

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50312599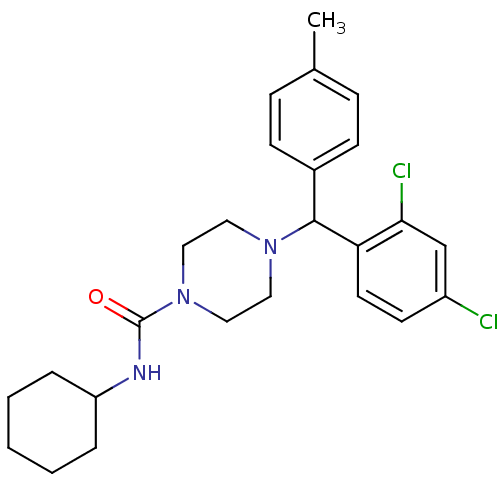 (CHEMBL1093735 | N-Cyclohexyl-4-[(2,4-dichloropheny...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55940 from human CB1 receptor expressed in CHO cells pretreated for 10 mins measured after 3 hrs by scintillation counting | Eur J Med Chem 46: 5310-6 (2011) Article DOI: 10.1016/j.ejmech.2011.08.030 BindingDB Entry DOI: 10.7270/Q22J6C85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50355892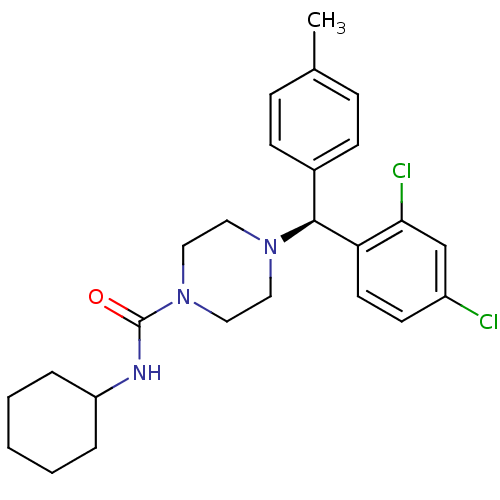 (CHEMBL1910035) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55940 from human CB1 receptor expressed in CHO cells pretreated for 10 mins measured after 3 hrs by scintillation counting | Eur J Med Chem 46: 5310-6 (2011) Article DOI: 10.1016/j.ejmech.2011.08.030 BindingDB Entry DOI: 10.7270/Q22J6C85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50355891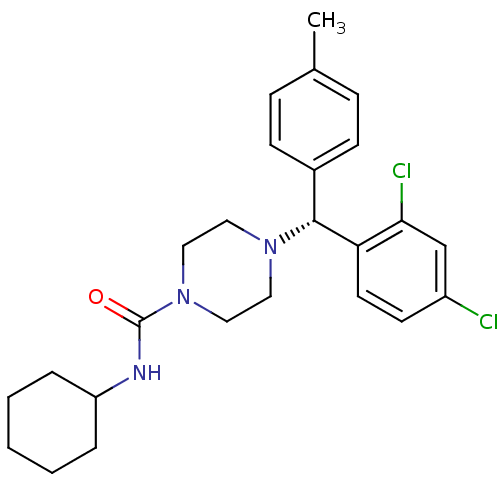 (CHEMBL1910034) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55940 from human CB1 receptor expressed in CHO cells pretreated for 10 mins measured after 3 hrs by scintillation counting | Eur J Med Chem 46: 5310-6 (2011) Article DOI: 10.1016/j.ejmech.2011.08.030 BindingDB Entry DOI: 10.7270/Q22J6C85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50154561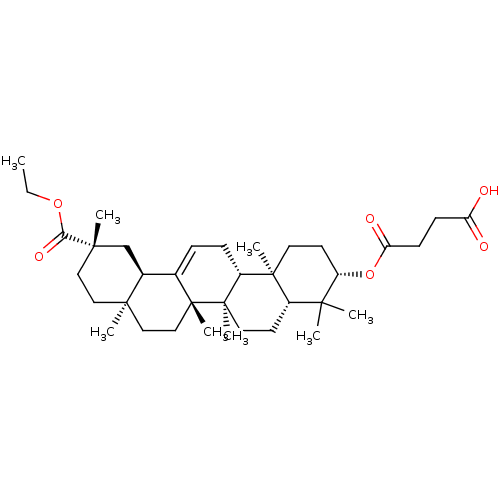 (CHEMBL3774603) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Competitive inhibition of CE2 in human liver microsomes using fluorescein diacetate as substrate preincubated for 10 mins followed by substrate addit... | Eur J Med Chem 112: 280-8 (2016) Article DOI: 10.1016/j.ejmech.2016.02.020 BindingDB Entry DOI: 10.7270/Q2TQ63D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM50450436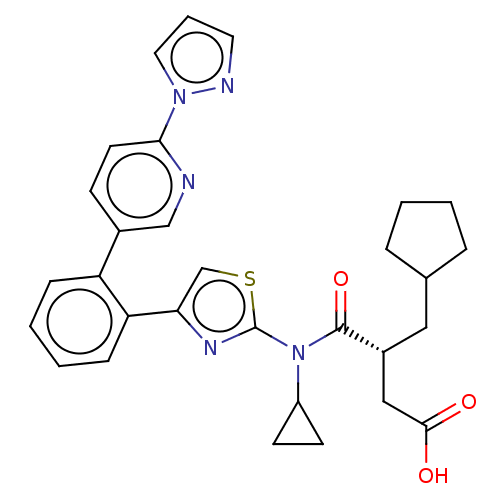 (CHEMBL4173663) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA Curated by ChEMBL | Assay Description Displacement of [3H]ES227703 from human FFA2R expressed in CHOK1 cells after 60 mins by TopCount scintillation counting method | Bioorg Med Chem 26: 5169-5180 (2018) Article DOI: 10.1016/j.bmc.2018.09.015 BindingDB Entry DOI: 10.7270/Q2RV0R8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50552232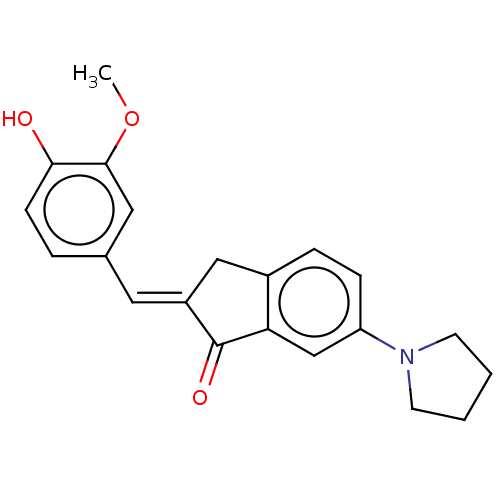 (CHEMBL4776624) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed inhibition of hCES2A in human liver microsome assessed as reduction in fluorescein diacetate hydrolysis preincubated for 10 mins followed by su... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112856 BindingDB Entry DOI: 10.7270/Q2NZ8CB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50571105 (CHEMBL4851375) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Non-competitive inhibition of CES2 in human liver microsomes using fluorescein diacetate as substrate by Lineweaver-Burk plot based Michelis-Menten e... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116187 BindingDB Entry DOI: 10.7270/Q2SN0DQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM50450440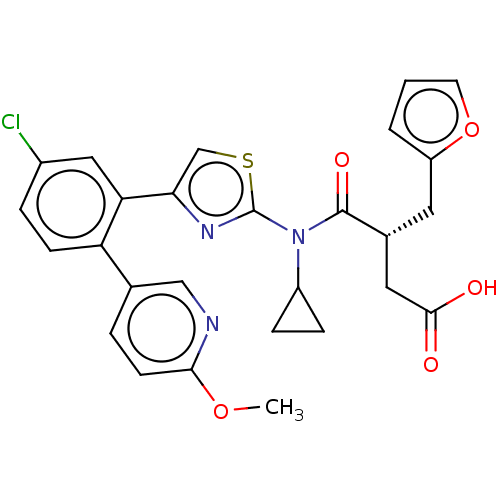 (CHEMBL4170829) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA Curated by ChEMBL | Assay Description Displacement of [3H]ES227703 from human FFA2R expressed in CHOK1 cells after 60 mins by TopCount scintillation counting method | Bioorg Med Chem 26: 5169-5180 (2018) Article DOI: 10.1016/j.bmc.2018.09.015 BindingDB Entry DOI: 10.7270/Q2RV0R8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM50450439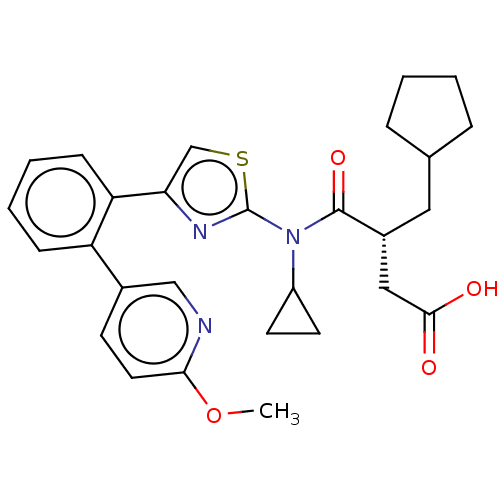 (CHEMBL4162307) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA Curated by ChEMBL | Assay Description Displacement of [3H]ES227703 from human FFA2R expressed in CHOK1 cells after 60 mins by TopCount scintillation counting method | Bioorg Med Chem 26: 5169-5180 (2018) Article DOI: 10.1016/j.bmc.2018.09.015 BindingDB Entry DOI: 10.7270/Q2RV0R8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM50450437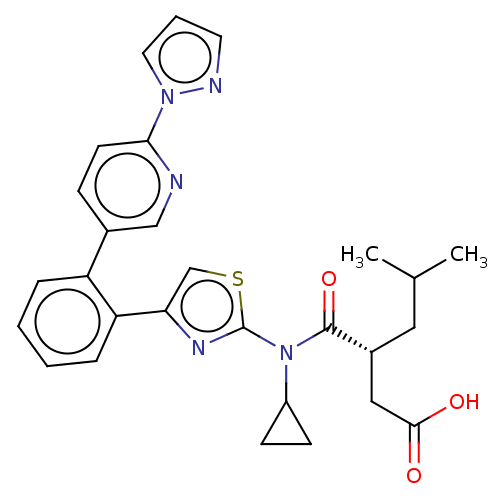 (CHEMBL4177037) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA Curated by ChEMBL | Assay Description Displacement of [3H]ES227703 from human FFA2R expressed in CHOK1 cells after 60 mins by TopCount scintillation counting method | Bioorg Med Chem 26: 5169-5180 (2018) Article DOI: 10.1016/j.bmc.2018.09.015 BindingDB Entry DOI: 10.7270/Q2RV0R8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM404212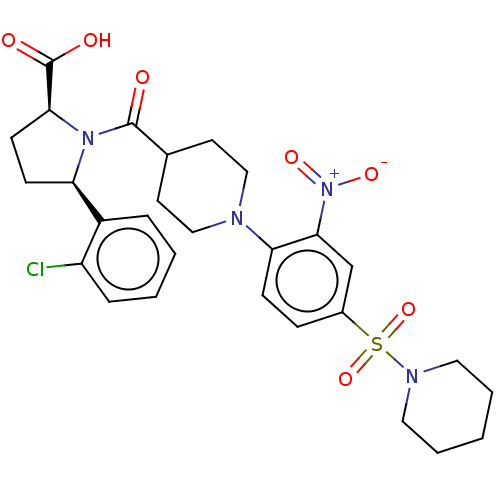 ((2S,5R)-5-(2-chlorophenyl)-1-(2'-cyano-4'-methoxy-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epics Therapeutics US Patent | Assay Description The [35S]GTPγS assay was incubated in 20 mM HEPES pH7.4, 100 mM NaCl, 10 μg/ml saponin, 30 mM of MgCl2, 10 μM of GDP, 5 μg membra... | US Patent US10358416 (2019) BindingDB Entry DOI: 10.7270/Q2TT4T9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM50450289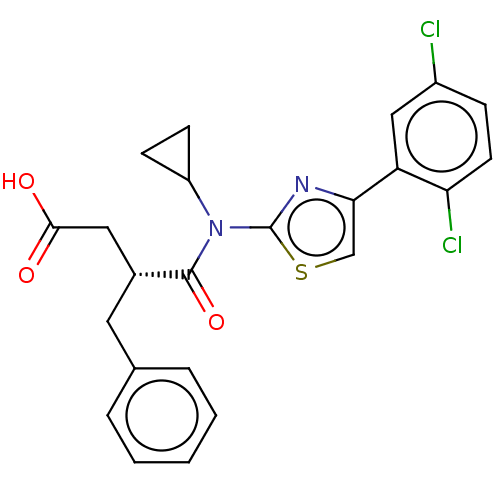 (CHEMBL4172928) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA Curated by ChEMBL | Assay Description Displacement of [3H]ES227703 from human FFA2R expressed in CHOK1 cells after 60 mins by TopCount scintillation counting method | Bioorg Med Chem 26: 5169-5180 (2018) Article DOI: 10.1016/j.bmc.2018.09.015 BindingDB Entry DOI: 10.7270/Q2RV0R8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM408766 ((2S,5R)-1-(1-(5-chloro-2-nitrophenyl)piperidine-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epics Therapeutics US Patent | Assay Description The [35S]GTPγS assay was incubated in 20 mM HEPES pH7.4, 100 mM NaCl, 10 μg/ml saponin, 30 mM of MgCl2, 10 μM of GDP, 5 μg membra... | US Patent US10358416 (2019) BindingDB Entry DOI: 10.7270/Q2TT4T9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50355892 (CHEMBL1910035) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 227 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55940 from human CB2 receptor expressed in CHO cells pretreated for 10 mins measured after 3 hrs by scintillation counting | Eur J Med Chem 46: 5310-6 (2011) Article DOI: 10.1016/j.ejmech.2011.08.030 BindingDB Entry DOI: 10.7270/Q22J6C85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50355891 (CHEMBL1910034) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 242 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55940 from human CB2 receptor expressed in CHO cells pretreated for 10 mins measured after 3 hrs by scintillation counting | Eur J Med Chem 46: 5310-6 (2011) Article DOI: 10.1016/j.ejmech.2011.08.030 BindingDB Entry DOI: 10.7270/Q22J6C85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50584760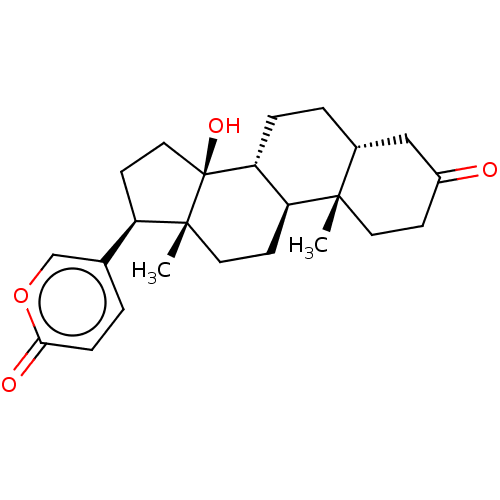 (CHEMBL2068968) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inactivation of recombinant human CYP3A4 assessed as inhibition constant using midazolam as substrate preincubated for 5 mins followed by beta-NADP a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01875 BindingDB Entry DOI: 10.7270/Q2PZ5DQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM50450441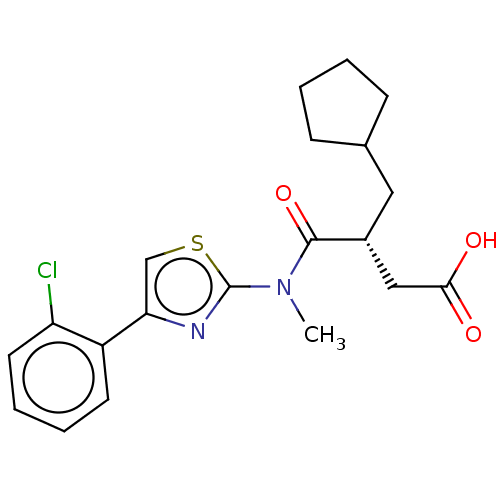 (CHEMBL4173387) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA Curated by ChEMBL | Assay Description Displacement of [3H]ES227703 from human FFA2R expressed in CHOK1 cells after 60 mins by TopCount scintillation counting method | Bioorg Med Chem 26: 5169-5180 (2018) Article DOI: 10.1016/j.bmc.2018.09.015 BindingDB Entry DOI: 10.7270/Q2RV0R8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM50450435 (CHEMBL4165748) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA Curated by ChEMBL | Assay Description Displacement of [3H]ES227703 from human FFA2R expressed in CHOK1 cells after 60 mins by TopCount scintillation counting method | Bioorg Med Chem 26: 5169-5180 (2018) Article DOI: 10.1016/j.bmc.2018.09.015 BindingDB Entry DOI: 10.7270/Q2RV0R8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50312599 (CHEMBL1093735 | N-Cyclohexyl-4-[(2,4-dichloropheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 329 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55940 from human CB2 receptor expressed in CHO cells pretreated for 10 mins measured after 3 hrs by scintillation counting | Eur J Med Chem 46: 5310-6 (2011) Article DOI: 10.1016/j.ejmech.2011.08.030 BindingDB Entry DOI: 10.7270/Q22J6C85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM50450438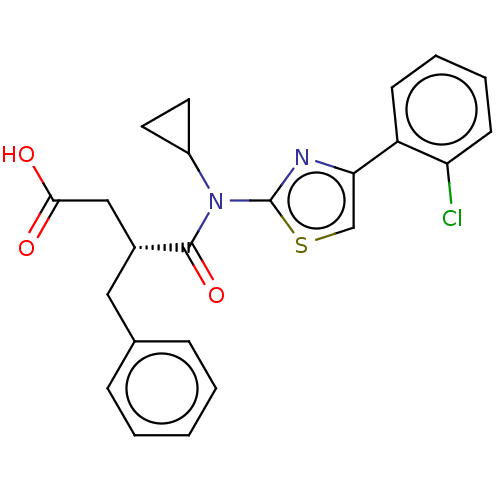 (CHEMBL4166818) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA Curated by ChEMBL | Assay Description Displacement of [3H]ES227703 from human FFA2R expressed in CHOK1 cells after 60 mins by TopCount scintillation counting method | Bioorg Med Chem 26: 5169-5180 (2018) Article DOI: 10.1016/j.bmc.2018.09.015 BindingDB Entry DOI: 10.7270/Q2RV0R8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM408762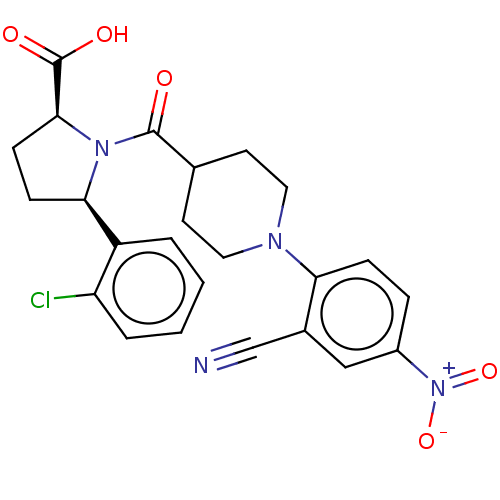 ((2S,5R)-5-(2-chlorophenyl)-1-(1-(2-cyano-4-nitroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epics Therapeutics US Patent | Assay Description The [35S]GTPγS assay was incubated in 20 mM HEPES pH7.4, 100 mM NaCl, 10 μg/ml saponin, 30 mM of MgCl2, 10 μM of GDP, 5 μg membra... | US Patent US10358416 (2019) BindingDB Entry DOI: 10.7270/Q2TT4T9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM404212 ((2S,5R)-5-(2-chlorophenyl)-1-(2'-cyano-4'-methoxy-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epics Therapeutics US Patent | Assay Description The [35S]GTPγS assay was incubated in 20 mM HEPES pH7.4, 100 mM NaCl, 10 μg/ml saponin, 30 mM of MgCl2, 10 μM of GDP, 5 μg membra... | US Patent US10358416 (2019) BindingDB Entry DOI: 10.7270/Q2TT4T9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM408761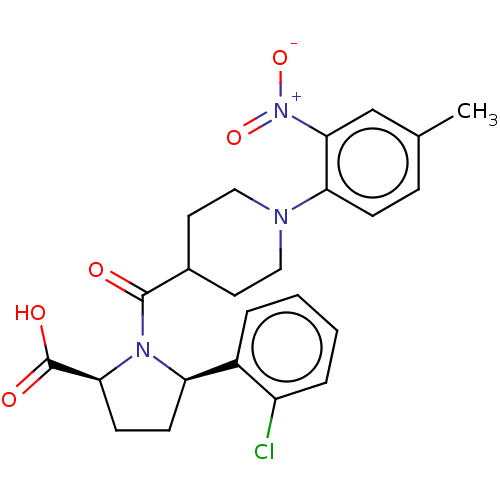 ((2S,5R)-5-(2-chlorophenyl)-1-(1-(4-methyl-2-nitrop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epics Therapeutics US Patent | Assay Description The [35S]GTPγS assay was incubated in 20 mM HEPES pH7.4, 100 mM NaCl, 10 μg/ml saponin, 30 mM of MgCl2, 10 μM of GDP, 5 μg membra... | US Patent US10358416 (2019) BindingDB Entry DOI: 10.7270/Q2TT4T9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50584760 (CHEMBL2068968) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inactivation of CYP3A4 in human liver microsomes assessed as inhibition constant using midazolam as substrate preincubated for 5 mins followed by bet... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01875 BindingDB Entry DOI: 10.7270/Q2PZ5DQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM50450443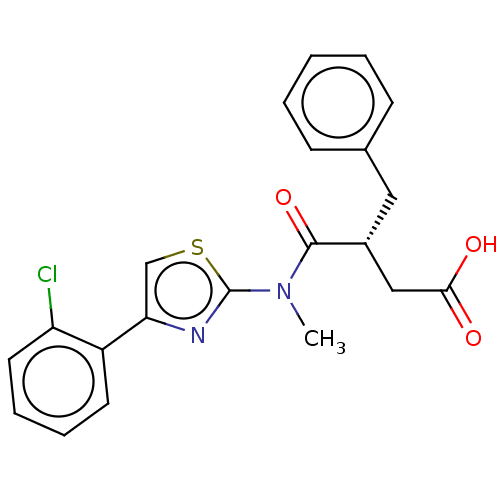 (CHEMBL4174307) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA Curated by ChEMBL | Assay Description Displacement of [3H]ES227703 from human FFA2R expressed in CHOK1 cells after 60 mins by TopCount scintillation counting method | Bioorg Med Chem 26: 5169-5180 (2018) Article DOI: 10.1016/j.bmc.2018.09.015 BindingDB Entry DOI: 10.7270/Q2RV0R8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM404211 ((2S,5R)-5-(2-cyanophenyl)-1-(2'-methoxy-[1,1'-biph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epics Therapeutics US Patent | Assay Description Human GPR43 radioligand binding assay is performed by adding successively in the wells of a 96 well plate (Master Block, Greiner, 786201), 50 ul of c... | US Patent US10781171 (2020) BindingDB Entry DOI: 10.7270/Q2XP780V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM408758 ( (2S,5R)-5-(2-chlorophenyl)-1-(1-(4-(morpholinosul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epics Therapeutics US Patent | Assay Description The [35S]GTPγS assay was incubated in 20 mM HEPES pH7.4, 100 mM NaCl, 10 μg/ml saponin, 30 mM of MgCl2, 10 μM of GDP, 5 μg membra... | US Patent US10358416 (2019) BindingDB Entry DOI: 10.7270/Q2TT4T9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM408760 ((2S,5R)-5-(2-chlorophenyl)-1-(1-(4-(N,N-diethylsul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epics Therapeutics US Patent | Assay Description The [35S]GTPγS assay was incubated in 20 mM HEPES pH7.4, 100 mM NaCl, 10 μg/ml saponin, 30 mM of MgCl2, 10 μM of GDP, 5 μg membra... | US Patent US10358416 (2019) BindingDB Entry DOI: 10.7270/Q2TT4T9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM408767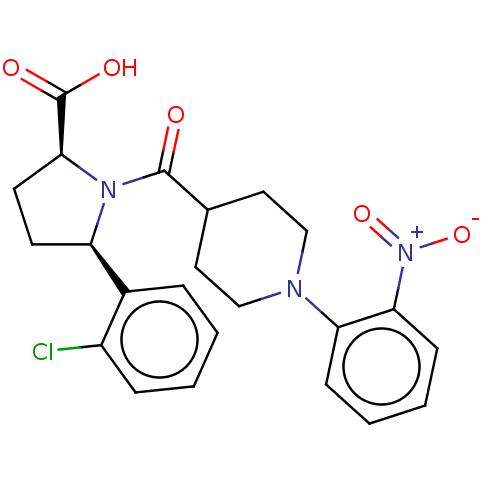 ((2S,5R)-5-(2-chlorophenyl)-1-(1-(2-nitrophenyl)pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epics Therapeutics US Patent | Assay Description The [35S]GTPγS assay was incubated in 20 mM HEPES pH7.4, 100 mM NaCl, 10 μg/ml saponin, 30 mM of MgCl2, 10 μM of GDP, 5 μg membra... | US Patent US10358416 (2019) BindingDB Entry DOI: 10.7270/Q2TT4T9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM408765 ( (2S,5R)-5-(2-chlorophenyl)-1-(1-(3-methoxy-4-nitr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epics Therapeutics US Patent | Assay Description The [35S]GTPγS assay was incubated in 20 mM HEPES pH7.4, 100 mM NaCl, 10 μg/ml saponin, 30 mM of MgCl2, 10 μM of GDP, 5 μg membra... | US Patent US10358416 (2019) BindingDB Entry DOI: 10.7270/Q2TT4T9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM408763 ( (2S,5R)-5-(2-chlorophenyl)-1-(1-(4-nitrophenyl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epics Therapeutics US Patent | Assay Description The [35S]GTPγS assay was incubated in 20 mM HEPES pH7.4, 100 mM NaCl, 10 μg/ml saponin, 30 mM of MgCl2, 10 μM of GDP, 5 μg membra... | US Patent US10358416 (2019) BindingDB Entry DOI: 10.7270/Q2TT4T9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM408764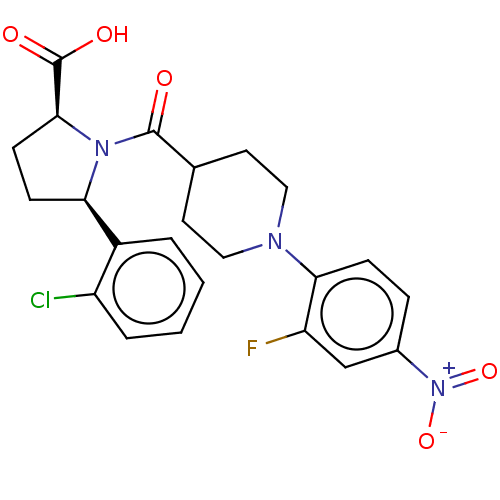 ((2S,5R)-5-(2-chlorophenyl)-1-(1-(2-fluoro-4-nitrop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epics Therapeutics US Patent | Assay Description The [35S]GTPγS assay was incubated in 20 mM HEPES pH7.4, 100 mM NaCl, 10 μg/ml saponin, 30 mM of MgCl2, 10 μM of GDP, 5 μg membra... | US Patent US10358416 (2019) BindingDB Entry DOI: 10.7270/Q2TT4T9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM408766 ((2S,5R)-1-(1-(5-chloro-2-nitrophenyl)piperidine-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epics Therapeutics US Patent | Assay Description Human GPR43 radioligand binding assay is performed by adding successively in the wells of a 96 well plate (Master Block, Greiner, 786201), 50 ul of c... | US Patent US10781171 (2020) BindingDB Entry DOI: 10.7270/Q2XP780V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50584761 (BUFALIN | Bufalin | CHEBI:517248) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inactivation of recombinant human CYP3A4 assessed as inhibition constant using midazolam as substrate preincubated for 5 mins followed by beta-NADP a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01875 BindingDB Entry DOI: 10.7270/Q2PZ5DQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50017698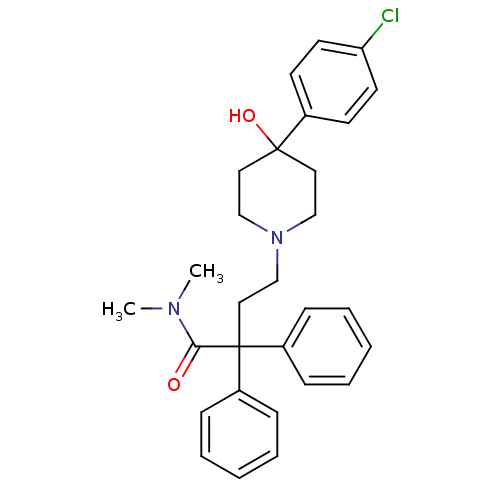 (4-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)-N,N...) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CES2A using 4-methylumbelliferone as a substrate | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112856 BindingDB Entry DOI: 10.7270/Q2NZ8CB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM408762 ((2S,5R)-5-(2-chlorophenyl)-1-(1-(2-cyano-4-nitroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epics Therapeutics US Patent | Assay Description Human GPR43 radioligand binding assay is performed by adding successively in the wells of a 96 well plate (Master Block, Greiner, 786201), 50 ul of c... | US Patent US10781171 (2020) BindingDB Entry DOI: 10.7270/Q2XP780V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM404212 ((2S,5R)-5-(2-chlorophenyl)-1-(2'-cyano-4'-methoxy-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epics Therapeutics US Patent | Assay Description Human GPR43 radioligand binding assay is performed by adding successively in the wells of a 96 well plate (Master Block, Greiner, 786201), 50 ul of c... | US Patent US10781171 (2020) BindingDB Entry DOI: 10.7270/Q2XP780V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM408761 ((2S,5R)-5-(2-chlorophenyl)-1-(1-(4-methyl-2-nitrop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epics Therapeutics US Patent | Assay Description Human GPR43 radioligand binding assay is performed by adding successively in the wells of a 96 well plate (Master Block, Greiner, 786201), 50 ul of c... | US Patent US10781171 (2020) BindingDB Entry DOI: 10.7270/Q2XP780V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM50450434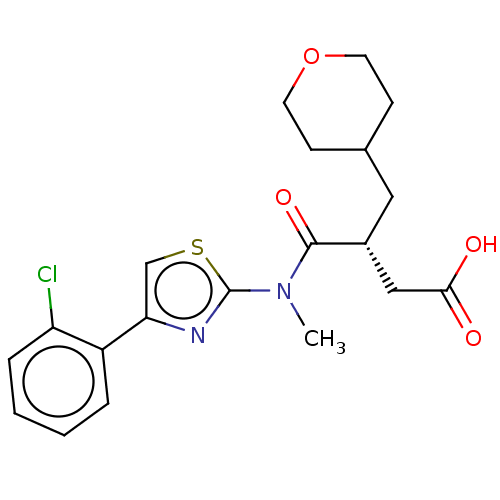 (CHEMBL4172349) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA Curated by ChEMBL | Assay Description Displacement of [3H]ES227703 from human FFA2R expressed in CHOK1 cells after 60 mins by TopCount scintillation counting method | Bioorg Med Chem 26: 5169-5180 (2018) Article DOI: 10.1016/j.bmc.2018.09.015 BindingDB Entry DOI: 10.7270/Q2RV0R8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM408758 ( (2S,5R)-5-(2-chlorophenyl)-1-(1-(4-(morpholinosul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epics Therapeutics US Patent | Assay Description Human GPR43 radioligand binding assay is performed by adding successively in the wells of a 96 well plate (Master Block, Greiner, 786201), 50 ul of c... | US Patent US10781171 (2020) BindingDB Entry DOI: 10.7270/Q2XP780V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM408765 ( (2S,5R)-5-(2-chlorophenyl)-1-(1-(3-methoxy-4-nitr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epics Therapeutics US Patent | Assay Description Human GPR43 radioligand binding assay is performed by adding successively in the wells of a 96 well plate (Master Block, Greiner, 786201), 50 ul of c... | US Patent US10781171 (2020) BindingDB Entry DOI: 10.7270/Q2XP780V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM408764 ((2S,5R)-5-(2-chlorophenyl)-1-(1-(2-fluoro-4-nitrop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epics Therapeutics US Patent | Assay Description Human GPR43 radioligand binding assay is performed by adding successively in the wells of a 96 well plate (Master Block, Greiner, 786201), 50 ul of c... | US Patent US10781171 (2020) BindingDB Entry DOI: 10.7270/Q2XP780V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM408763 ( (2S,5R)-5-(2-chlorophenyl)-1-(1-(4-nitrophenyl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epics Therapeutics US Patent | Assay Description Human GPR43 radioligand binding assay is performed by adding successively in the wells of a 96 well plate (Master Block, Greiner, 786201), 50 ul of c... | US Patent US10781171 (2020) BindingDB Entry DOI: 10.7270/Q2XP780V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM408767 ((2S,5R)-5-(2-chlorophenyl)-1-(1-(2-nitrophenyl)pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epics Therapeutics US Patent | Assay Description Human GPR43 radioligand binding assay is performed by adding successively in the wells of a 96 well plate (Master Block, Greiner, 786201), 50 ul of c... | US Patent US10781171 (2020) BindingDB Entry DOI: 10.7270/Q2XP780V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM408760 ((2S,5R)-5-(2-chlorophenyl)-1-(1-(4-(N,N-diethylsul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epics Therapeutics US Patent | Assay Description Human GPR43 radioligand binding assay is performed by adding successively in the wells of a 96 well plate (Master Block, Greiner, 786201), 50 ul of c... | US Patent US10781171 (2020) BindingDB Entry DOI: 10.7270/Q2XP780V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 2 (Homo sapiens (Human)) | BDBM50450442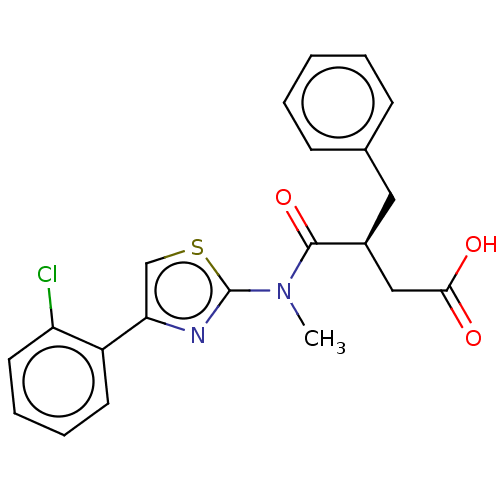 (CHEMBL4170872) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA Curated by ChEMBL | Assay Description Displacement of [3H]ES227703 from human FFA2R expressed in CHOK1 cells after 60 mins by TopCount scintillation counting method | Bioorg Med Chem 26: 5169-5180 (2018) Article DOI: 10.1016/j.bmc.2018.09.015 BindingDB Entry DOI: 10.7270/Q2RV0R8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50584761 (BUFALIN | Bufalin | CHEBI:517248) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 2.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inactivation of CYP3A4 in human liver microsomes assessed as inhibition constant using midazolam as substrate preincubated for 5 mins followed by bet... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01875 BindingDB Entry DOI: 10.7270/Q2PZ5DQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50355892 (CHEMBL1910035) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in CHO cells coexpressing Galpha15/16 assessed as inhibition of CP55940-induced Ca2+ release afte... | Eur J Med Chem 46: 5310-6 (2011) Article DOI: 10.1016/j.ejmech.2011.08.030 BindingDB Entry DOI: 10.7270/Q22J6C85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50602881 (CHEMBL5204688) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01864 BindingDB Entry DOI: 10.7270/Q2VT1X5F | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50602873 (CHEMBL5191045) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01864 BindingDB Entry DOI: 10.7270/Q2VT1X5F | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1609 total ) | Next | Last >> |