 Found 100 hits with Last Name = 'zimmerman' and Initial = 'km'
Found 100 hits with Last Name = 'zimmerman' and Initial = 'km' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50228078
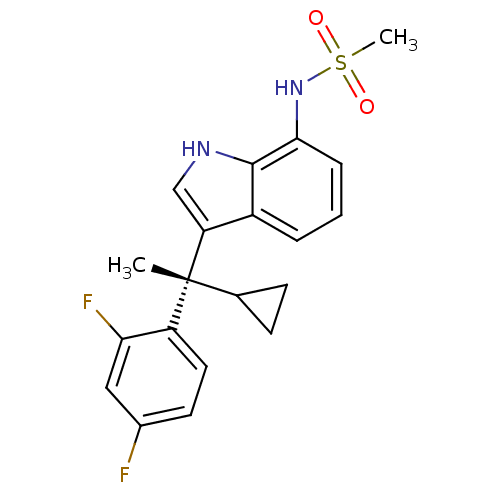
((S)-N-(3-(1-cyclopropyl-1-(2,4-difluorophenyl)ethy...)Show SMILES C[C@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1F Show InChI InChI=1S/C20H20F2N2O2S/c1-20(12-6-7-12,15-9-8-13(21)10-17(15)22)16-11-23-19-14(16)4-3-5-18(19)24-27(2,25)26/h3-5,8-12,23-24H,6-7H2,1-2H3/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.494 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human mineralocorticoid receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50228079
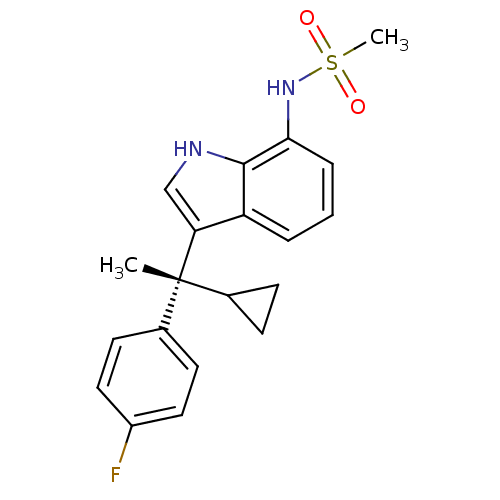
((R)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...)Show SMILES C[C@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1 Show InChI InChI=1S/C20H21FN2O2S/c1-20(13-6-7-13,14-8-10-15(21)11-9-14)17-12-22-19-16(17)4-3-5-18(19)23-26(2,24)25/h3-5,8-13,22-23H,6-7H2,1-2H3/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human mineralocorticoid receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50228076
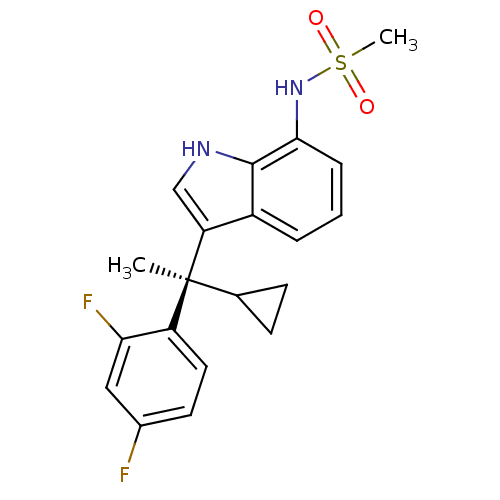
((R)-N-(3-(1-cyclopropyl-1-(2,4-difluorophenyl)ethy...)Show SMILES C[C@@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1F Show InChI InChI=1S/C20H20F2N2O2S/c1-20(12-6-7-12,15-9-8-13(21)10-17(15)22)16-11-23-19-14(16)4-3-5-18(19)24-27(2,25)26/h3-5,8-12,23-24H,6-7H2,1-2H3/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human mineralocorticoid receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50228080

(2',15'-dimethyl-5,5'-dioxo-(9'R)-spiro[tetrahydrof...)Show SMILES CC(=O)S[C@@H]1CC2=CC(=O)CC[C@]2(C)[C@H]2CC[C@@]3(C)[C@@H](CC[C@@]33CCC(=O)O3)[C@H]12 |r,t:6| Show InChI InChI=1S/C24H32O4S/c1-14(25)29-19-13-15-12-16(26)4-8-22(15,2)17-5-9-23(3)18(21(17)19)6-10-24(23)11-7-20(27)28-24/h12,17-19,21H,4-11,13H2,1-3H3/t17-,18-,19+,21+,22-,23-,24+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 2.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human mineralocorticoid receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50228081

((S)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...)Show SMILES C[C@@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1 Show InChI InChI=1S/C20H21FN2O2S/c1-20(13-6-7-13,14-8-10-15(21)11-9-14)17-12-22-19-16(17)4-3-5-18(19)23-26(2,24)25/h3-5,8-13,22-23H,6-7H2,1-2H3/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human mineralocorticoid receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50228078

((S)-N-(3-(1-cyclopropyl-1-(2,4-difluorophenyl)ethy...)Show SMILES C[C@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1F Show InChI InChI=1S/C20H20F2N2O2S/c1-20(12-6-7-12,15-9-8-13(21)10-17(15)22)16-11-23-19-14(16)4-3-5-18(19)24-27(2,25)26/h3-5,8-12,23-24H,6-7H2,1-2H3/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human glucocorticoid receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50228080

(2',15'-dimethyl-5,5'-dioxo-(9'R)-spiro[tetrahydrof...)Show SMILES CC(=O)S[C@@H]1CC2=CC(=O)CC[C@]2(C)[C@H]2CC[C@@]3(C)[C@@H](CC[C@@]33CCC(=O)O3)[C@H]12 |r,t:6| Show InChI InChI=1S/C24H32O4S/c1-14(25)29-19-13-15-12-16(26)4-8-22(15,2)17-5-9-23(3)18(21(17)19)6-10-24(23)11-7-20(27)28-24/h12,17-19,21H,4-11,13H2,1-3H3/t17-,18-,19+,21+,22-,23-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 32.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human glucocorticoid receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50228080

(2',15'-dimethyl-5,5'-dioxo-(9'R)-spiro[tetrahydrof...)Show SMILES CC(=O)S[C@@H]1CC2=CC(=O)CC[C@]2(C)[C@H]2CC[C@@]3(C)[C@@H](CC[C@@]33CCC(=O)O3)[C@H]12 |r,t:6| Show InChI InChI=1S/C24H32O4S/c1-14(25)29-19-13-15-12-16(26)4-8-22(15,2)17-5-9-23(3)18(21(17)19)6-10-24(23)11-7-20(27)28-24/h12,17-19,21H,4-11,13H2,1-3H3/t17-,18-,19+,21+,22-,23-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 39.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human androgen receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50228077
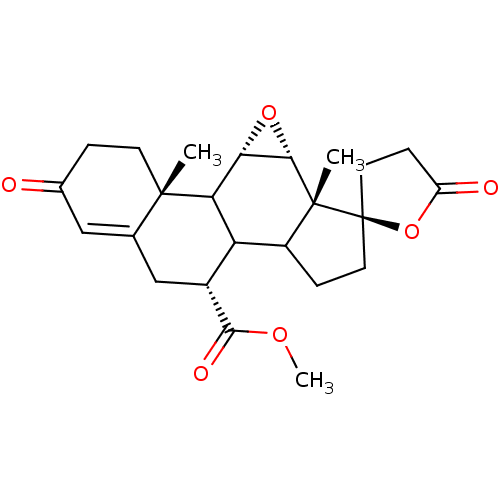
(CHEMBL237122 | epierenone)Show SMILES COC(=O)[C@@H]1CC2=CC(=O)CC[C@]2(C)C2[C@@H]3O[C@@H]3[C@@]3(C)C(CC[C@@]33CCC(=O)O3)C12 |t:6| Show InChI InChI=1S/C24H30O6/c1-22-7-4-13(25)10-12(22)11-14(21(27)28-3)17-15-5-8-24(9-6-16(26)30-24)23(15,2)20-19(29-20)18(17)22/h10,14-15,17-20H,4-9,11H2,1-3H3/t14-,15?,17?,18?,19+,20+,22+,23-,24-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human mineralocorticoid receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50228079

((R)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...)Show SMILES C[C@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1 Show InChI InChI=1S/C20H21FN2O2S/c1-20(13-6-7-13,14-8-10-15(21)11-9-14)17-12-22-19-16(17)4-3-5-18(19)23-26(2,24)25/h3-5,8-13,22-23H,6-7H2,1-2H3/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human progesterone receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50228078

((S)-N-(3-(1-cyclopropyl-1-(2,4-difluorophenyl)ethy...)Show SMILES C[C@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1F Show InChI InChI=1S/C20H20F2N2O2S/c1-20(12-6-7-12,15-9-8-13(21)10-17(15)22)16-11-23-19-14(16)4-3-5-18(19)24-27(2,25)26/h3-5,8-12,23-24H,6-7H2,1-2H3/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human progesterone receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50228079

((R)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...)Show SMILES C[C@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1 Show InChI InChI=1S/C20H21FN2O2S/c1-20(13-6-7-13,14-8-10-15(21)11-9-14)17-12-22-19-16(17)4-3-5-18(19)23-26(2,24)25/h3-5,8-13,22-23H,6-7H2,1-2H3/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human glucocorticoid receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50228081

((S)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...)Show SMILES C[C@@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1 Show InChI InChI=1S/C20H21FN2O2S/c1-20(13-6-7-13,14-8-10-15(21)11-9-14)17-12-22-19-16(17)4-3-5-18(19)23-26(2,24)25/h3-5,8-13,22-23H,6-7H2,1-2H3/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 313 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human progesterone receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50228081

((S)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...)Show SMILES C[C@@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1 Show InChI InChI=1S/C20H21FN2O2S/c1-20(13-6-7-13,14-8-10-15(21)11-9-14)17-12-22-19-16(17)4-3-5-18(19)23-26(2,24)25/h3-5,8-13,22-23H,6-7H2,1-2H3/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 387 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human glucocorticoid receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50228080

(2',15'-dimethyl-5,5'-dioxo-(9'R)-spiro[tetrahydrof...)Show SMILES CC(=O)S[C@@H]1CC2=CC(=O)CC[C@]2(C)[C@H]2CC[C@@]3(C)[C@@H](CC[C@@]33CCC(=O)O3)[C@H]12 |r,t:6| Show InChI InChI=1S/C24H32O4S/c1-14(25)29-19-13-15-12-16(26)4-8-22(15,2)17-5-9-23(3)18(21(17)19)6-10-24(23)11-7-20(27)28-24/h12,17-19,21H,4-11,13H2,1-3H3/t17-,18-,19+,21+,22-,23-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human progesterone receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50228081

((S)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...)Show SMILES C[C@@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1 Show InChI InChI=1S/C20H21FN2O2S/c1-20(13-6-7-13,14-8-10-15(21)11-9-14)17-12-22-19-16(17)4-3-5-18(19)23-26(2,24)25/h3-5,8-13,22-23H,6-7H2,1-2H3/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human androgen receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50228078

((S)-N-(3-(1-cyclopropyl-1-(2,4-difluorophenyl)ethy...)Show SMILES C[C@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1F Show InChI InChI=1S/C20H20F2N2O2S/c1-20(12-6-7-12,15-9-8-13(21)10-17(15)22)16-11-23-19-14(16)4-3-5-18(19)24-27(2,25)26/h3-5,8-12,23-24H,6-7H2,1-2H3/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human androgen receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50228079

((R)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...)Show SMILES C[C@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1 Show InChI InChI=1S/C20H21FN2O2S/c1-20(13-6-7-13,14-8-10-15(21)11-9-14)17-12-22-19-16(17)4-3-5-18(19)23-26(2,24)25/h3-5,8-13,22-23H,6-7H2,1-2H3/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 849 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human androgen receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50228078

((S)-N-(3-(1-cyclopropyl-1-(2,4-difluorophenyl)ethy...)Show SMILES C[C@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1F Show InChI InChI=1S/C20H20F2N2O2S/c1-20(12-6-7-12,15-9-8-13(21)10-17(15)22)16-11-23-19-14(16)4-3-5-18(19)24-27(2,25)26/h3-5,8-12,23-24H,6-7H2,1-2H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human estrogen receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50228077

(CHEMBL237122 | epierenone)Show SMILES COC(=O)[C@@H]1CC2=CC(=O)CC[C@]2(C)C2[C@@H]3O[C@@H]3[C@@]3(C)C(CC[C@@]33CCC(=O)O3)C12 |t:6| Show InChI InChI=1S/C24H30O6/c1-22-7-4-13(25)10-12(22)11-14(21(27)28-3)17-15-5-8-24(9-6-16(26)30-24)23(15,2)20-19(29-20)18(17)22/h10,14-15,17-20H,4-9,11H2,1-3H3/t14-,15?,17?,18?,19+,20+,22+,23-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human estrogen receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50228080

(2',15'-dimethyl-5,5'-dioxo-(9'R)-spiro[tetrahydrof...)Show SMILES CC(=O)S[C@@H]1CC2=CC(=O)CC[C@]2(C)[C@H]2CC[C@@]3(C)[C@@H](CC[C@@]33CCC(=O)O3)[C@H]12 |r,t:6| Show InChI InChI=1S/C24H32O4S/c1-14(25)29-19-13-15-12-16(26)4-8-22(15,2)17-5-9-23(3)18(21(17)19)6-10-24(23)11-7-20(27)28-24/h12,17-19,21H,4-11,13H2,1-3H3/t17-,18-,19+,21+,22-,23-,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human estrogen receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50228081

((S)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...)Show SMILES C[C@@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1 Show InChI InChI=1S/C20H21FN2O2S/c1-20(13-6-7-13,14-8-10-15(21)11-9-14)17-12-22-19-16(17)4-3-5-18(19)23-26(2,24)25/h3-5,8-13,22-23H,6-7H2,1-2H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human estrogen receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50228079

((R)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...)Show SMILES C[C@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1 Show InChI InChI=1S/C20H21FN2O2S/c1-20(13-6-7-13,14-8-10-15(21)11-9-14)17-12-22-19-16(17)4-3-5-18(19)23-26(2,24)25/h3-5,8-13,22-23H,6-7H2,1-2H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human estrogen receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50228077

(CHEMBL237122 | epierenone)Show SMILES COC(=O)[C@@H]1CC2=CC(=O)CC[C@]2(C)C2[C@@H]3O[C@@H]3[C@@]3(C)C(CC[C@@]33CCC(=O)O3)C12 |t:6| Show InChI InChI=1S/C24H30O6/c1-22-7-4-13(25)10-12(22)11-14(21(27)28-3)17-15-5-8-24(9-6-16(26)30-24)23(15,2)20-19(29-20)18(17)22/h10,14-15,17-20H,4-9,11H2,1-3H3/t14-,15?,17?,18?,19+,20+,22+,23-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human glucocorticoid receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50228077

(CHEMBL237122 | epierenone)Show SMILES COC(=O)[C@@H]1CC2=CC(=O)CC[C@]2(C)C2[C@@H]3O[C@@H]3[C@@]3(C)C(CC[C@@]33CCC(=O)O3)C12 |t:6| Show InChI InChI=1S/C24H30O6/c1-22-7-4-13(25)10-12(22)11-14(21(27)28-3)17-15-5-8-24(9-6-16(26)30-24)23(15,2)20-19(29-20)18(17)22/h10,14-15,17-20H,4-9,11H2,1-3H3/t14-,15?,17?,18?,19+,20+,22+,23-,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human androgen receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50228077

(CHEMBL237122 | epierenone)Show SMILES COC(=O)[C@@H]1CC2=CC(=O)CC[C@]2(C)C2[C@@H]3O[C@@H]3[C@@]3(C)C(CC[C@@]33CCC(=O)O3)C12 |t:6| Show InChI InChI=1S/C24H30O6/c1-22-7-4-13(25)10-12(22)11-14(21(27)28-3)17-15-5-8-24(9-6-16(26)30-24)23(15,2)20-19(29-20)18(17)22/h10,14-15,17-20H,4-9,11H2,1-3H3/t14-,15?,17?,18?,19+,20+,22+,23-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human progesterone receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Mus musculus (mouse)) | BDBM26333
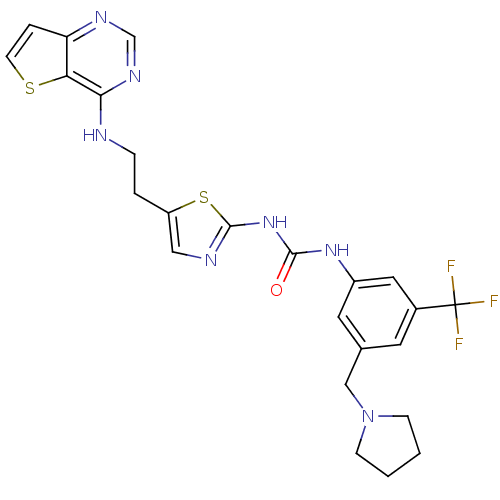
(1-[3-(pyrrolidin-1-ylmethyl)-5-(trifluoromethyl)ph...)Show SMILES FC(F)(F)c1cc(CN2CCCC2)cc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)c1 Show InChI InChI=1S/C24H24F3N7OS2/c25-24(26,27)16-9-15(13-34-6-1-2-7-34)10-17(11-16)32-22(35)33-23-29-12-18(37-23)3-5-28-21-20-19(4-8-36-20)30-14-31-21/h4,8-12,14H,1-3,5-7,13H2,(H,28,30,31)(H2,29,32,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 21 |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50310621

(CHEMBL1079538 | N-(4-(6-(trifluoromethyl)-3H-imida...)Show SMILES FC(F)(F)c1cnc2nc(Nc3ccc(CCNc4ncnc5ccsc45)cc3)[nH]c2c1 Show InChI InChI=1S/C21H16F3N7S/c22-21(23,24)13-9-16-18(26-10-13)31-20(30-16)29-14-3-1-12(2-4-14)5-7-25-19-17-15(6-8-32-17)27-11-28-19/h1-4,6,8-11H,5,7H2,(H,25,27,28)(H2,26,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B after 60 mins |
Bioorg Med Chem Lett 19: 5158-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.016
BindingDB Entry DOI: 10.7270/Q2T72HK2 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50310608

(CHEMBL1077729 | N-(4-(5-(trifluoromethyl)-1H-benzo...)Show SMILES FC(F)(F)c1ccc2nc(Nc3ccc(CCNc4ncnc5ccsc45)cc3)[nH]c2c1 Show InChI InChI=1S/C22H17F3N6S/c23-22(24,25)14-3-6-16-18(11-14)31-21(30-16)29-15-4-1-13(2-5-15)7-9-26-20-19-17(8-10-32-19)27-12-28-20/h1-6,8,10-12H,7,9H2,(H,26,27,28)(H2,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B after 60 mins |
Bioorg Med Chem Lett 19: 5158-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.016
BindingDB Entry DOI: 10.7270/Q2T72HK2 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50310607
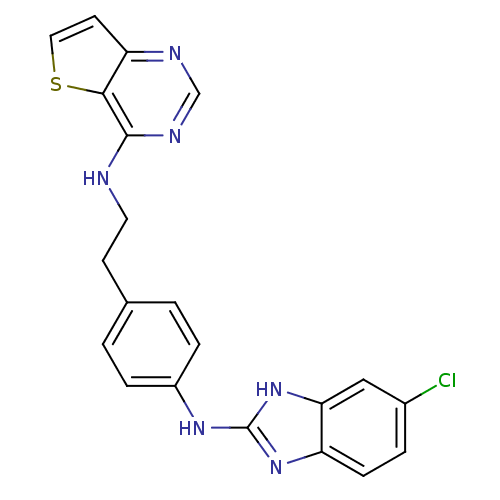
(CHEMBL1078060 | N-(4-(5-chloro-1H-benzo[d]imidazol...)Show SMILES Clc1ccc2nc(Nc3ccc(CCNc4ncnc5ccsc45)cc3)[nH]c2c1 Show InChI InChI=1S/C21H17ClN6S/c22-14-3-6-16-18(11-14)28-21(27-16)26-15-4-1-13(2-5-15)7-9-23-20-19-17(8-10-29-19)24-12-25-20/h1-6,8,10-12H,7,9H2,(H,23,24,25)(H2,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B after 60 mins |
Bioorg Med Chem Lett 19: 5158-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.016
BindingDB Entry DOI: 10.7270/Q2T72HK2 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Mus musculus (mouse)) | BDBM26326
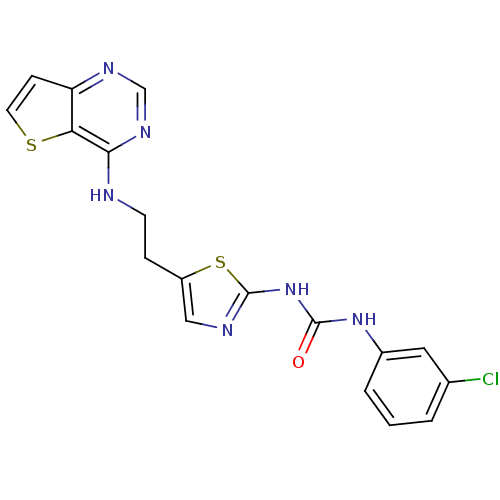
(1-(3-chlorophenyl)-3-[5-(2-{thieno[3,2-d]pyrimidin...)Show SMILES Clc1cccc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)c1 Show InChI InChI=1S/C18H15ClN6OS2/c19-11-2-1-3-12(8-11)24-17(26)25-18-21-9-13(28-18)4-6-20-16-15-14(5-7-27-15)22-10-23-16/h1-3,5,7-10H,4,6H2,(H,20,22,23)(H2,21,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.2 | 21 |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Mus musculus (mouse)) | BDBM26322
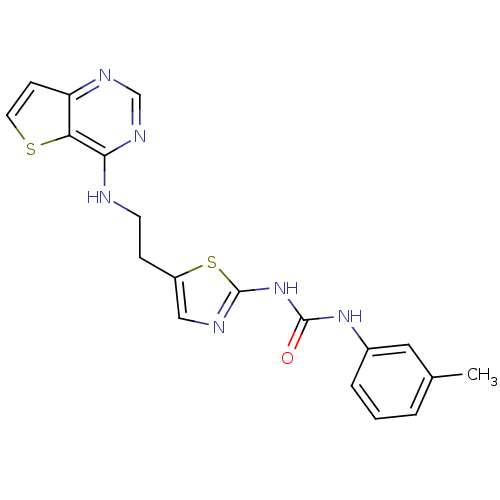
(1-(3-methylphenyl)-3-[5-(2-{thieno[3,2-d]pyrimidin...)Show SMILES Cc1cccc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)c1 Show InChI InChI=1S/C19H18N6OS2/c1-12-3-2-4-13(9-12)24-18(26)25-19-21-10-14(28-19)5-7-20-17-16-15(6-8-27-16)22-11-23-17/h2-4,6,8-11H,5,7H2,1H3,(H,20,22,23)(H2,21,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.2 | 21 |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM26326

(1-(3-chlorophenyl)-3-[5-(2-{thieno[3,2-d]pyrimidin...)Show SMILES Clc1cccc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)c1 Show InChI InChI=1S/C18H15ClN6OS2/c19-11-2-1-3-12(8-11)24-17(26)25-18-21-9-13(28-18)4-6-20-16-15-14(5-7-27-15)22-10-23-16/h1-3,5,7-10H,4,6H2,(H,20,22,23)(H2,21,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A after 60 mins |
Bioorg Med Chem Lett 19: 5158-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.016
BindingDB Entry DOI: 10.7270/Q2T72HK2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50310620
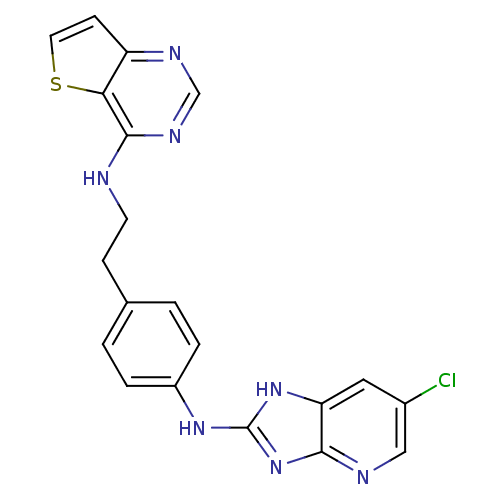
(CHEMBL1079713 | N-(4-(6-chloro-3H-imidazo[4,5-b]py...)Show SMILES Clc1cnc2nc(Nc3ccc(CCNc4ncnc5ccsc45)cc3)[nH]c2c1 Show InChI InChI=1S/C20H16ClN7S/c21-13-9-16-18(23-10-13)28-20(27-16)26-14-3-1-12(2-4-14)5-7-22-19-17-15(6-8-29-17)24-11-25-19/h1-4,6,8-11H,5,7H2,(H,22,24,25)(H2,23,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B after 60 mins |
Bioorg Med Chem Lett 19: 5158-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.016
BindingDB Entry DOI: 10.7270/Q2T72HK2 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50310614
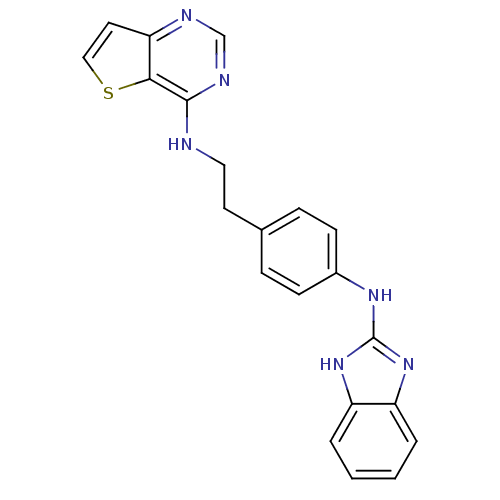
(CHEMBL1078906 | N-(4-(1H-benzo[d]imidazol-2-ylamin...)Show SMILES C(Cc1ccc(Nc2nc3ccccc3[nH]2)cc1)Nc1ncnc2ccsc12 Show InChI InChI=1S/C21H18N6S/c1-2-4-17-16(3-1)26-21(27-17)25-15-7-5-14(6-8-15)9-11-22-20-19-18(10-12-28-19)23-13-24-20/h1-8,10,12-13H,9,11H2,(H,22,23,24)(H2,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B after 60 mins |
Bioorg Med Chem Lett 19: 5158-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.016
BindingDB Entry DOI: 10.7270/Q2T72HK2 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Mus musculus (mouse)) | BDBM26330
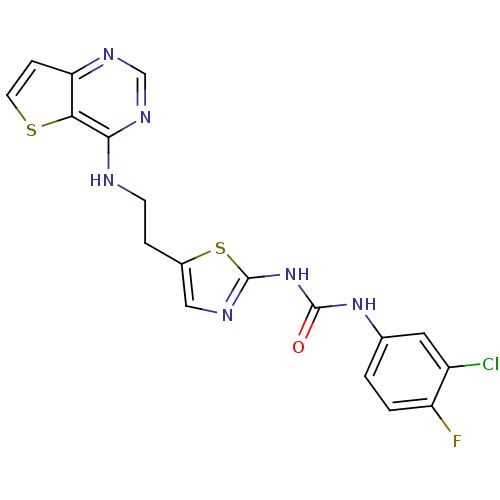
(1-(3-chloro-4-fluorophenyl)-3-[5-(2-{thieno[3,2-d]...)Show SMILES Fc1ccc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)cc1Cl Show InChI InChI=1S/C18H14ClFN6OS2/c19-12-7-10(1-2-13(12)20)25-17(27)26-18-22-8-11(29-18)3-5-21-16-15-14(4-6-28-15)23-9-24-16/h1-2,4,6-9H,3,5H2,(H,21,23,24)(H2,22,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.2 | 21 |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM26329
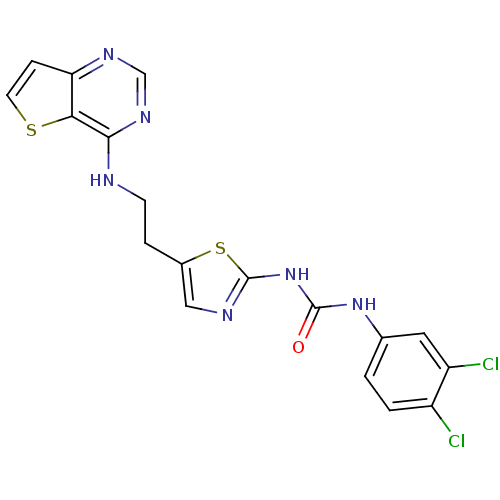
(1-(3,4-dichlorophenyl)-3-[5-(2-{thieno[3,2-d]pyrim...)Show SMILES Clc1ccc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)cc1Cl Show InChI InChI=1S/C18H14Cl2N6OS2/c19-12-2-1-10(7-13(12)20)25-17(27)26-18-22-8-11(29-18)3-5-21-16-15-14(4-6-28-15)23-9-24-16/h1-2,4,6-9H,3,5H2,(H,21,23,24)(H2,22,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM26315
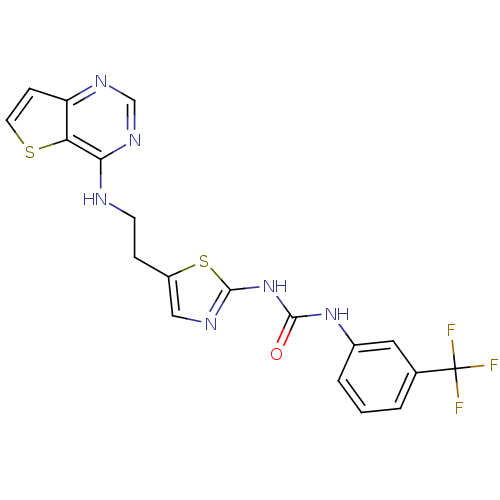
(3-[5-(2-{thieno[3,2-d]pyrimidin-4-ylamino}ethyl)-1...)Show SMILES FC(F)(F)c1cccc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)c1 Show InChI InChI=1S/C19H15F3N6OS2/c20-19(21,22)11-2-1-3-12(8-11)27-17(29)28-18-24-9-13(31-18)4-6-23-16-15-14(5-7-30-15)25-10-26-16/h1-3,5,7-10H,4,6H2,(H,23,25,26)(H2,24,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B after 60 mins |
Bioorg Med Chem Lett 19: 5158-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.016
BindingDB Entry DOI: 10.7270/Q2T72HK2 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM26315

(3-[5-(2-{thieno[3,2-d]pyrimidin-4-ylamino}ethyl)-1...)Show SMILES FC(F)(F)c1cccc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)c1 Show InChI InChI=1S/C19H15F3N6OS2/c20-19(21,22)11-2-1-3-12(8-11)27-17(29)28-18-24-9-13(31-18)4-6-23-16-15-14(5-7-30-15)25-10-26-16/h1-3,5,7-10H,4,6H2,(H,23,25,26)(H2,24,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50310621

(CHEMBL1079538 | N-(4-(6-(trifluoromethyl)-3H-imida...)Show SMILES FC(F)(F)c1cnc2nc(Nc3ccc(CCNc4ncnc5ccsc45)cc3)[nH]c2c1 Show InChI InChI=1S/C21H16F3N7S/c22-21(23,24)13-9-16-18(26-10-13)31-20(30-16)29-14-3-1-12(2-4-14)5-7-25-19-17-15(6-8-32-17)27-11-28-19/h1-4,6,8-11H,5,7H2,(H,25,27,28)(H2,26,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A after 60 mins |
Bioorg Med Chem Lett 19: 5158-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.016
BindingDB Entry DOI: 10.7270/Q2T72HK2 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM26325
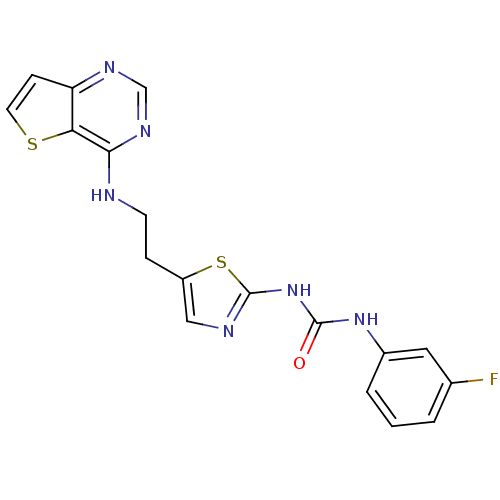
(1-(3-fluorophenyl)-3-[5-(2-{thieno[3,2-d]pyrimidin...)Show SMILES Fc1cccc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)c1 Show InChI InChI=1S/C18H15FN6OS2/c19-11-2-1-3-12(8-11)24-17(26)25-18-21-9-13(28-18)4-6-20-16-15-14(5-7-27-15)22-10-23-16/h1-3,5,7-10H,4,6H2,(H,20,22,23)(H2,21,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM26331
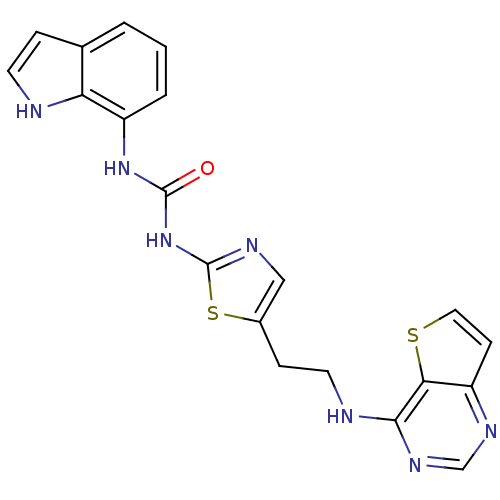
(1-1H-indol-7-yl-3-[5-(2-{thieno[3,2-d]pyrimidin-4-...)Show SMILES O=C(Nc1ncc(CCNc2ncnc3ccsc23)s1)Nc1cccc2cc[nH]c12 Show InChI InChI=1S/C20H17N7OS2/c28-19(26-14-3-1-2-12-4-7-21-16(12)14)27-20-23-10-13(30-20)5-8-22-18-17-15(6-9-29-17)24-11-25-18/h1-4,6-7,9-11,21H,5,8H2,(H,22,24,25)(H2,23,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50310620

(CHEMBL1079713 | N-(4-(6-chloro-3H-imidazo[4,5-b]py...)Show SMILES Clc1cnc2nc(Nc3ccc(CCNc4ncnc5ccsc45)cc3)[nH]c2c1 Show InChI InChI=1S/C20H16ClN7S/c21-13-9-16-18(23-10-13)28-20(27-16)26-14-3-1-12(2-4-14)5-7-22-19-17-15(6-8-29-17)24-11-25-19/h1-4,6,8-11H,5,7H2,(H,22,24,25)(H2,23,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A after 60 mins |
Bioorg Med Chem Lett 19: 5158-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.016
BindingDB Entry DOI: 10.7270/Q2T72HK2 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50310622
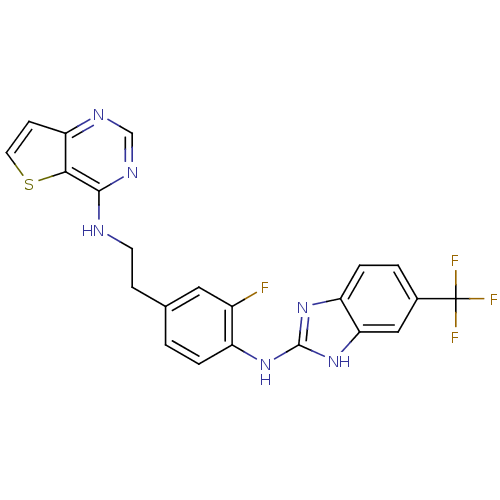
(CHEMBL1078061 | N-(3-fluoro-4-(5-(trifluoromethyl)...)Show SMILES Fc1cc(CCNc2ncnc3ccsc23)ccc1Nc1nc2ccc(cc2[nH]1)C(F)(F)F Show InChI InChI=1S/C22H16F4N6S/c23-14-9-12(5-7-27-20-19-17(6-8-33-19)28-11-29-20)1-3-15(14)30-21-31-16-4-2-13(22(24,25)26)10-18(16)32-21/h1-4,6,8-11H,5,7H2,(H,27,28,29)(H2,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B after 60 mins |
Bioorg Med Chem Lett 19: 5158-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.016
BindingDB Entry DOI: 10.7270/Q2T72HK2 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Mus musculus (mouse)) | BDBM26315

(3-[5-(2-{thieno[3,2-d]pyrimidin-4-ylamino}ethyl)-1...)Show SMILES FC(F)(F)c1cccc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)c1 Show InChI InChI=1S/C19H15F3N6OS2/c20-19(21,22)11-2-1-3-12(8-11)27-17(29)28-18-24-9-13(31-18)4-6-23-16-15-14(5-7-30-15)25-10-26-16/h1-3,5,7-10H,4,6H2,(H,23,25,26)(H2,24,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.2 | 21 |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase B
(Homo sapiens (Human)) | BDBM26333

(1-[3-(pyrrolidin-1-ylmethyl)-5-(trifluoromethyl)ph...)Show SMILES FC(F)(F)c1cc(CN2CCCC2)cc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)c1 Show InChI InChI=1S/C24H24F3N7OS2/c25-24(26,27)16-9-15(13-34-6-1-2-7-34)10-17(11-16)32-22(35)33-23-29-12-18(37-23)3-5-28-21-20-19(4-8-36-20)30-14-31-21/h4,8-12,14H,1-3,5-7,13H2,(H,28,30,31)(H2,29,32,33,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM26315

(3-[5-(2-{thieno[3,2-d]pyrimidin-4-ylamino}ethyl)-1...)Show SMILES FC(F)(F)c1cccc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)c1 Show InChI InChI=1S/C19H15F3N6OS2/c20-19(21,22)11-2-1-3-12(8-11)27-17(29)28-18-24-9-13(31-18)4-6-23-16-15-14(5-7-30-15)25-10-26-16/h1-3,5,7-10H,4,6H2,(H,23,25,26)(H2,24,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A after 60 mins |
Bioorg Med Chem Lett 19: 5158-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.016
BindingDB Entry DOI: 10.7270/Q2T72HK2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Mus musculus (mouse)) | BDBM26325

(1-(3-fluorophenyl)-3-[5-(2-{thieno[3,2-d]pyrimidin...)Show SMILES Fc1cccc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)c1 Show InChI InChI=1S/C18H15FN6OS2/c19-11-2-1-3-12(8-11)24-17(26)25-18-21-9-13(28-18)4-6-20-16-15-14(5-7-27-15)22-10-23-16/h1-3,5,7-10H,4,6H2,(H,20,22,23)(H2,21,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.2 | 21 |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50310607

(CHEMBL1078060 | N-(4-(5-chloro-1H-benzo[d]imidazol...)Show SMILES Clc1ccc2nc(Nc3ccc(CCNc4ncnc5ccsc45)cc3)[nH]c2c1 Show InChI InChI=1S/C21H17ClN6S/c22-14-3-6-16-18(11-14)28-21(27-16)26-15-4-1-13(2-5-15)7-9-23-20-19-17(8-10-29-19)24-12-25-20/h1-6,8,10-12H,7,9H2,(H,23,24,25)(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A after 60 mins |
Bioorg Med Chem Lett 19: 5158-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.016
BindingDB Entry DOI: 10.7270/Q2T72HK2 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50310614

(CHEMBL1078906 | N-(4-(1H-benzo[d]imidazol-2-ylamin...)Show SMILES C(Cc1ccc(Nc2nc3ccccc3[nH]2)cc1)Nc1ncnc2ccsc12 Show InChI InChI=1S/C21H18N6S/c1-2-4-17-16(3-1)26-21(27-17)25-15-7-5-14(6-8-15)9-11-22-20-19-18(10-12-28-19)23-13-24-20/h1-8,10,12-13H,9,11H2,(H,22,23,24)(H2,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A after 60 mins |
Bioorg Med Chem Lett 19: 5158-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.016
BindingDB Entry DOI: 10.7270/Q2T72HK2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data