

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50527134 (CHEMBL4471306 | US20230295213, Compound a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human CD73 | J Med Chem 63: 2941-2957 (2020) Article DOI: 10.1021/acs.jmedchem.9b01611 BindingDB Entry DOI: 10.7270/Q2NS0ZBH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50527135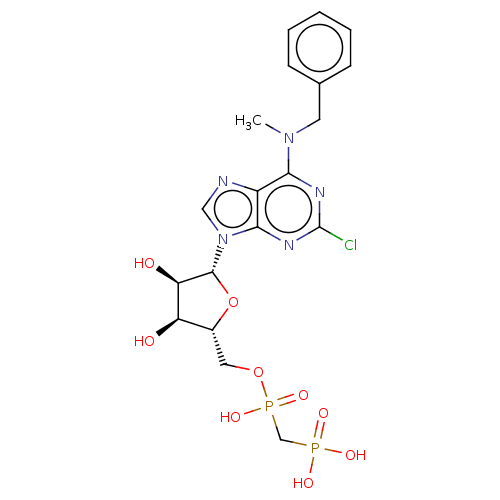 (CHEMBL4452072) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.318 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human CD73 | J Med Chem 63: 2941-2957 (2020) Article DOI: 10.1021/acs.jmedchem.9b01611 BindingDB Entry DOI: 10.7270/Q2NS0ZBH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50527135 (CHEMBL4452072) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.381 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human C-terminal His-tagged CD73 (27 to 549 residues) expressed in Sf9 cells using [2,8-3H]-AMP as substrate incubated for ... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00391 BindingDB Entry DOI: 10.7270/Q2CF9TTM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50527135 (CHEMBL4452072) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.746 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat CD73 | J Med Chem 63: 2941-2957 (2020) Article DOI: 10.1021/acs.jmedchem.9b01611 BindingDB Entry DOI: 10.7270/Q2NS0ZBH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50527135 (CHEMBL4452072) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.746 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant rat CD73 expressed in Sf9 cells using [2,8-3H]-AMP as substrate incubated for 25 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00391 BindingDB Entry DOI: 10.7270/Q2CF9TTM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50378656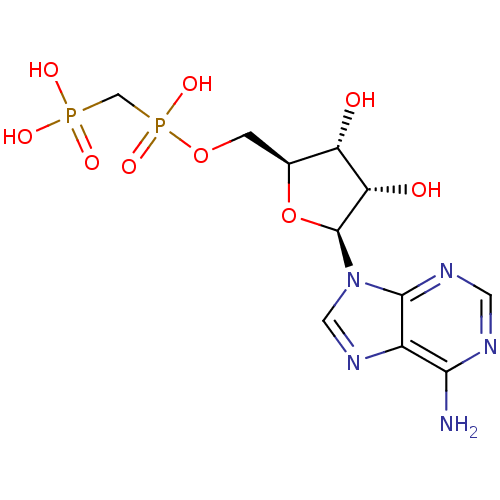 (CHEMBL598619) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat ecto-5'-nucleotidase expressed in Sf9 cells by capillary electrophoresis method | J Med Chem 53: 2076-86 (2010) Article DOI: 10.1021/jm901851t BindingDB Entry DOI: 10.7270/Q2DZ097V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50237495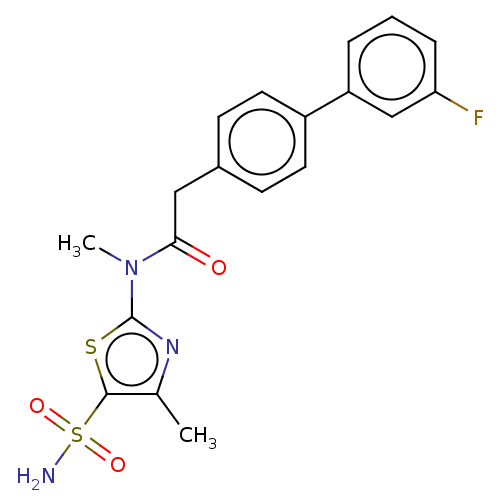 (CHEMBL4092504) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human Carbonic anhydrase 9 assessed as reduction in CO2 hydration preincubated for 15 mins prior to testing measured for 10... | J Med Chem 60: 3154-3164 (2017) Article DOI: 10.1021/acs.jmedchem.7b00183 BindingDB Entry DOI: 10.7270/Q2CF9SCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50237365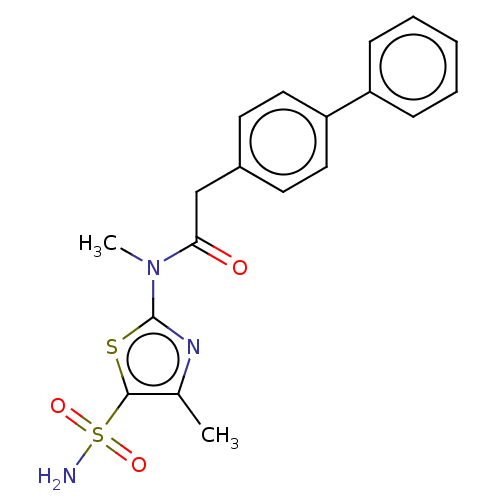 (CHEMBL4090611) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human Carbonic anhydrase 2 assessed as reduction in CO2 hydration preincubated for 15 mins prior to testing measured for 10... | J Med Chem 60: 3154-3164 (2017) Article DOI: 10.1021/acs.jmedchem.7b00183 BindingDB Entry DOI: 10.7270/Q2CF9SCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50237365 (CHEMBL4090611) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human Carbonic anhydrase 9 assessed as reduction in CO2 hydration preincubated for 15 mins prior to testing measured for 10... | J Med Chem 60: 3154-3164 (2017) Article DOI: 10.1021/acs.jmedchem.7b00183 BindingDB Entry DOI: 10.7270/Q2CF9SCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50368125 (ADENOSINE DIPHOSPHATE | ADP) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat ecto-5'-nucleotidase expressed in Sf9 cells by capillary electrophoresis method | J Med Chem 53: 2076-86 (2010) Article DOI: 10.1021/jm901851t BindingDB Entry DOI: 10.7270/Q2DZ097V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50237369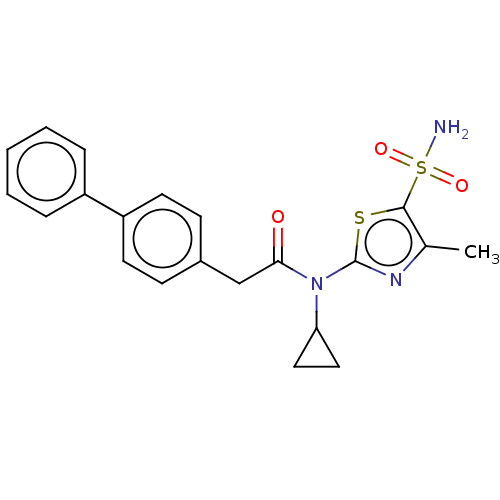 (CHEMBL4084758) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human Carbonic anhydrase 9 assessed as reduction in CO2 hydration preincubated for 15 mins prior to testing measured for 10... | J Med Chem 60: 3154-3164 (2017) Article DOI: 10.1021/acs.jmedchem.7b00183 BindingDB Entry DOI: 10.7270/Q2CF9SCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50237363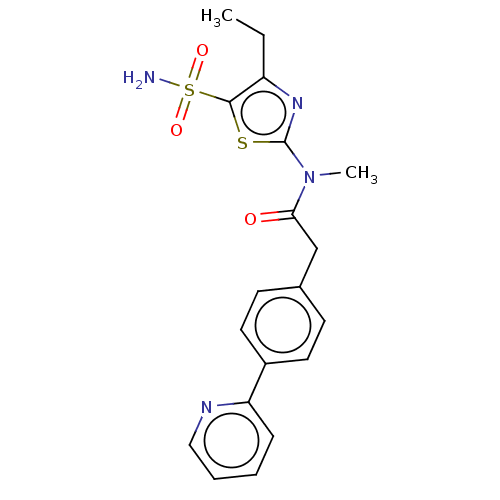 (CHEMBL4100366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human Carbonic anhydrase 2 assessed as reduction in CO2 hydration preincubated for 15 mins prior to testing measured for 10... | J Med Chem 60: 3154-3164 (2017) Article DOI: 10.1021/acs.jmedchem.7b00183 BindingDB Entry DOI: 10.7270/Q2CF9SCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50561892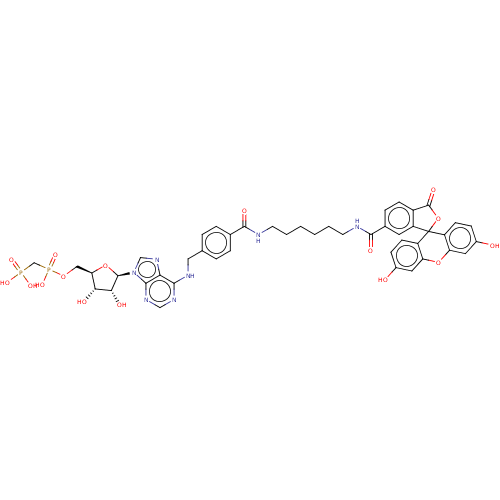 (CHEMBL4795486) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human C-terminal His-tagged CD73 (27 to 549 residues) expressed in Sf9 cells using [2,8-3H]-AMP as substrate incubated for ... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00391 BindingDB Entry DOI: 10.7270/Q2CF9TTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50237369 (CHEMBL4084758) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of Dexamethasone binding to Glucocorticoid receptor | J Med Chem 60: 3154-3164 (2017) Article DOI: 10.1021/acs.jmedchem.7b00183 BindingDB Entry DOI: 10.7270/Q2CF9SCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50527131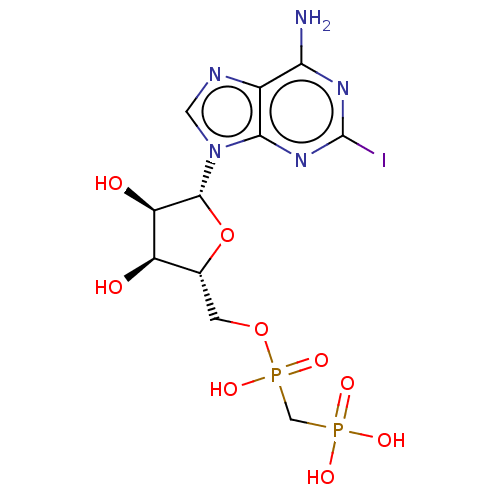 (CHEMBL4585872) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of native CD73 in human MDA-MB-231 cell membrane preparations [3H]AMP as substrate incubated for 25 mins by scintillation counting method | J Med Chem 63: 2941-2957 (2020) Article DOI: 10.1021/acs.jmedchem.9b01611 BindingDB Entry DOI: 10.7270/Q2NS0ZBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50527131 (CHEMBL4585872) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of purified recombinant soluble human CD73 expressed in Sf9 cells [3H]AMP as substrate incubated for 25 mins by scintillation counting met... | J Med Chem 63: 2941-2957 (2020) Article DOI: 10.1021/acs.jmedchem.9b01611 BindingDB Entry DOI: 10.7270/Q2NS0ZBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50523511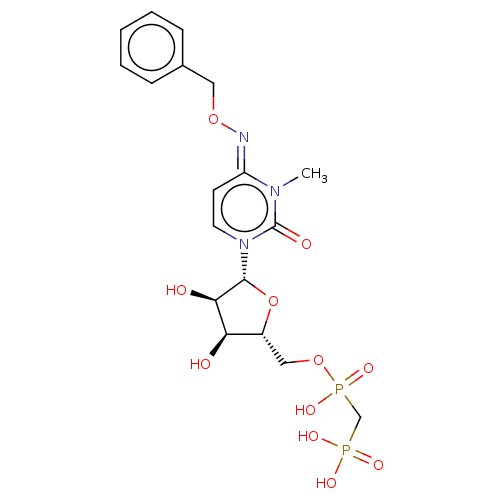 (CHEMBL4443977) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50237372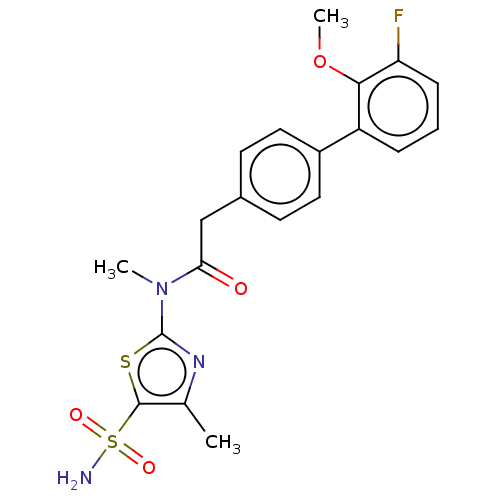 (CHEMBL4089153) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Compound was tested for inhibition of [3H]5-HT binding to Serotonin transporter in HEK cells | J Med Chem 60: 3154-3164 (2017) Article DOI: 10.1021/acs.jmedchem.7b00183 BindingDB Entry DOI: 10.7270/Q2CF9SCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50523526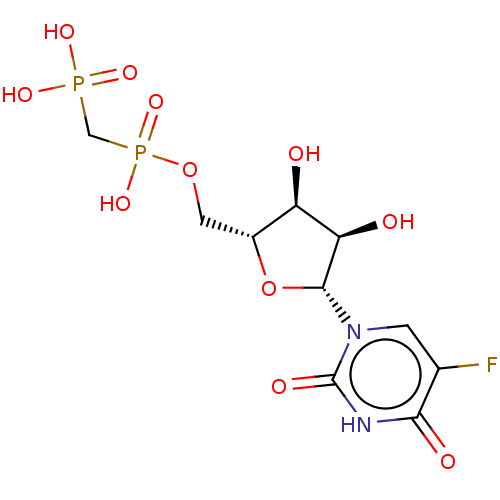 (CHEMBL3606064) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of CD73 in human MDA-MB-231 cell membranes using [2,8-3H]AMP as substrate measured after 25 mins by scintillation counting method | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50561892 (CHEMBL4795486) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CD73 in human MDA-MB-231 cells using [2,8-3H]-AMP as substrate incubated for 25 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00391 BindingDB Entry DOI: 10.7270/Q2CF9TTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50527138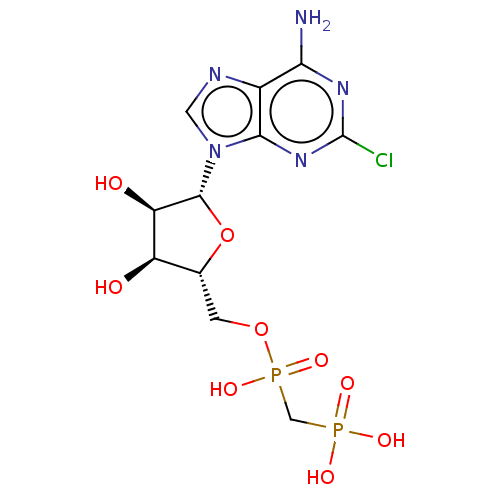 (CHEMBL4475460) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of native CD73 in human MDA-MB-231 cell membrane preparations [3H]AMP as substrate incubated for 25 mins by scintillation counting method | J Med Chem 63: 2941-2957 (2020) Article DOI: 10.1021/acs.jmedchem.9b01611 BindingDB Entry DOI: 10.7270/Q2NS0ZBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50523510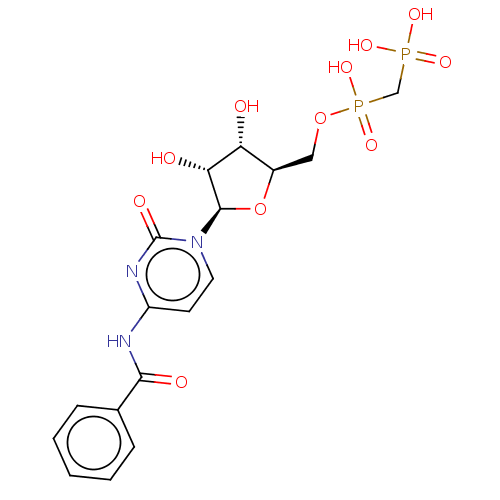 (CHEMBL4483379) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as su... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50237370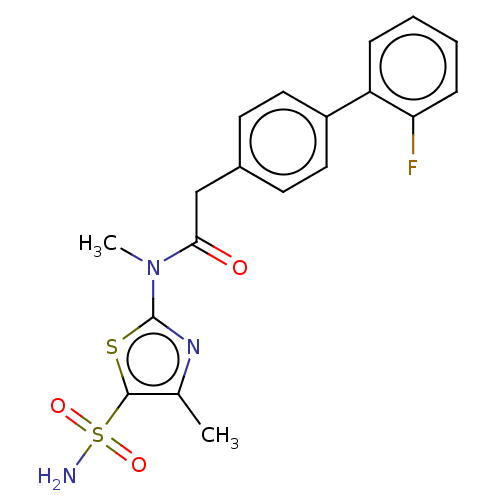 (CHEMBL4071722) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human Carbonic anhydrase 2 assessed as reduction in CO2 hydration preincubated for 15 mins prior to testing measured for 10... | J Med Chem 60: 3154-3164 (2017) Article DOI: 10.1021/acs.jmedchem.7b00183 BindingDB Entry DOI: 10.7270/Q2CF9SCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50237365 (CHEMBL4090611) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human Carbonic anhydrase 12 assessed as reduction in CO2 hydration preincubated for 15 mins prior to testing measured for 1... | J Med Chem 60: 3154-3164 (2017) Article DOI: 10.1021/acs.jmedchem.7b00183 BindingDB Entry DOI: 10.7270/Q2CF9SCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50523526 (CHEMBL3606064) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as su... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50237495 (CHEMBL4092504) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human Carbonic anhydrase 12 assessed as reduction in CO2 hydration preincubated for 15 mins prior to testing measured for 1... | J Med Chem 60: 3154-3164 (2017) Article DOI: 10.1021/acs.jmedchem.7b00183 BindingDB Entry DOI: 10.7270/Q2CF9SCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human Carbonic anhydrase 12 assessed as reduction in CO2 hydration preincubated for 15 mins prior to testing measured for 1... | J Med Chem 60: 3154-3164 (2017) Article DOI: 10.1021/acs.jmedchem.7b00183 BindingDB Entry DOI: 10.7270/Q2CF9SCC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50523510 (CHEMBL4483379) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of CD73 in human MDA-MB-231 cell membranes using [2,8-3H]AMP as substrate measured after 25 mins by scintillation counting method | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50527138 (CHEMBL4475460) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of purified recombinant soluble human CD73 expressed in Sf9 cells [3H]AMP as substrate incubated for 25 mins by scintillation counting met... | J Med Chem 63: 2941-2957 (2020) Article DOI: 10.1021/acs.jmedchem.9b01611 BindingDB Entry DOI: 10.7270/Q2NS0ZBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50523527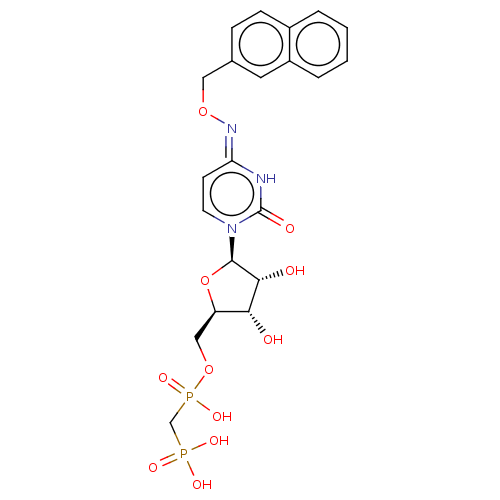 (CHEMBL4443094) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of CD73 in human MDA-MB-231 cell membranes using [2,8-3H]AMP as substrate measured after 25 mins by scintillation counting method | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50523527 (CHEMBL4443094) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as su... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50523511 (CHEMBL4443977) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of CD73 in human MDA-MB-231 cell membranes using [2,8-3H]AMP as substrate measured after 25 mins by scintillation counting method | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50237370 (CHEMBL4071722) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human Carbonic anhydrase 9 assessed as reduction in CO2 hydration preincubated for 15 mins prior to testing measured for 10... | J Med Chem 60: 3154-3164 (2017) Article DOI: 10.1021/acs.jmedchem.7b00183 BindingDB Entry DOI: 10.7270/Q2CF9SCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50237364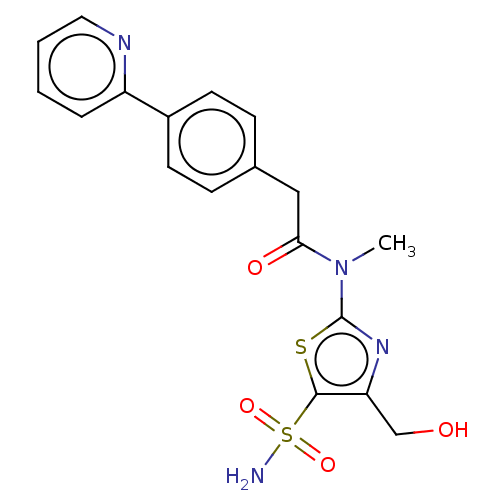 (CHEMBL4073528) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human Carbonic anhydrase 2 assessed as reduction in CO2 hydration preincubated for 15 mins prior to testing measured for 10... | J Med Chem 60: 3154-3164 (2017) Article DOI: 10.1021/acs.jmedchem.7b00183 BindingDB Entry DOI: 10.7270/Q2CF9SCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50523529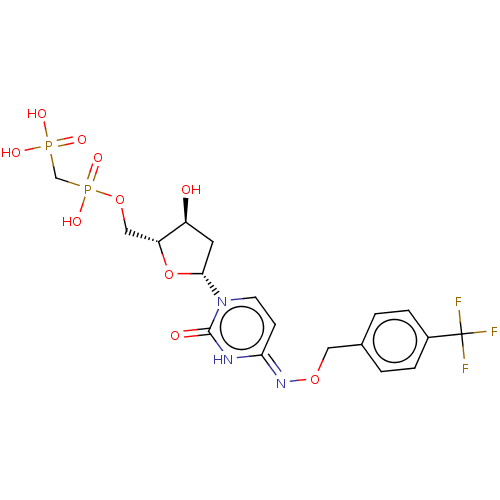 (CHEMBL4551648) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of CD73 in human MDA-MB-231 cell membranes using [2,8-3H]AMP as substrate measured after 25 mins by scintillation counting method | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50523511 (CHEMBL4443977) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as su... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human Carbonic anhydrase 2 assessed as reduction in CO2 hydration preincubated for 15 mins prior to testing measured for 10... | J Med Chem 60: 3154-3164 (2017) Article DOI: 10.1021/acs.jmedchem.7b00183 BindingDB Entry DOI: 10.7270/Q2CF9SCC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50237366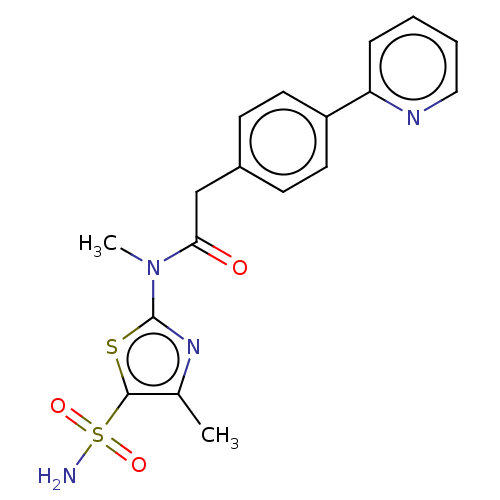 (CHEMBL4069597) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human Carbonic anhydrase 2 assessed as reduction in CO2 hydration preincubated for 15 mins prior to testing measured for 10... | J Med Chem 60: 3154-3164 (2017) Article DOI: 10.1021/acs.jmedchem.7b00183 BindingDB Entry DOI: 10.7270/Q2CF9SCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50561893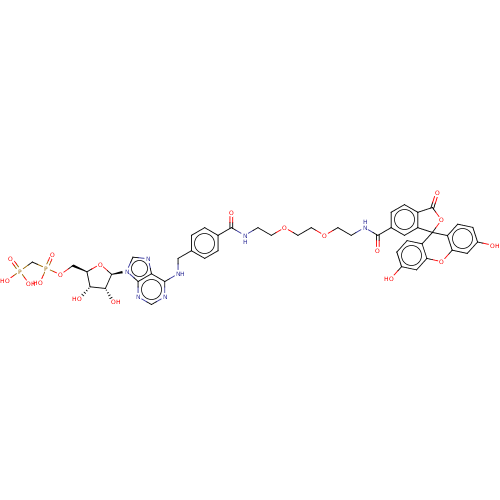 (CHEMBL4761798) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human C-terminal His-tagged CD73 (27 to 549 residues) expressed in Sf9 cells using [2,8-3H]-AMP as substrate incubated for ... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00391 BindingDB Entry DOI: 10.7270/Q2CF9TTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50237361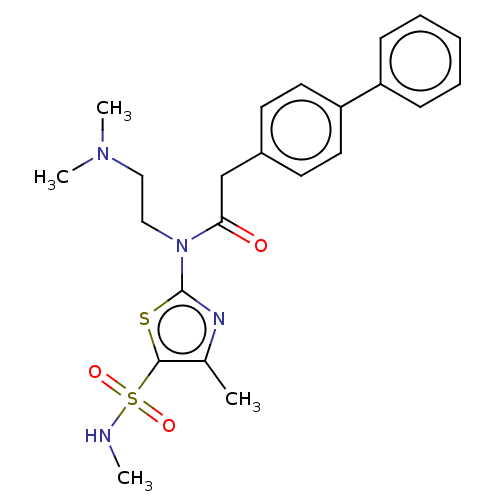 (CHEMBL4102820) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human Carbonic anhydrase 9 assessed as reduction in CO2 hydration preincubated for 15 mins prior to testing measured for 10... | J Med Chem 60: 3154-3164 (2017) Article DOI: 10.1021/acs.jmedchem.7b00183 BindingDB Entry DOI: 10.7270/Q2CF9SCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50237366 (CHEMBL4069597) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human Carbonic anhydrase 2 assessed as reduction in CO2 hydration preincubated for 15 mins prior to testing measured for 10... | J Med Chem 60: 3154-3164 (2017) Article DOI: 10.1021/acs.jmedchem.7b00183 BindingDB Entry DOI: 10.7270/Q2CF9SCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50523529 (CHEMBL4551648) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as su... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50523510 (CHEMBL4483379) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50527139 (CHEMBL4591580) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of native CD73 in human MDA-MB-231 cell membrane preparations [3H]AMP as substrate incubated for 25 mins by scintillation counting method | J Med Chem 63: 2941-2957 (2020) Article DOI: 10.1021/acs.jmedchem.9b01611 BindingDB Entry DOI: 10.7270/Q2NS0ZBH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50527131 (CHEMBL4585872) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of purified recombinant soluble rat CD73 expressed in Sf9 cells [3H]AMP as substrate incubated for 25 mins by scintillation counting metho... | J Med Chem 63: 2941-2957 (2020) Article DOI: 10.1021/acs.jmedchem.9b01611 BindingDB Entry DOI: 10.7270/Q2NS0ZBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50523526 (CHEMBL3606064) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50527139 (CHEMBL4591580) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of purified recombinant soluble human CD73 expressed in Sf9 cells [3H]AMP as substrate incubated for 25 mins by scintillation counting met... | J Med Chem 63: 2941-2957 (2020) Article DOI: 10.1021/acs.jmedchem.9b01611 BindingDB Entry DOI: 10.7270/Q2NS0ZBH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50237496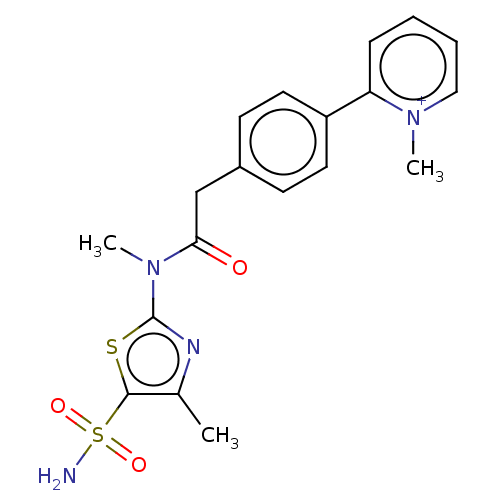 (CHEMBL4100256) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human Carbonic anhydrase 2 assessed as reduction in CO2 hydration preincubated for 15 mins prior to testing measured for 10... | J Med Chem 60: 3154-3164 (2017) Article DOI: 10.1021/acs.jmedchem.7b00183 BindingDB Entry DOI: 10.7270/Q2CF9SCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50523528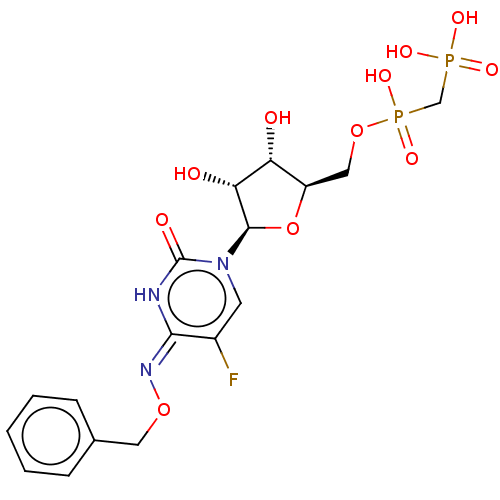 (CHEMBL4470616) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as su... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50523528 (CHEMBL4470616) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of CD73 in human MDA-MB-231 cell membranes using [2,8-3H]AMP as substrate measured after 25 mins by scintillation counting method | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 413 total ) | Next | Last >> |