 Found 1717 hits with Last Name = 'zonzini' and Initial = 'l'
Found 1717 hits with Last Name = 'zonzini' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417035

(CHEMBL1257993)Show SMILES Fc1ccccc1-c1cnc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3ccccn3)CC2)cn1 |r,wU:13.13,wD:16.22,(15.52,-7.53,;14.76,-6.18,;13.23,-6.17,;12.47,-4.82,;13.26,-3.49,;14.8,-3.52,;15.55,-4.86,;17.09,-4.88,;17.88,-3.56,;19.42,-3.58,;20.17,-4.93,;21.71,-4.96,;22.45,-6.3,;23.99,-6.33,;24.79,-5.01,;26.33,-5.03,;27.07,-6.38,;28.2,-5.34,;29.54,-6.12,;29.22,-7.62,;30.25,-8.77,;27.69,-7.78,;30.95,-5.5,;32.19,-6.4,;33.6,-5.78,;33.76,-4.25,;32.51,-3.34,;31.11,-3.97,;26.27,-7.7,;24.74,-7.67,;19.38,-6.25,;17.85,-6.23,)| Show InChI InChI=1S/C24H24FN5O2/c25-19-6-2-1-5-18(19)20-14-29-21(15-27-20)28-13-17-8-10-24(11-9-17)16-30(23(31)32-24)22-7-3-4-12-26-22/h1-7,12,14-15,17H,8-11,13,16H2,(H,28,29)/t17-,24- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417033
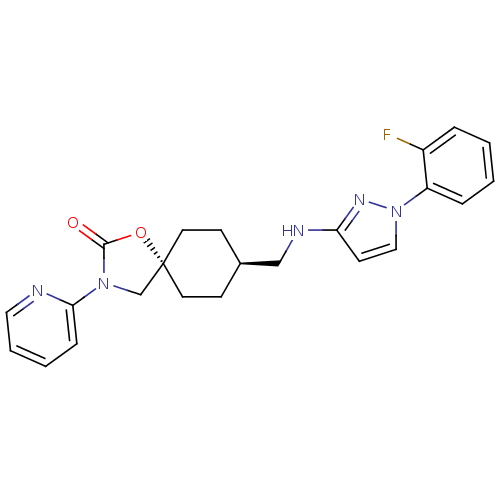
(CHEMBL1258111)Show SMILES Fc1ccccc1-n1ccc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3ccccn3)CC2)n1 |r,wU:13.13,wD:16.22,(15.91,-.57,;14.51,.08,;13.26,-.81,;11.85,-.16,;11.72,1.37,;12.98,2.26,;14.38,1.6,;15.63,2.49,;15.65,4.03,;17.12,4.48,;18.01,3.23,;19.55,3.2,;20.3,1.86,;21.84,1.83,;22.63,3.15,;24.17,3.13,;24.91,1.78,;26.05,2.82,;27.39,2.04,;27.06,.54,;28.09,-.61,;25.53,.38,;28.79,2.66,;30.04,1.75,;31.44,2.38,;31.61,3.91,;30.35,4.82,;28.95,4.19,;24.12,.46,;22.59,.49,;17.08,1.99,)| Show InChI InChI=1S/C23H24FN5O2/c24-18-5-1-2-6-19(18)29-14-10-20(27-29)26-15-17-8-11-23(12-9-17)16-28(22(30)31-23)21-7-3-4-13-25-21/h1-7,10,13-14,17H,8-9,11-12,15-16H2,(H,26,27)/t17-,23- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417056
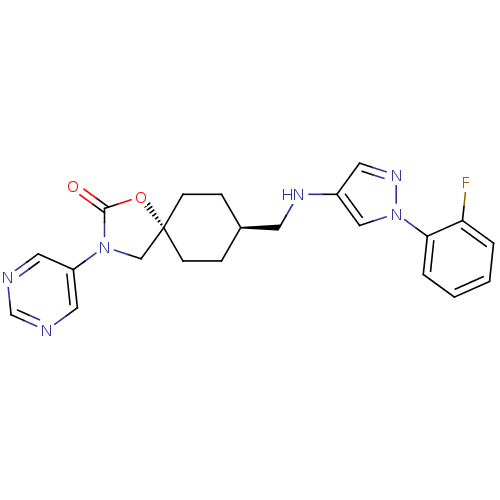
(CHEMBL1258225)Show SMILES Fc1ccccc1-n1cc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cncnc3)CC2)cn1 |r,wU:12.12,wD:15.21,(-5.79,-9.02,;-7.19,-8.37,;-8.44,-9.26,;-9.85,-8.61,;-9.98,-7.07,;-8.73,-6.19,;-7.33,-6.84,;-6.08,-5.96,;-4.62,-6.46,;-3.69,-5.22,;-2.15,-5.25,;-1.4,-6.59,;.14,-6.62,;.94,-5.3,;2.48,-5.31,;3.21,-6.67,;4.35,-5.63,;5.69,-6.41,;5.37,-7.91,;6.4,-9.06,;3.84,-8.08,;7.1,-5.79,;8.34,-6.69,;9.75,-6.07,;9.91,-4.54,;8.66,-3.63,;7.26,-4.26,;2.42,-7.99,;.89,-7.96,;-4.58,-3.96,;-6.05,-4.42,)| Show InChI InChI=1S/C22H23FN6O2/c23-19-3-1-2-4-20(19)29-13-17(10-27-29)26-9-16-5-7-22(8-6-16)14-28(21(30)31-22)18-11-24-15-25-12-18/h1-4,10-13,15-16,26H,5-9,14H2/t16-,22- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50408664
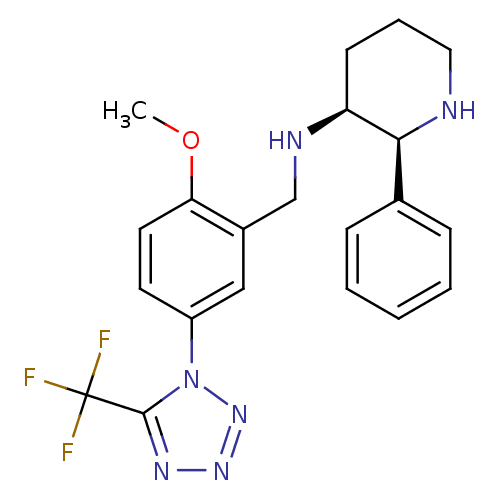
(GR-205171 | VOFOPITANT)Show SMILES COc1ccc(cc1CN[C@H]1CCCN[C@H]1c1ccccc1)-n1nnnc1C(F)(F)F Show InChI InChI=1S/C21H23F3N6O/c1-31-18-10-9-16(30-20(21(22,23)24)27-28-29-30)12-15(18)13-26-17-8-5-11-25-19(17)14-6-3-2-4-7-14/h2-4,6-7,9-10,12,17,19,25-26H,5,8,11,13H2,1H3/t17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human recombinant NK1 receptor expressed in CHO cells |
J Med Chem 52: 3238-47 (2009)
Article DOI: 10.1021/jm900023b
BindingDB Entry DOI: 10.7270/Q2BP0425 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413549
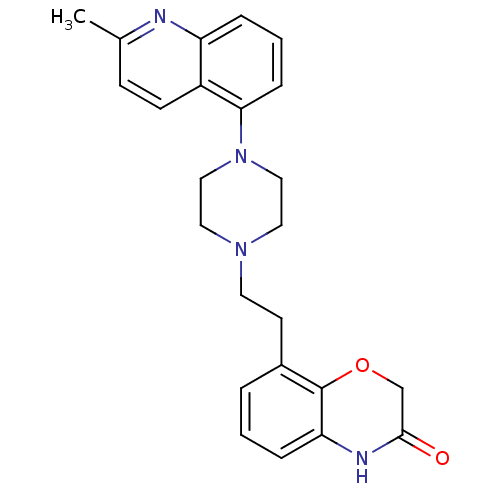
(CHEMBL513715)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc3NC(=O)COc23)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-8-9-19-20(25-17)5-3-7-22(19)28-14-12-27(13-15-28)11-10-18-4-2-6-21-24(18)30-16-23(29)26-21/h2-9H,10-16H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413549

(CHEMBL513715)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc3NC(=O)COc23)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-8-9-19-20(25-17)5-3-7-22(19)28-14-12-27(13-15-28)11-10-18-4-2-6-21-24(18)30-16-23(29)26-21/h2-9H,10-16H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1D assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50412441
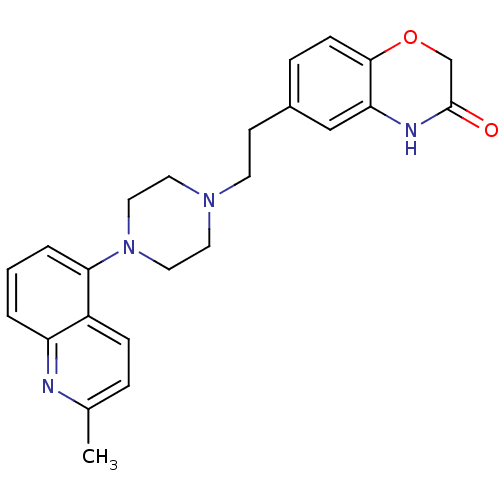
(CHEMBL490417 | SB-744185)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-5-7-19-20(25-17)3-2-4-22(19)28-13-11-27(12-14-28)10-9-18-6-8-23-21(15-18)26-24(29)16-30-23/h2-8,15H,9-14,16H2,1H3,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1A assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417051

(CHEMBL1258787)Show SMILES Cc1ccccc1-c1ccc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cccnn3)CC2)nn1 |r,wU:13.13,wD:16.22,(17.45,-6.84,;16.7,-5.5,;15.16,-5.48,;14.41,-4.13,;15.2,-2.81,;16.74,-2.83,;17.48,-4.18,;19.03,-4.2,;19.78,-5.55,;21.32,-5.57,;22.1,-4.25,;23.64,-4.27,;24.39,-5.62,;25.93,-5.64,;26.72,-4.32,;28.26,-4.34,;29,-5.69,;30.14,-4.66,;31.48,-5.43,;31.16,-6.94,;32.19,-8.08,;29.62,-7.1,;32.88,-4.81,;34.13,-5.72,;35.54,-5.1,;35.7,-3.56,;34.44,-2.66,;33.04,-3.29,;28.21,-7.01,;26.68,-6.98,;21.36,-2.9,;19.82,-2.87,)| Show InChI InChI=1S/C24H26N6O2/c1-17-5-2-3-6-19(17)20-8-9-21(28-27-20)25-15-18-10-12-24(13-11-18)16-30(23(31)32-24)22-7-4-14-26-29-22/h2-9,14,18H,10-13,15-16H2,1H3,(H,25,28)/t18-,24- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417045

(CHEMBL1258341)Show SMILES Fc1ccccc1-n1cc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cnccn3)CC2)cn1 |r,wU:12.12,wD:15.21,(-6.54,-17.19,;-7.94,-16.54,;-9.19,-17.43,;-10.59,-16.78,;-10.73,-15.24,;-9.48,-14.36,;-8.08,-15.01,;-6.82,-14.13,;-5.37,-14.63,;-4.44,-13.39,;-2.9,-13.41,;-2.15,-14.76,;-.61,-14.79,;.19,-13.47,;1.73,-13.48,;2.46,-14.84,;3.6,-13.8,;4.94,-14.58,;4.62,-16.08,;5.64,-17.23,;3.09,-16.24,;6.35,-13.96,;7.58,-14.86,;8.99,-14.24,;9.15,-12.71,;7.91,-11.8,;6.5,-12.43,;1.67,-16.16,;.14,-16.13,;-5.33,-12.13,;-6.8,-12.59,)| Show InChI InChI=1S/C22H23FN6O2/c23-18-3-1-2-4-19(18)29-14-17(12-27-29)26-11-16-5-7-22(8-6-16)15-28(21(30)31-22)20-13-24-9-10-25-20/h1-4,9-10,12-14,16,26H,5-8,11,15H2/t16-,22- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417036

(CHEMBL1257992)Show SMILES Fc1ccccc1-c1ccc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3ccccn3)CC2)nn1 |r,wU:13.13,wD:16.22,(-7.93,-14.34,;-8.69,-13,;-10.22,-12.98,;-10.98,-11.63,;-10.19,-10.31,;-8.65,-10.33,;-7.9,-11.68,;-6.36,-11.69,;-5.6,-13.04,;-4.07,-13.06,;-3.28,-11.75,;-1.74,-11.77,;-1,-13.12,;.54,-13.14,;1.34,-11.82,;2.88,-11.84,;3.62,-13.19,;4.75,-12.16,;6.09,-12.93,;5.77,-14.44,;6.8,-15.58,;4.24,-14.6,;7.5,-12.31,;8.74,-13.22,;10.15,-12.59,;10.31,-11.06,;9.06,-10.15,;7.66,-10.78,;2.82,-14.51,;1.29,-14.48,;-4.03,-10.4,;-5.57,-10.37,)| Show InChI InChI=1S/C24H24FN5O2/c25-19-6-2-1-5-18(19)20-8-9-21(29-28-20)27-15-17-10-12-24(13-11-17)16-30(23(31)32-24)22-7-3-4-14-26-22/h1-9,14,17H,10-13,15-16H2,(H,27,29)/t17-,24- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50192240
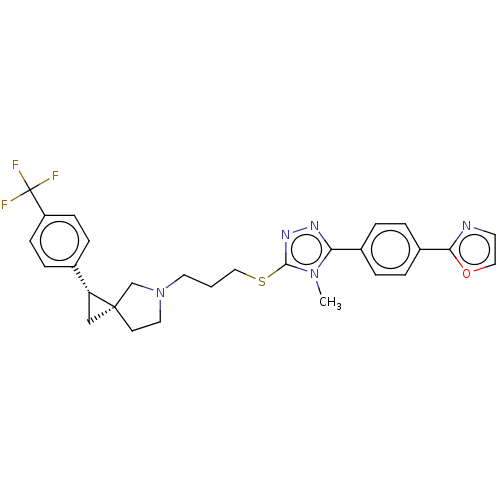
(CHEMBL3941818 | US10239870, Example 278)Show SMILES Cn1c(SCCCN2CC[C@]3(C[C@@H]3c3ccc(cc3)C(F)(F)F)C2)nnc1-c1ccc(cc1)-c1ncco1 |r| Show InChI InChI=1S/C28H28F3N5OS/c1-35-24(20-3-5-21(6-4-20)25-32-12-15-37-25)33-34-26(35)38-16-2-13-36-14-11-27(18-36)17-23(27)19-7-9-22(10-8-19)28(29,30)31/h3-10,12,15,23H,2,11,13-14,16-18H2,1H3/t23-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine D3 receptor expressed in CHO-K1 cell membranes after 90 mins by liquid scintillation counting |
J Med Chem 59: 8549-76 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00972
BindingDB Entry DOI: 10.7270/Q2SQ9298 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50192240

(CHEMBL3941818 | US10239870, Example 278)Show SMILES Cn1c(SCCCN2CC[C@]3(C[C@@H]3c3ccc(cc3)C(F)(F)F)C2)nnc1-c1ccc(cc1)-c1ncco1 |r| Show InChI InChI=1S/C28H28F3N5OS/c1-35-24(20-3-5-21(6-4-20)25-32-12-15-37-25)33-34-26(35)38-16-2-13-36-14-11-27(18-36)17-23(27)19-7-9-22(10-8-19)28(29,30)31/h3-10,12,15,23H,2,11,13-14,16-18H2,1H3/t23-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l.
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D3 receptor expressed in CHO cell membranes after 90 mins in presence of quinelorane by [35S]-GTPgammaS binding... |
J Med Chem 59: 8549-76 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00972
BindingDB Entry DOI: 10.7270/Q2SQ9298 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50192240

(CHEMBL3941818 | US10239870, Example 278)Show SMILES Cn1c(SCCCN2CC[C@]3(C[C@@H]3c3ccc(cc3)C(F)(F)F)C2)nnc1-c1ccc(cc1)-c1ncco1 |r| Show InChI InChI=1S/C28H28F3N5OS/c1-35-24(20-3-5-21(6-4-20)25-32-12-15-37-25)33-34-26(35)38-16-2-13-36-14-11-27(18-36)17-23(27)19-7-9-22(10-8-19)28(29,30)31/h3-10,12,15,23H,2,11,13-14,16-18H2,1H3/t23-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l.
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D3 receptor expressed in CHO cell membranes after 90 mins in presence of quinelorane by [35S]-GTPgammaS binding... |
J Med Chem 59: 8549-76 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00972
BindingDB Entry DOI: 10.7270/Q2SQ9298 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413077
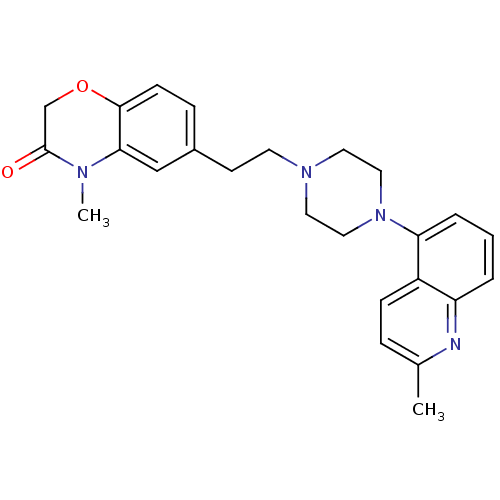
(CHEMBL522257)Show SMILES CN1C(=O)COc2ccc(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cc12 Show InChI InChI=1S/C25H28N4O2/c1-18-6-8-20-21(26-18)4-3-5-22(20)29-14-12-28(13-15-29)11-10-19-7-9-24-23(16-19)27(2)25(30)17-31-24/h3-9,16H,10-15,17H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1A assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417032

(CHEMBL1258110)Show SMILES O=C1O[C@@]2(CN1c1ccccn1)CC[C@H](CNc1nc(cs1)-c1ccccn1)CC2 |r,wU:14.16,wD:3.2,(5.76,.15,;4.73,1.3,;3.2,1.14,;2.57,2.54,;3.71,3.58,;5.05,2.8,;6.45,3.43,;7.7,2.52,;9.11,3.14,;9.27,4.67,;8.02,5.58,;6.61,4.95,;1.83,3.89,;.29,3.91,;-.5,2.59,;-2.04,2.62,;-2.79,3.96,;-4.33,3.99,;-5.25,2.75,;-6.71,3.25,;-6.69,4.79,;-5.21,5.24,;-7.96,2.37,;-7.82,.84,;-9.08,-.05,;-10.48,.6,;-10.62,2.14,;-9.36,3.02,;.25,1.25,;1.78,1.22,)| Show InChI InChI=1S/C22H23N5O2S/c28-21-27(19-6-2-4-12-24-19)15-22(29-21)9-7-16(8-10-22)13-25-20-26-18(14-30-20)17-5-1-3-11-23-17/h1-6,11-12,14,16H,7-10,13,15H2,(H,25,26)/t16-,22- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50417409

(CHEMBL1290487)Show SMILES CN1C(=O)CCc2c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C26H30N4O/c1-19-9-10-22-23(27-19)6-4-8-25(22)30-17-15-29(16-18-30)14-13-20-5-3-7-24-21(20)11-12-26(31)28(24)2/h3-10H,11-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413560
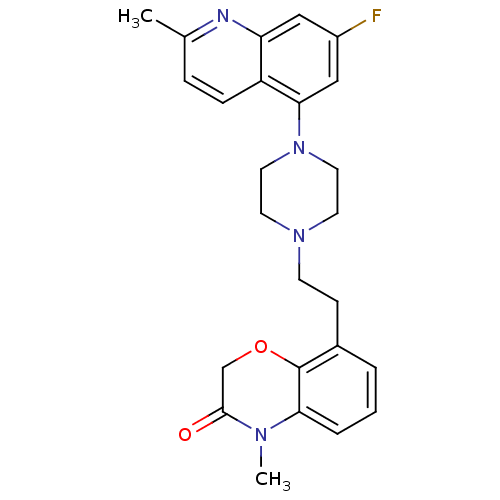
(CHEMBL469374)Show SMILES CN1C(=O)COc2c(CCN3CCN(CC3)c3cc(F)cc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C25H27FN4O2/c1-17-6-7-20-21(27-17)14-19(26)15-23(20)30-12-10-29(11-13-30)9-8-18-4-3-5-22-25(18)32-16-24(31)28(22)2/h3-7,14-15H,8-13,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1D assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417977
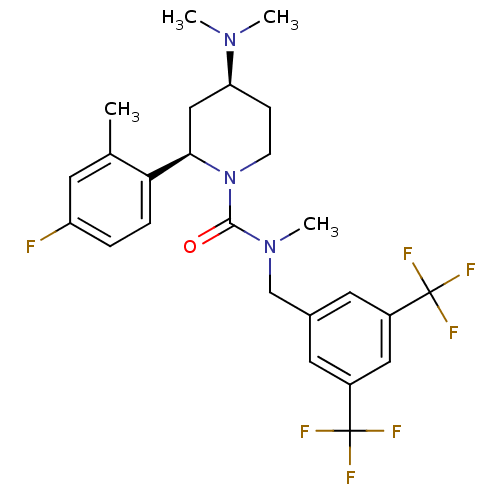
(CHEMBL1672044)Show SMILES CN(C)[C@H]1CCN([C@H](C1)c1ccc(F)cc1C)C(=O)N(C)Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C25H28F7N3O/c1-15-9-19(26)5-6-21(15)22-13-20(33(2)3)7-8-35(22)23(36)34(4)14-16-10-17(24(27,28)29)12-18(11-16)25(30,31)32/h5-6,9-12,20,22H,7-8,13-14H2,1-4H3/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417978
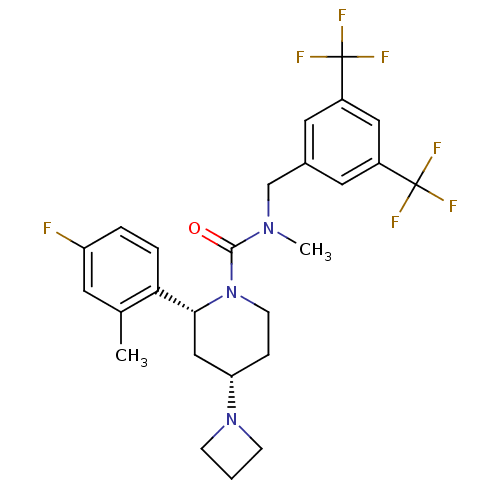
(CHEMBL1672047)Show SMILES CN(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N1CCC1 |r| Show InChI InChI=1S/C26H28F7N3O/c1-16-10-20(27)4-5-22(16)23-14-21(35-7-3-8-35)6-9-36(23)24(37)34(2)15-17-11-18(25(28,29)30)13-19(12-17)26(31,32)33/h4-5,10-13,21,23H,3,6-9,14-15H2,1-2H3/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0813 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413555
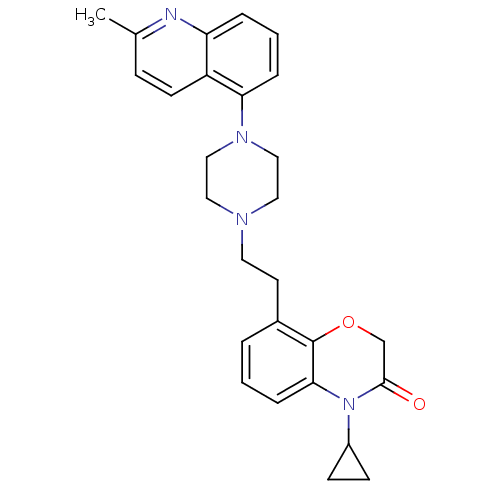
(CHEMBL469568)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc3N(C4CC4)C(=O)COc23)CC1 Show InChI InChI=1S/C27H30N4O2/c1-19-8-11-22-23(28-19)5-3-6-24(22)30-16-14-29(15-17-30)13-12-20-4-2-7-25-27(20)33-18-26(32)31(25)21-9-10-21/h2-8,11,21H,9-10,12-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1A assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413077

(CHEMBL522257)Show SMILES CN1C(=O)COc2ccc(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cc12 Show InChI InChI=1S/C25H28N4O2/c1-18-6-8-20-21(26-18)4-3-5-22(20)29-14-12-28(13-15-29)11-10-19-7-9-24-23(16-19)27(2)25(30)17-31-24/h3-9,16H,10-15,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1D assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417050

(CHEMBL1258674)Show SMILES Fc1cc(F)cc(c1)-c1ccc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cccnn3)CC2)nn1 |r,wU:14.14,wD:17.23,(-9.07,-1.62,;-8.32,-2.96,;-9.11,-4.29,;-8.36,-5.64,;-9.14,-6.96,;-6.82,-5.65,;-6.04,-4.34,;-6.78,-2.99,;-4.49,-4.35,;-3.74,-5.7,;-2.2,-5.72,;-1.42,-4.4,;.12,-4.43,;.87,-5.77,;2.41,-5.8,;3.2,-4.48,;4.74,-4.5,;5.48,-5.85,;6.62,-4.81,;7.96,-5.59,;7.64,-7.09,;8.67,-8.24,;6.1,-7.25,;9.36,-4.97,;10.61,-5.88,;12.02,-5.25,;12.18,-3.72,;10.92,-2.81,;9.52,-3.44,;4.69,-7.17,;3.16,-7.14,;-2.16,-3.06,;-3.7,-3.03,)| Show InChI InChI=1S/C23H22F2N6O2/c24-17-10-16(11-18(25)12-17)19-3-4-20(29-28-19)26-13-15-5-7-23(8-6-15)14-31(22(32)33-23)21-2-1-9-27-30-21/h1-4,9-12,15H,5-8,13-14H2,(H,26,29)/t15-,23- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417046

(CHEMBL1258453)Show SMILES Fc1ccccc1-n1cc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cccnn3)CC2)cn1 |r,wU:12.12,wD:15.21,(16.1,-16.72,;14.7,-16.07,;13.45,-16.97,;12.04,-16.31,;11.91,-14.78,;13.16,-13.9,;14.56,-14.55,;15.81,-13.67,;17.27,-14.16,;18.2,-12.92,;19.74,-12.95,;20.49,-14.3,;22.03,-14.32,;22.82,-13,;24.36,-13.02,;25.1,-14.37,;26.24,-13.33,;27.58,-14.11,;27.26,-15.62,;28.28,-16.76,;25.73,-15.78,;28.99,-13.49,;30.23,-14.4,;31.63,-13.78,;31.8,-12.24,;30.55,-11.33,;29.15,-11.97,;24.31,-15.69,;22.78,-15.66,;17.31,-11.67,;15.84,-12.13,)| Show InChI InChI=1S/C22H23FN6O2/c23-18-4-1-2-5-19(18)29-14-17(13-26-29)24-12-16-7-9-22(10-8-16)15-28(21(30)31-22)20-6-3-11-25-27-20/h1-6,11,13-14,16,24H,7-10,12,15H2/t16-,22- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50417420
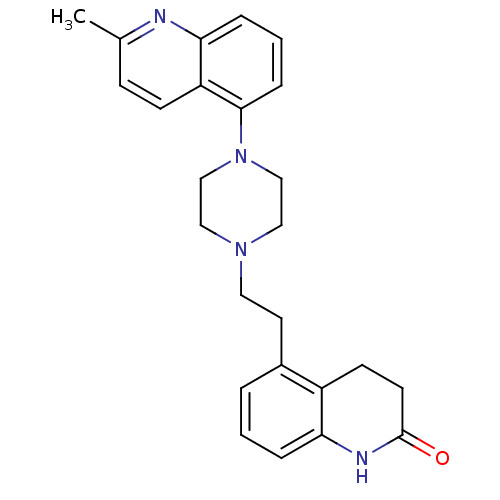
(CHEMBL1290486)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc3NC(=O)CCc23)CC1 Show InChI InChI=1S/C25H28N4O/c1-18-8-9-21-23(26-18)6-3-7-24(21)29-16-14-28(15-17-29)13-12-19-4-2-5-22-20(19)10-11-25(30)27-22/h2-9H,10-17H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417042

(CHEMBL1257636)Show SMILES Fc1ccccc1-n1cc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cccnc3)CC2)cn1 |r,wU:12.12,wD:15.21,(-7.01,-.66,;-8.41,-.01,;-9.65,-.9,;-11.06,-.25,;-11.21,1.29,;-9.94,2.17,;-8.54,1.52,;-7.29,2.4,;-5.83,1.9,;-4.9,3.14,;-3.36,3.12,;-2.62,1.77,;-1.08,1.74,;-.28,3.07,;1.26,3.05,;2,1.7,;3.14,2.73,;4.48,1.96,;4.16,.45,;5.18,-.7,;2.62,.29,;5.88,2.57,;7.12,1.67,;8.53,2.29,;8.69,3.82,;7.44,4.73,;6.04,4.1,;1.21,.37,;-.33,.41,;-5.79,4.4,;-7.27,3.94,)| Show InChI InChI=1S/C23H24FN5O2/c24-20-5-1-2-6-21(20)29-15-18(13-27-29)26-12-17-7-9-23(10-8-17)16-28(22(30)31-23)19-4-3-11-25-14-19/h1-6,11,13-15,17,26H,7-10,12,16H2/t17-,23- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417044
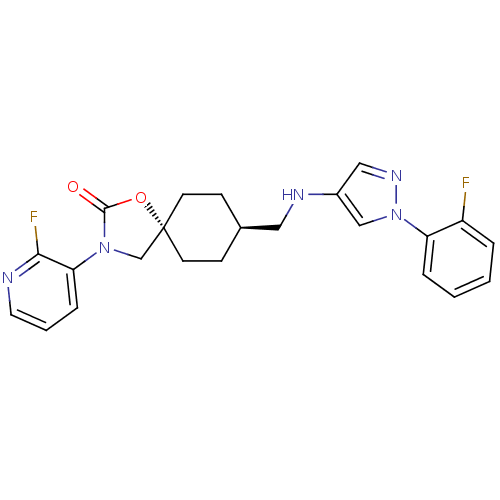
(CHEMBL1258340)Show SMILES Fc1ccccc1-n1cc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cccnc3F)CC2)cn1 |r,wU:12.12,wD:15.21,(17.14,-9.3,;15.74,-8.65,;14.5,-9.54,;13.09,-8.89,;12.95,-7.35,;14.21,-6.47,;15.61,-7.12,;16.86,-6.24,;18.32,-6.74,;19.25,-5.5,;20.79,-5.52,;21.54,-6.87,;23.08,-6.9,;23.87,-5.57,;25.41,-5.59,;26.15,-6.95,;27.29,-5.92,;28.63,-6.69,;28.31,-8.19,;29.34,-9.34,;26.78,-8.35,;30.04,-6.07,;31.28,-6.97,;32.69,-6.35,;32.85,-4.82,;31.6,-3.91,;30.2,-4.54,;28.95,-3.63,;25.36,-8.27,;23.83,-8.24,;18.36,-4.24,;16.89,-4.7,)| Show InChI InChI=1S/C23H23F2N5O2/c24-18-4-1-2-5-19(18)30-14-17(13-28-30)27-12-16-7-9-23(10-8-16)15-29(22(31)32-23)20-6-3-11-26-21(20)25/h1-6,11,13-14,16,27H,7-10,12,15H2/t16-,23- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413550
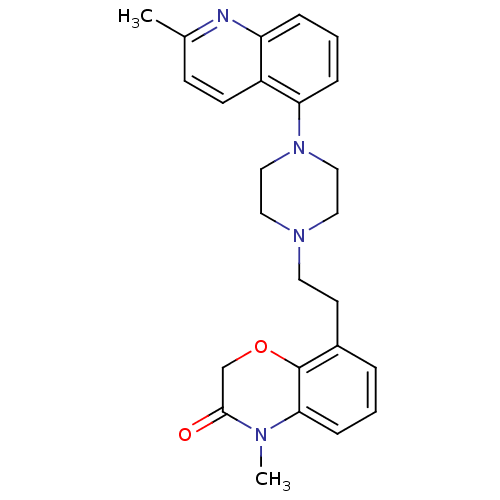
(CHEMBL469345)Show SMILES CN1C(=O)COc2c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C25H28N4O2/c1-18-9-10-20-21(26-18)6-4-7-22(20)29-15-13-28(14-16-29)12-11-19-5-3-8-23-25(19)31-17-24(30)27(23)2/h3-10H,11-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417973
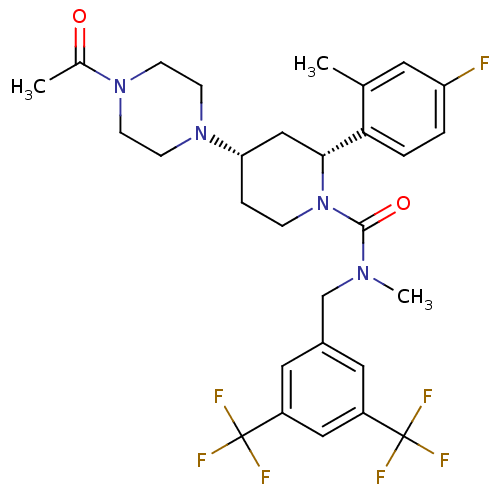
(CHEMBL1672053)Show SMILES CN(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N1CCN(CC1)C(C)=O |r| Show InChI InChI=1S/C29H33F7N4O2/c1-18-12-23(30)4-5-25(18)26-16-24(39-10-8-38(9-11-39)19(2)41)6-7-40(26)27(42)37(3)17-20-13-21(28(31,32)33)15-22(14-20)29(34,35)36/h4-5,12-15,24,26H,6-11,16-17H2,1-3H3/t24-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50336575
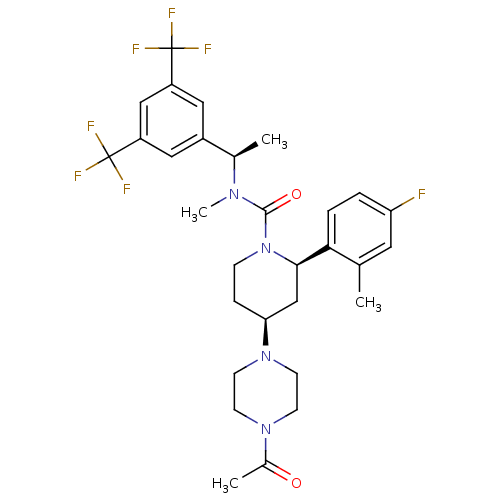
(CHEMBL1672054 | cis-(1'-Acetyl-N-{(1R)-1-[3,5-bis(...)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N1CCN(CC1)C(C)=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C30H35F7N4O2/c1-18-13-24(31)5-6-26(18)27-17-25(40-11-9-39(10-12-40)20(3)42)7-8-41(27)28(43)38(4)19(2)21-14-22(29(32,33)34)16-23(15-21)30(35,36)37/h5-6,13-16,19,25,27H,7-12,17H2,1-4H3/t19-,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR205171 from human NK1 receptor in cortex homogenate by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417041

(CHEMBL1257637)Show SMILES O=C1O[C@@]2(CN1c1ccccn1)CC[C@H](CNc1ccc(cn1)-c1nccs1)CC2 |r,wU:14.16,wD:3.2,(30.13,-31.33,;29.1,-30.19,;27.57,-30.35,;26.95,-28.94,;28.09,-27.91,;29.43,-28.68,;30.83,-28.06,;32.08,-28.97,;33.48,-28.34,;33.65,-26.81,;32.39,-25.9,;30.99,-26.53,;26.21,-27.59,;24.67,-27.57,;23.88,-28.89,;22.34,-28.87,;21.59,-27.52,;20.05,-27.5,;19.26,-28.81,;17.73,-28.79,;16.97,-27.44,;17.77,-26.12,;19.3,-26.15,;15.43,-27.43,;14.51,-28.66,;13.05,-28.17,;13.06,-26.63,;14.53,-26.17,;24.62,-30.23,;26.16,-30.26,)| Show InChI InChI=1S/C22H23N5O2S/c28-21-27(19-3-1-2-10-23-19)15-22(29-21)8-6-16(7-9-22)13-25-18-5-4-17(14-26-18)20-24-11-12-30-20/h1-5,10-12,14,16H,6-9,13,15H2,(H,25,26)/t16-,22- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50417411
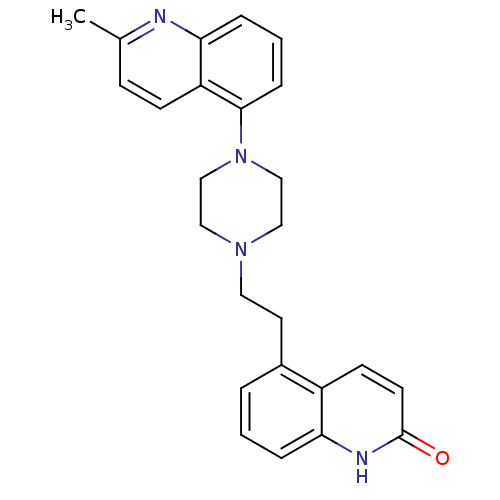
(CHEMBL1290715)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc3[nH]c(=O)ccc23)CC1 Show InChI InChI=1S/C25H26N4O/c1-18-8-9-21-23(26-18)6-3-7-24(21)29-16-14-28(15-17-29)13-12-19-4-2-5-22-20(19)10-11-25(30)27-22/h2-11H,12-17H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50417424
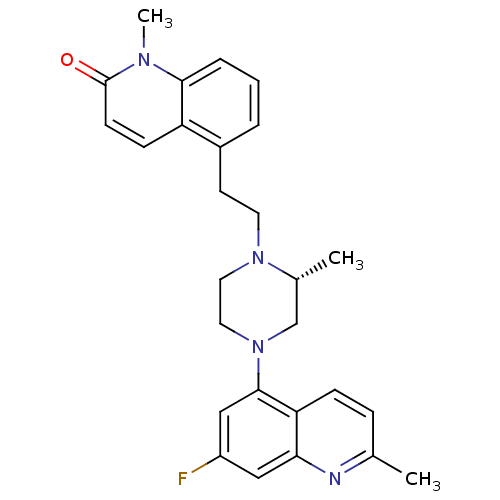
(CHEMBL1289394)Show SMILES C[C@@H]1CN(CCN1CCc1cccc2n(C)c(=O)ccc12)c1cc(F)cc2nc(C)ccc12 |r| Show InChI InChI=1S/C27H29FN4O/c1-18-7-8-23-24(29-18)15-21(28)16-26(23)32-14-13-31(19(2)17-32)12-11-20-5-4-6-25-22(20)9-10-27(33)30(25)3/h4-10,15-16,19H,11-14,17H2,1-3H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413086
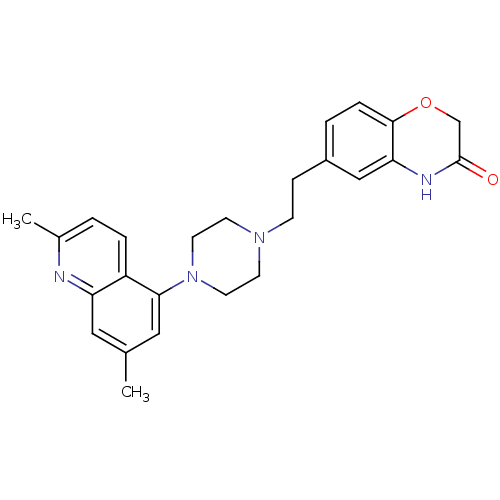
(CHEMBL484260)Show SMILES Cc1cc(N2CCN(CCc3ccc4OCC(=O)Nc4c3)CC2)c2ccc(C)nc2c1 Show InChI InChI=1S/C25H28N4O2/c1-17-13-21-20(5-3-18(2)26-21)23(14-17)29-11-9-28(10-12-29)8-7-19-4-6-24-22(15-19)27-25(30)16-31-24/h3-6,13-15H,7-12,16H2,1-2H3,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]WAY-100635 from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413553
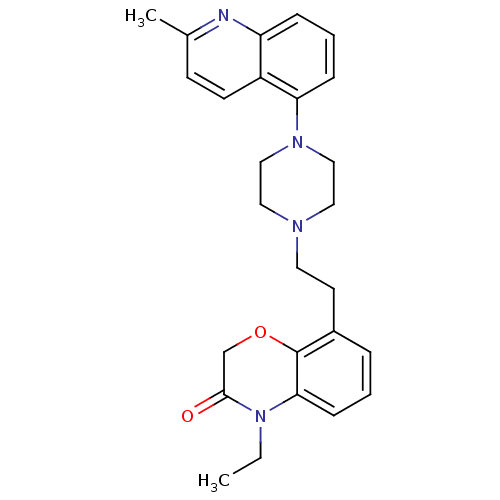
(CHEMBL472290)Show SMILES CCN1C(=O)COc2c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C26H30N4O2/c1-3-30-24-9-4-6-20(26(24)32-18-25(30)31)12-13-28-14-16-29(17-15-28)23-8-5-7-22-21(23)11-10-19(2)27-22/h4-11H,3,12-18H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1A assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus (rat)) | BDBM50308250
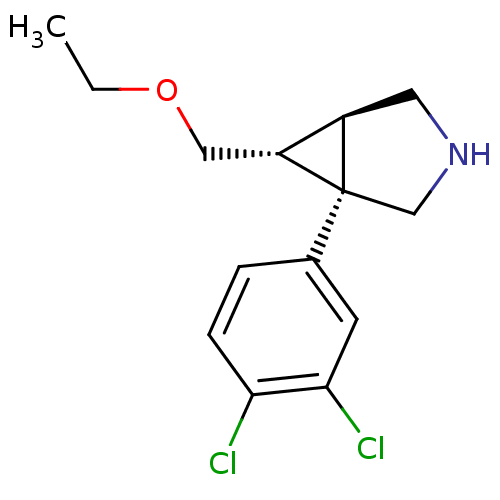
((1R,5R,6R)-1-(3,4-dichlorophenyl)-6-(ethoxymethyl)...)Show SMILES CCOC[C@@H]1[C@H]2CNC[C@@]12c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C14H17Cl2NO/c1-2-18-7-11-10-6-17-8-14(10,11)9-3-4-12(15)13(16)5-9/h3-5,10-11,17H,2,6-8H2,1H3/t10-,11-,14+/m1/s1 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [N-methyl-3H]nisoxetine from rat hippocampus NET by filtration binding assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
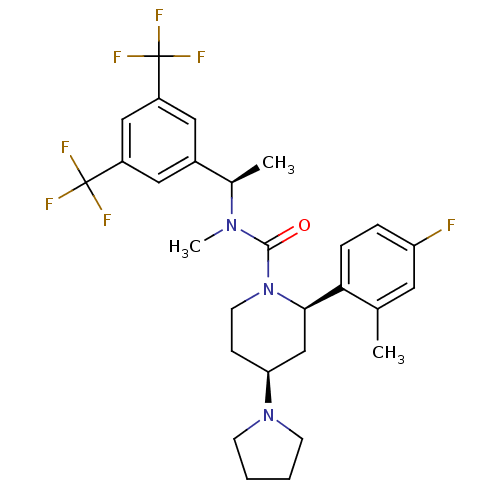
(Homo sapiens (Human)) | BDBM50417972
(CHEMBL1672051)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N1CCCC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C28H32F7N3O/c1-17-12-22(29)6-7-24(17)25-16-23(37-9-4-5-10-37)8-11-38(25)26(39)36(3)18(2)19-13-20(27(30,31)32)15-21(14-19)28(33,34)35/h6-7,12-15,18,23,25H,4-5,8-11,16H2,1-3H3/t18-,23+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412441

(CHEMBL490417 | SB-744185)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-5-7-19-20(25-17)3-2-4-22(19)28-13-11-27(12-14-28)10-9-18-6-8-23-21(15-18)26-24(29)16-30-23/h2-8,15H,9-14,16H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413550

(CHEMBL469345)Show SMILES CN1C(=O)COc2c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C25H28N4O2/c1-18-9-10-20-21(26-18)6-4-7-22(20)29-15-13-28(14-16-29)12-11-19-5-3-8-23-25(19)31-17-24(30)27(23)2/h3-10H,11-17H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1A assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413555

(CHEMBL469568)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc3N(C4CC4)C(=O)COc23)CC1 Show InChI InChI=1S/C27H30N4O2/c1-19-8-11-22-23(28-19)5-3-6-24(22)30-16-14-29(15-17-30)13-12-20-4-2-7-25-27(20)33-18-26(32)31(25)21-9-10-21/h2-8,11,21H,9-10,12-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1D assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50413083
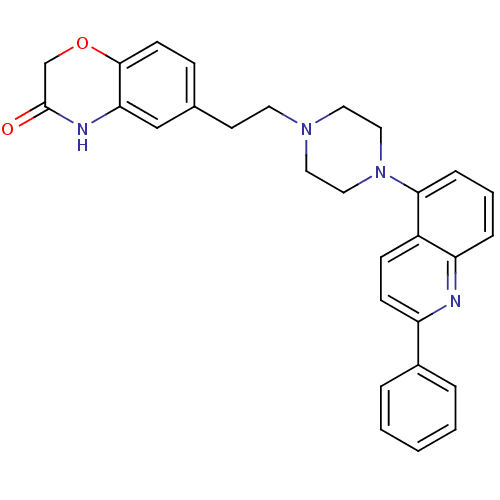
(CHEMBL484059)Show SMILES O=C1COc2ccc(CCN3CCN(CC3)c3cccc4nc(ccc34)-c3ccccc3)cc2N1 Show InChI InChI=1S/C29H28N4O2/c34-29-20-35-28-12-9-21(19-26(28)31-29)13-14-32-15-17-33(18-16-32)27-8-4-7-25-23(27)10-11-24(30-25)22-5-2-1-3-6-22/h1-12,19H,13-18,20H2,(H,31,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from human SerT expressed pig LLCPK cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413078
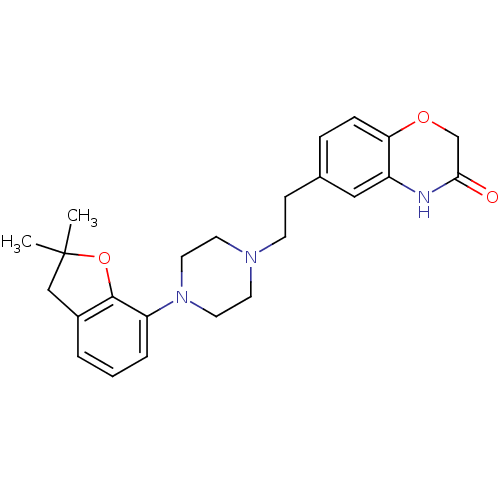
(CHEMBL491839)Show SMILES CC1(C)Cc2cccc(N3CCN(CCc4ccc5OCC(=O)Nc5c4)CC3)c2O1 Show InChI InChI=1S/C24H29N3O3/c1-24(2)15-18-4-3-5-20(23(18)30-24)27-12-10-26(11-13-27)9-8-17-6-7-21-19(14-17)25-22(28)16-29-21/h3-7,14H,8-13,15-16H2,1-2H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417390
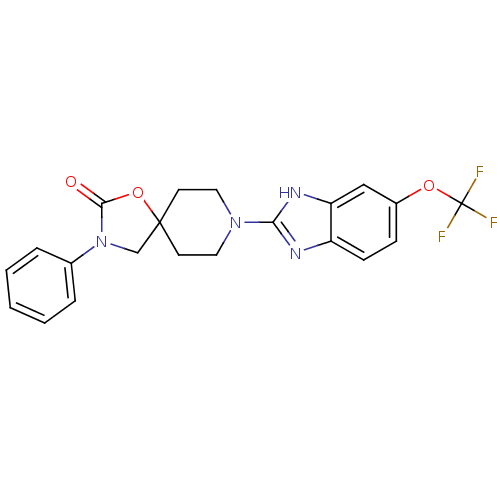
(CHEMBL1289154)Show SMILES FC(F)(F)Oc1ccc2nc([nH]c2c1)N1CCC2(CN(C(=O)O2)c2ccccc2)CC1 Show InChI InChI=1S/C21H19F3N4O3/c22-21(23,24)30-15-6-7-16-17(12-15)26-18(25-16)27-10-8-20(9-11-27)13-28(19(29)31-20)14-4-2-1-3-5-14/h1-7,12H,8-11,13H2,(H,25,26) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonistic activity at human NPY Y5 receptor expressed in HEK293 cells assessed as inhibition of calcium level by FLIPR assay |
Bioorg Med Chem Lett 20: 7120-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.064
BindingDB Entry DOI: 10.7270/Q2R49S2N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413084
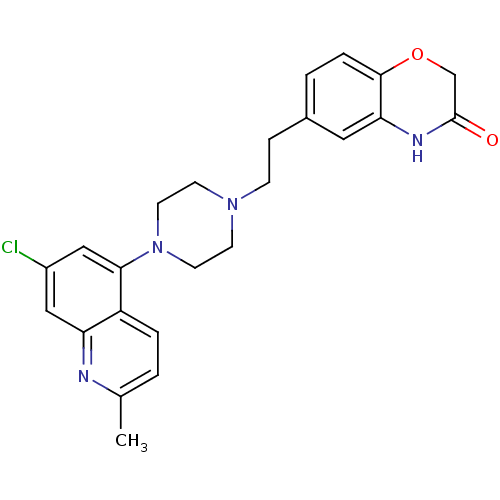
(CHEMBL521506)Show SMILES Cc1ccc2c(cc(Cl)cc2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H25ClN4O2/c1-16-2-4-19-20(26-16)13-18(25)14-22(19)29-10-8-28(9-11-29)7-6-17-3-5-23-21(12-17)27-24(30)15-31-23/h2-5,12-14H,6-11,15H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417054

(CHEMBL1258673)Show SMILES Fc1ccccc1-c1ccc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cccnn3)CC2)nn1 |r,wU:13.13,wD:16.22,(14.72,.44,;13.96,1.78,;12.43,1.8,;11.67,3.15,;12.46,4.47,;14,4.45,;14.75,3.1,;16.29,3.08,;17.04,1.73,;18.58,1.71,;19.36,3.03,;20.9,3.01,;21.65,1.66,;23.19,1.64,;23.99,2.96,;25.53,2.94,;26.26,1.59,;27.4,2.62,;28.74,1.85,;28.42,.34,;29.45,-.8,;26.89,.18,;30.15,2.47,;31.39,1.56,;32.8,2.18,;32.96,3.72,;31.71,4.62,;30.31,3.99,;25.47,.27,;23.94,.3,;18.62,4.38,;17.08,4.41,)| Show InChI InChI=1S/C23H23FN6O2/c24-18-5-2-1-4-17(18)19-7-8-20(28-27-19)25-14-16-9-11-23(12-10-16)15-30(22(31)32-23)21-6-3-13-26-29-21/h1-8,13,16H,9-12,14-15H2,(H,25,28)/t16-,23- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417039
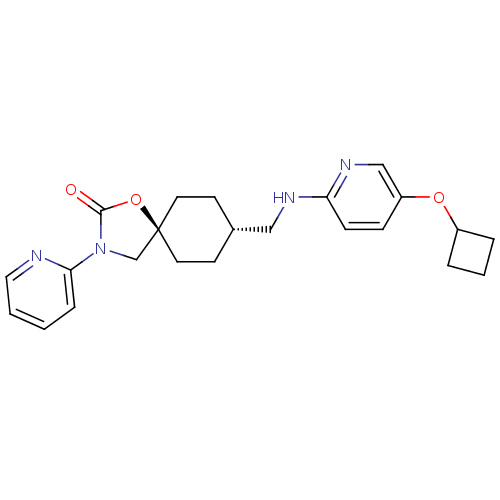
(CHEMBL1257761)Show SMILES O=C1O[C@@]2(CN1c1ccccn1)CC[C@H](CNc1ccc(OC3CCC3)cn1)CC2 |r,wU:14.16,wD:3.2,(29.67,-25.14,;28.64,-23.99,;27.1,-24.16,;26.48,-22.75,;27.62,-21.72,;28.96,-22.49,;30.36,-21.87,;31.61,-22.78,;33.02,-22.15,;33.18,-20.62,;31.92,-19.71,;30.52,-20.34,;25.74,-21.4,;24.2,-21.38,;23.41,-22.7,;21.87,-22.68,;21.12,-21.33,;19.58,-21.3,;18.8,-22.62,;17.26,-22.6,;16.51,-21.25,;14.96,-21.24,;14.22,-19.89,;14.64,-18.41,;13.16,-17.99,;12.74,-19.47,;17.3,-19.93,;18.84,-19.96,;24.16,-24.04,;25.69,-24.07,)| Show InChI InChI=1S/C23H28N4O3/c28-22-27(21-6-1-2-13-24-21)16-23(30-22)11-9-17(10-12-23)14-25-20-8-7-19(15-26-20)29-18-4-3-5-18/h1-2,6-8,13,15,17-18H,3-5,9-12,14,16H2,(H,25,26)/t17-,23- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417040
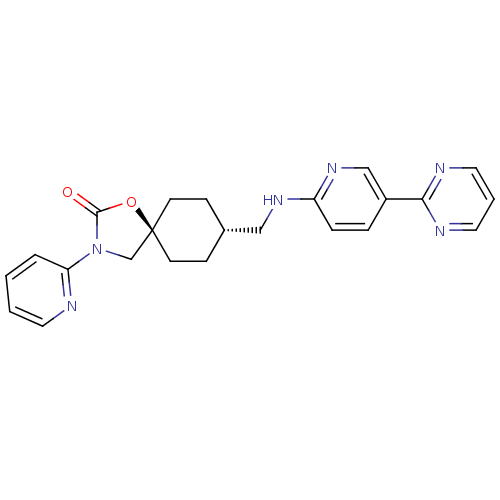
(CHEMBL1257760)Show SMILES O=C1O[C@@]2(CN1c1ccccn1)CC[C@H](CNc1ccc(cn1)-c1ncccn1)CC2 |r,wU:14.16,wD:3.2,(7.1,-28.95,;6.07,-27.81,;4.54,-27.97,;3.92,-26.56,;5.06,-25.53,;6.4,-26.3,;7.8,-25.68,;9.05,-26.59,;10.45,-25.96,;10.62,-24.43,;9.36,-23.52,;7.96,-24.15,;3.18,-25.21,;1.64,-25.19,;.85,-26.51,;-.69,-26.49,;-1.44,-25.14,;-2.98,-25.12,;-3.77,-26.43,;-5.3,-26.41,;-6.06,-25.06,;-5.26,-23.74,;-3.73,-23.77,;-7.6,-25.05,;-8.35,-23.7,;-9.89,-23.68,;-10.68,-25,;-9.92,-26.35,;-8.38,-26.37,;1.59,-27.85,;3.13,-27.88,)| Show InChI InChI=1S/C23H24N6O2/c30-22-29(20-4-1-2-11-24-20)16-23(31-22)9-7-17(8-10-23)14-27-19-6-5-18(15-28-19)21-25-12-3-13-26-21/h1-6,11-13,15,17H,7-10,14,16H2,(H,27,28)/t17-,23- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413550

(CHEMBL469345)Show SMILES CN1C(=O)COc2c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C25H28N4O2/c1-18-9-10-20-21(26-18)6-4-7-22(20)29-15-13-28(14-16-29)12-11-19-5-3-8-23-25(19)31-17-24(30)27(23)2/h3-10H,11-17H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1A receptor expressed in HEK293 cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50192317

(CHEMBL3899125 | US10239870, Example 281)Show SMILES Cn1c(SCCCN2CC[C@]3(C[C@@H]3c3ccc(cc3)C(F)(F)F)C2)nnc1-c1ccc(cc1)C(N)=O |r| Show InChI InChI=1S/C26H28F3N5OS/c1-33-23(19-5-3-18(4-6-19)22(30)35)31-32-24(33)36-14-2-12-34-13-11-25(16-34)15-21(25)17-7-9-20(10-8-17)26(27,28)29/h3-10,21H,2,11-16H2,1H3,(H2,30,35)/t21-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine D3 receptor expressed in CHO-K1 cell membranes after 90 mins by liquid scintillation counting |
J Med Chem 59: 8549-76 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00972
BindingDB Entry DOI: 10.7270/Q2SQ9298 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417982
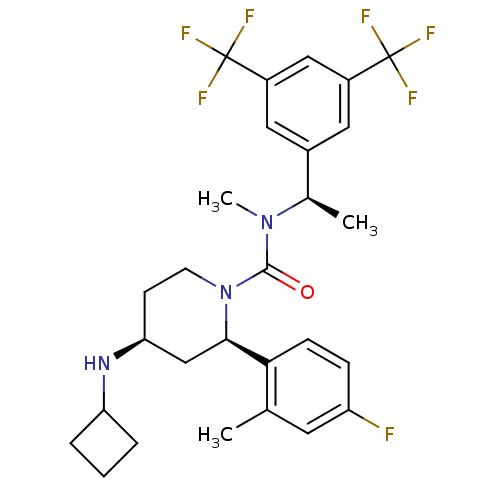
(CHEMBL1672058)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)NC1CCC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C28H32F7N3O/c1-16-11-21(29)7-8-24(16)25-15-23(36-22-5-4-6-22)9-10-38(25)26(39)37(3)17(2)18-12-19(27(30,31)32)14-20(13-18)28(33,34)35/h7-8,11-14,17,22-23,25,36H,4-6,9-10,15H2,1-3H3/t17-,23+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417970
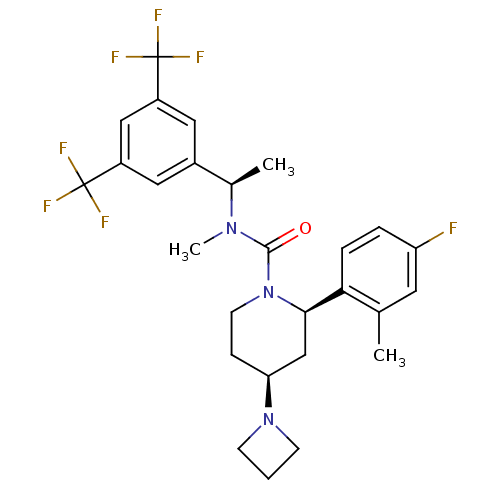
(CHEMBL1672048)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N1CCC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C27H30F7N3O/c1-16-11-21(28)5-6-23(16)24-15-22(36-8-4-9-36)7-10-37(24)25(38)35(3)17(2)18-12-19(26(29,30)31)14-20(13-18)27(32,33)34/h5-6,11-14,17,22,24H,4,7-10,15H2,1-3H3/t17-,22+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data