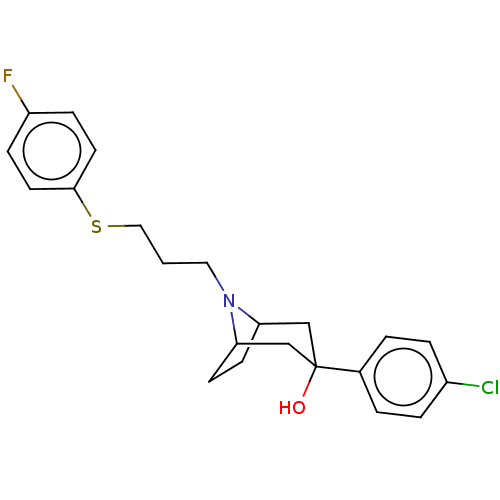 (CHEMBL3321790) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D3 receptor by liquid scintillation counting | Bioorg Med Chem Lett 24: 4294-7 (2014) Article DOI: 10.1016/j.bmcl.2014.07.018 BindingDB Entry DOI: 10.7270/Q2222WF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50185473 (4-(3-(4-chlorophenyl)-3-hydroxy-8-aza-bicyclo[3.2....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D3 receptor by liquid scintillation counting | Bioorg Med Chem Lett 24: 4294-7 (2014) Article DOI: 10.1016/j.bmcl.2014.07.018 BindingDB Entry DOI: 10.7270/Q2222WF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 12385 total ) | Next | Last >> |