 Found 72 hits with Last Name = 'zugnoni' and Initial = 'p'
Found 72 hits with Last Name = 'zugnoni' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327930

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES C[C@@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C25H25N5O3S/c1-16(17-5-3-2-4-6-17)26-25(32)21-15-20-22(34-21)23(29-28-20)27-24(31)18-7-9-19(10-8-18)30-11-13-33-14-12-30/h2-10,15-16H,11-14H2,1H3,(H,26,32)(H2,27,28,29,31)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12983
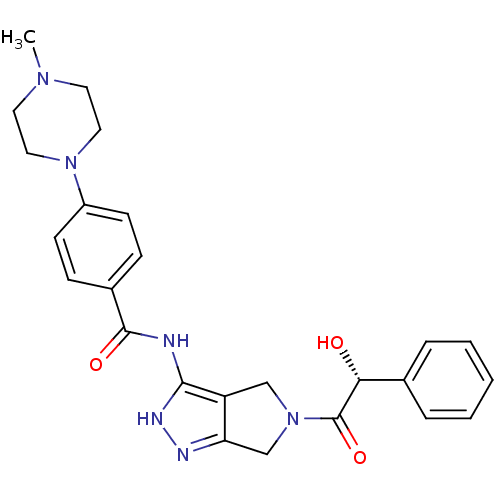
(5-Amido-pyrrolopyrazole 9b | CHEMBL385872 | N-{5-[...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2CN(Cc12)C(=O)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C25H28N6O3/c1-29-11-13-30(14-12-29)19-9-7-18(8-10-19)24(33)26-23-20-15-31(16-21(20)27-28-23)25(34)22(32)17-5-3-2-4-6-17/h2-10,22,32H,11-16H2,1H3,(H2,26,27,28,33)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 49: 7247-51 (2006)
Article DOI: 10.1021/jm060897w
BindingDB Entry DOI: 10.7270/Q2NS0S4R |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327929
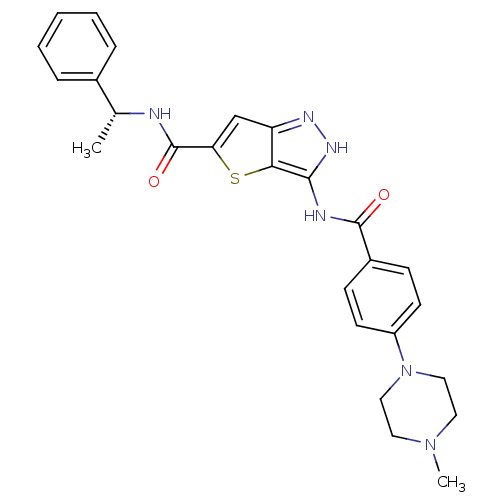
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES C[C@@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C26H28N6O2S/c1-17(18-6-4-3-5-7-18)27-26(34)22-16-21-23(35-22)24(30-29-21)28-25(33)19-8-10-20(11-9-19)32-14-12-31(2)13-15-32/h3-11,16-17H,12-15H2,1-2H3,(H,27,34)(H2,28,29,30,33)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327928
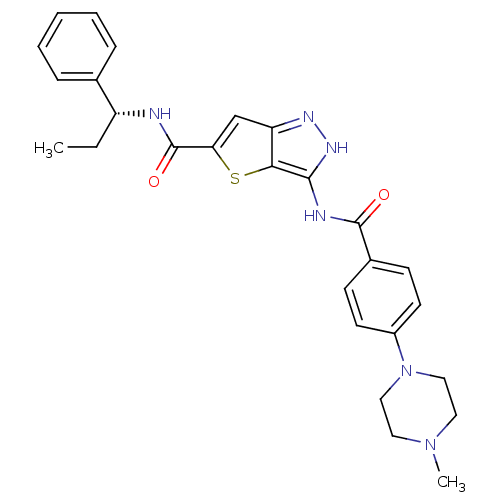
(3-({[4-(4-METHYLPIPERAZIN-1-YL)PHENYL]CARBONYL}AMI...)Show SMILES CC[C@@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C27H30N6O2S/c1-3-21(18-7-5-4-6-8-18)28-27(35)23-17-22-24(36-23)25(31-30-22)29-26(34)19-9-11-20(12-10-19)33-15-13-32(2)14-16-33/h4-12,17,21H,3,13-16H2,1-2H3,(H,28,35)(H2,29,30,31,34)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12982
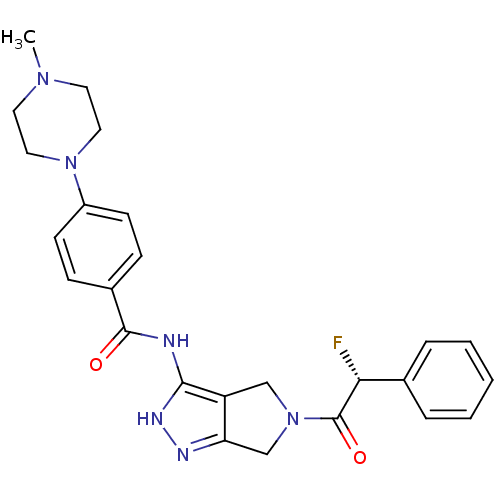
(5-Amido-pyrrolopyrazole 9a | CHEMBL385266 | N-{5-[...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2CN(Cc12)C(=O)[C@H](F)c1ccccc1 |r| Show InChI InChI=1S/C25H27FN6O2/c1-30-11-13-31(14-12-30)19-9-7-18(8-10-19)24(33)27-23-20-15-32(16-21(20)28-29-23)25(34)22(26)17-5-3-2-4-6-17/h2-10,22H,11-16H2,1H3,(H2,27,28,29,33)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 49: 7247-51 (2006)
Article DOI: 10.1021/jm060897w
BindingDB Entry DOI: 10.7270/Q2NS0S4R |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327923
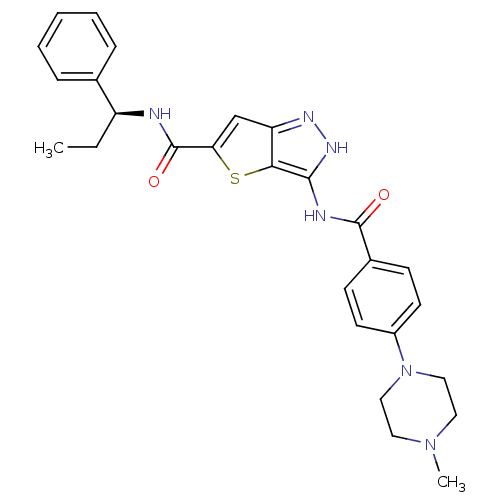
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES CC[C@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C27H30N6O2S/c1-3-21(18-7-5-4-6-8-18)28-27(35)23-17-22-24(36-23)25(31-30-22)29-26(34)19-9-11-20(12-10-19)33-15-13-32(2)14-16-33/h4-12,17,21H,3,13-16H2,1-2H3,(H,28,35)(H2,29,30,31,34)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327927
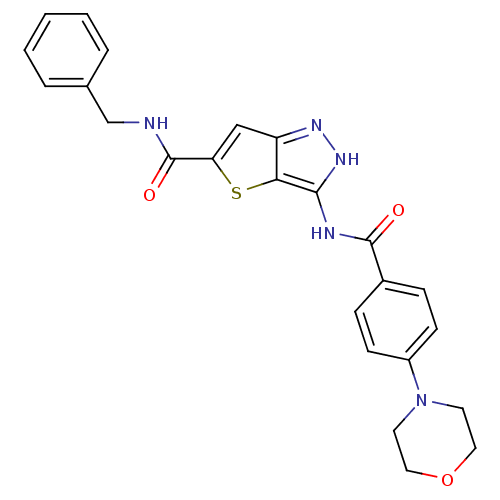
(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(NCc1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 Show InChI InChI=1S/C24H23N5O3S/c30-23(17-6-8-18(9-7-17)29-10-12-32-13-11-29)26-22-21-19(27-28-22)14-20(33-21)24(31)25-15-16-4-2-1-3-5-16/h1-9,14H,10-13,15H2,(H,25,31)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12985
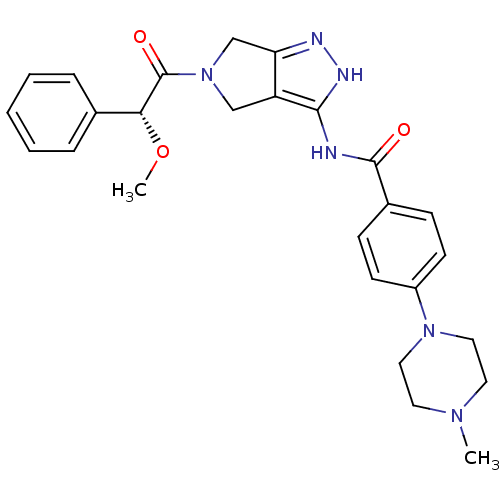
(5-Amido-pyrrolopyrazole 9d | CHEMBL402548 | N-{5-[...)Show SMILES CO[C@@H](C(=O)N1Cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2C1)c1ccccc1 |r| Show InChI InChI=1S/C26H30N6O3/c1-30-12-14-31(15-13-30)20-10-8-19(9-11-20)25(33)27-24-21-16-32(17-22(21)28-29-24)26(34)23(35-2)18-6-4-3-5-7-18/h3-11,23H,12-17H2,1-2H3,(H2,27,28,29,33)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 49: 7247-51 (2006)
Article DOI: 10.1021/jm060897w
BindingDB Entry DOI: 10.7270/Q2NS0S4R |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327915
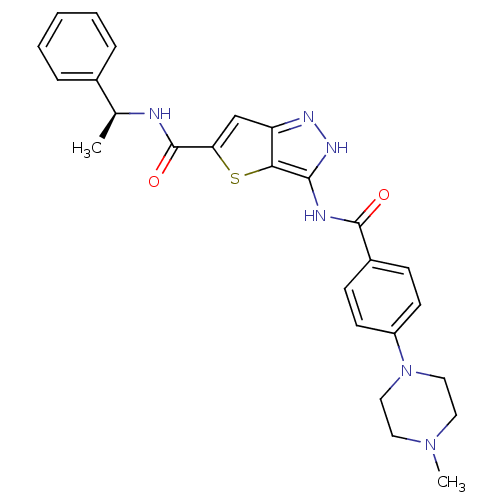
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES C[C@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C26H28N6O2S/c1-17(18-6-4-3-5-7-18)27-26(34)22-16-21-23(35-22)24(30-29-21)28-25(33)19-8-10-20(11-9-19)32-14-12-31(2)13-15-32/h3-11,16-17H,12-15H2,1-2H3,(H,27,34)(H2,28,29,30,33)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50327912
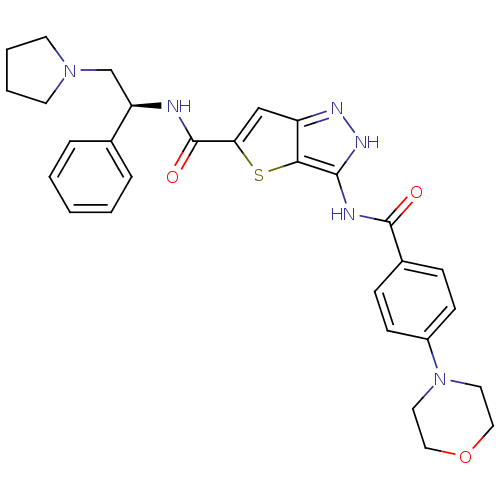
(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327912

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327926
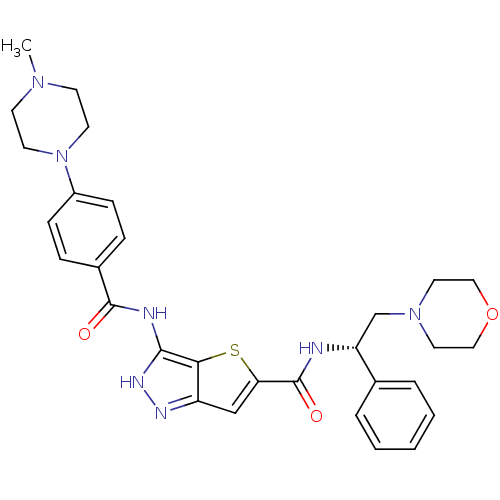
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2cc(sc12)C(=O)N[C@H](CN1CCOCC1)c1ccccc1 |r| Show InChI InChI=1S/C30H35N7O3S/c1-35-11-13-37(14-12-35)23-9-7-22(8-10-23)29(38)32-28-27-24(33-34-28)19-26(41-27)30(39)31-25(21-5-3-2-4-6-21)20-36-15-17-40-18-16-36/h2-10,19,25H,11-18,20H2,1H3,(H,31,39)(H2,32,33,34,38)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM50327912

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR3 |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12984
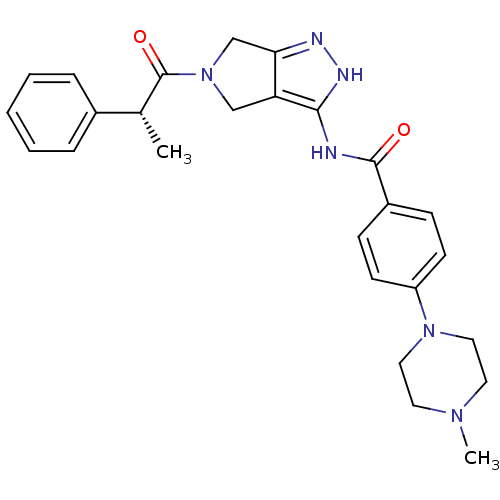
(4-(4-methylpiperazin-1-yl)-N-{5-[(2R)-2-phenylprop...)Show SMILES C[C@@H](C(=O)N1Cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2C1)c1ccccc1 |r| Show InChI InChI=1S/C26H30N6O2/c1-18(19-6-4-3-5-7-19)26(34)32-16-22-23(17-32)28-29-24(22)27-25(33)20-8-10-21(11-9-20)31-14-12-30(2)13-15-31/h3-11,18H,12-17H2,1-2H3,(H2,27,28,29,33)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 49: 7247-51 (2006)
Article DOI: 10.1021/jm060897w
BindingDB Entry DOI: 10.7270/Q2NS0S4R |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327925
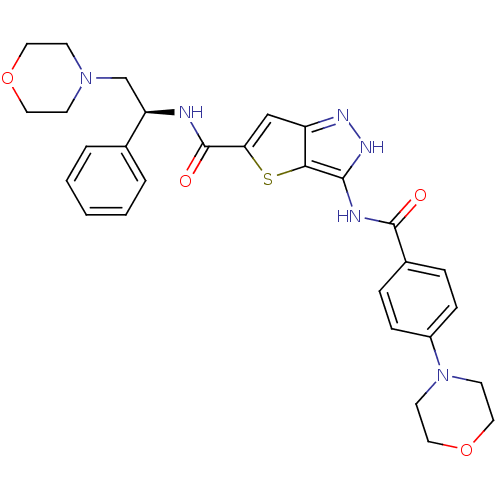
(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCOCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O4S/c36-28(21-6-8-22(9-7-21)35-12-16-39-17-13-35)31-27-26-23(32-33-27)18-25(40-26)29(37)30-24(20-4-2-1-3-5-20)19-34-10-14-38-15-11-34/h1-9,18,24H,10-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12110
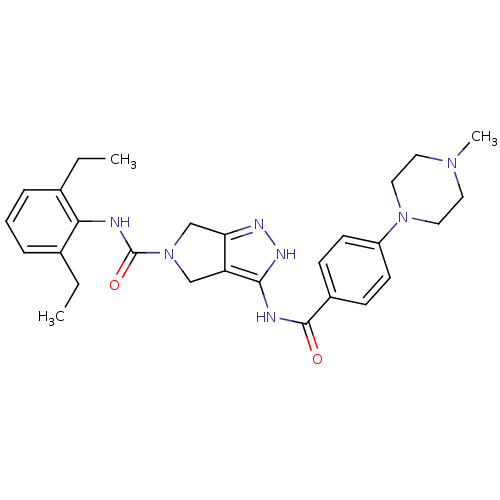
(1,4,5,6-Tetrahydropyrrolo[3,4-c]pyrazole 18 | 5-N-...)Show SMILES CCc1cccc(CC)c1NC(=O)N1Cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2C1 Show InChI InChI=1S/C28H35N7O2/c1-4-19-7-6-8-20(5-2)25(19)29-28(37)35-17-23-24(18-35)31-32-26(23)30-27(36)21-9-11-22(12-10-21)34-15-13-33(3)14-16-34/h6-12H,4-5,13-18H2,1-3H3,(H,29,37)(H2,30,31,32,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 49: 7247-51 (2006)
Article DOI: 10.1021/jm060897w
BindingDB Entry DOI: 10.7270/Q2NS0S4R |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327924
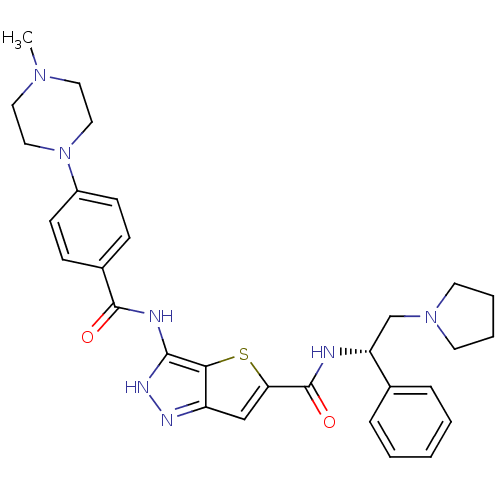
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2cc(sc12)C(=O)N[C@H](CN1CCCC1)c1ccccc1 |r| Show InChI InChI=1S/C30H35N7O2S/c1-35-15-17-37(18-16-35)23-11-9-22(10-12-23)29(38)32-28-27-24(33-34-28)19-26(40-27)30(39)31-25(20-36-13-5-6-14-36)21-7-3-2-4-8-21/h2-4,7-12,19,25H,5-6,13-18,20H2,1H3,(H,31,39)(H2,32,33,34,38)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327923

(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES CC[C@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C27H30N6O2S/c1-3-21(18-7-5-4-6-8-18)28-27(35)23-17-22-24(36-23)25(31-30-22)29-26(34)19-9-11-20(12-10-19)33-15-13-32(2)14-16-33/h4-12,17,21H,3,13-16H2,1-2H3,(H,28,35)(H2,29,30,31,34)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327922
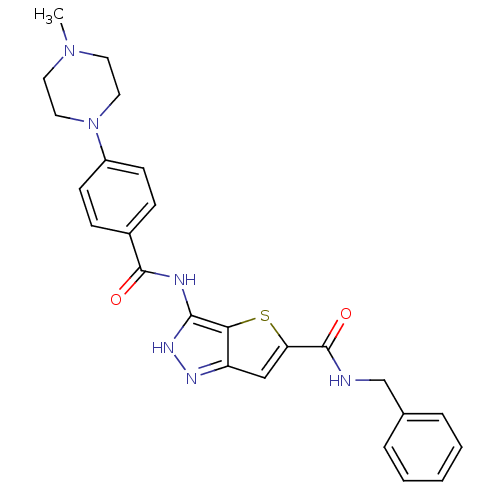
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2cc(sc12)C(=O)NCc1ccccc1 Show InChI InChI=1S/C25H26N6O2S/c1-30-11-13-31(14-12-30)19-9-7-18(8-10-19)24(32)27-23-22-20(28-29-23)15-21(34-22)25(33)26-16-17-5-3-2-4-6-17/h2-10,15H,11-14,16H2,1H3,(H,26,33)(H2,27,28,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50327912

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of KIT |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327921
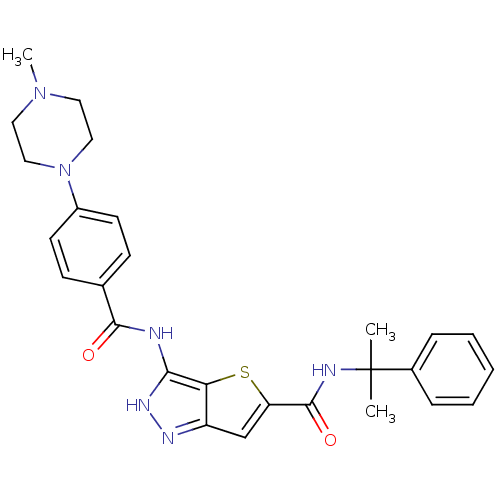
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2cc(sc12)C(=O)NC(C)(C)c1ccccc1 Show InChI InChI=1S/C27H30N6O2S/c1-27(2,19-7-5-4-6-8-19)29-26(35)22-17-21-23(36-22)24(31-30-21)28-25(34)18-9-11-20(12-10-18)33-15-13-32(3)14-16-33/h4-12,17H,13-16H2,1-3H3,(H,29,35)(H2,28,30,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327920
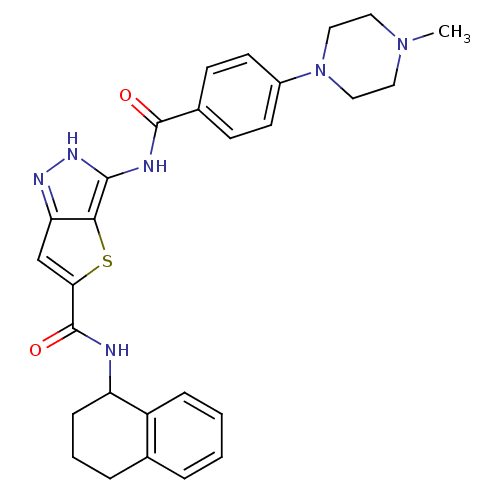
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2cc(sc12)C(=O)NC1CCCc2ccccc12 Show InChI InChI=1S/C28H30N6O2S/c1-33-13-15-34(16-14-33)20-11-9-19(10-12-20)27(35)30-26-25-23(31-32-26)17-24(37-25)28(36)29-22-8-4-6-18-5-2-3-7-21(18)22/h2-3,5,7,9-12,17,22H,4,6,8,13-16H2,1H3,(H,29,36)(H2,30,31,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50327912

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of aurora B |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327918
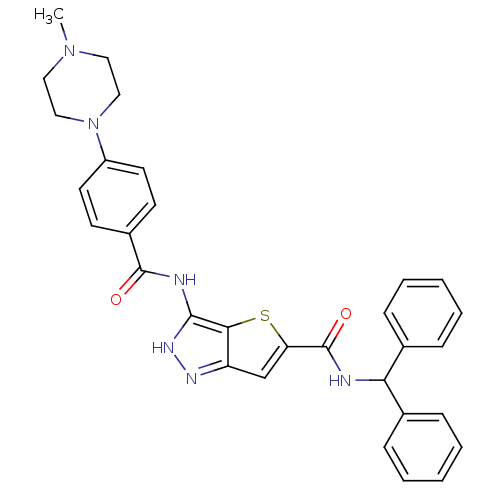
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2cc(sc12)C(=O)NC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C31H30N6O2S/c1-36-16-18-37(19-17-36)24-14-12-23(13-15-24)30(38)33-29-28-25(34-35-29)20-26(40-28)31(39)32-27(21-8-4-2-5-9-21)22-10-6-3-7-11-22/h2-15,20,27H,16-19H2,1H3,(H,32,39)(H2,33,34,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327919

(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES CCNC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2s1 Show InChI InChI=1S/C20H24N6O2S/c1-3-21-20(28)16-12-15-17(29-16)18(24-23-15)22-19(27)13-4-6-14(7-5-13)26-10-8-25(2)9-11-26/h4-7,12H,3,8-11H2,1-2H3,(H,21,28)(H2,22,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM12983

(5-Amido-pyrrolopyrazole 9b | CHEMBL385872 | N-{5-[...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2CN(Cc12)C(=O)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C25H28N6O3/c1-29-11-13-30(14-12-29)19-9-7-18(8-10-19)24(33)26-23-20-15-31(16-21(20)27-28-23)25(34)22(32)17-5-3-2-4-6-17/h2-10,22,32H,11-16H2,1H3,(H2,26,27,28,33)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 49: 7247-51 (2006)
Article DOI: 10.1021/jm060897w
BindingDB Entry DOI: 10.7270/Q2NS0S4R |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327917
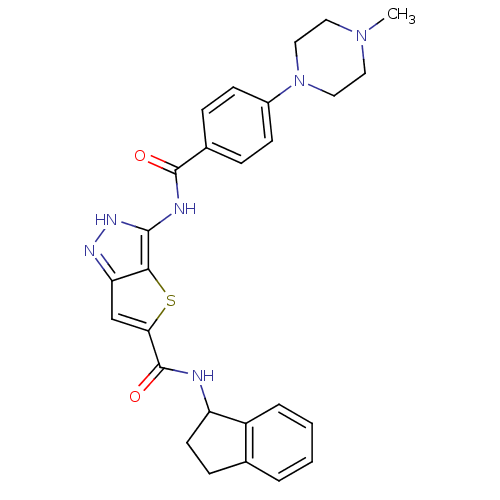
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2cc(sc12)C(=O)NC1CCc2ccccc12 Show InChI InChI=1S/C27H28N6O2S/c1-32-12-14-33(15-13-32)19-9-6-18(7-10-19)26(34)29-25-24-22(30-31-25)16-23(36-24)27(35)28-21-11-8-17-4-2-3-5-20(17)21/h2-7,9-10,16,21H,8,11-15H2,1H3,(H,28,35)(H2,29,30,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327916
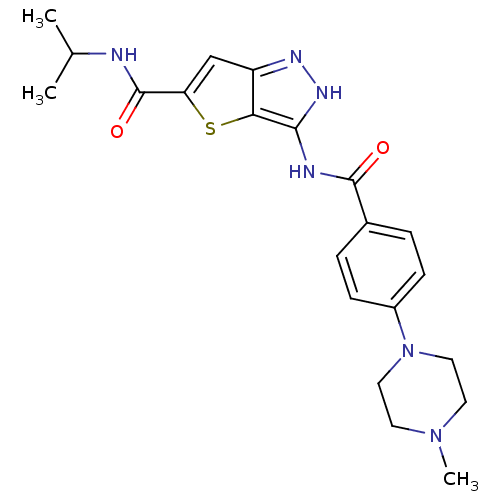
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES CC(C)NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2s1 Show InChI InChI=1S/C21H26N6O2S/c1-13(2)22-21(29)17-12-16-18(30-17)19(25-24-16)23-20(28)14-4-6-15(7-5-14)27-10-8-26(3)9-11-27/h4-7,12-13H,8-11H2,1-3H3,(H,22,29)(H2,23,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase C
(Homo sapiens (Human)) | BDBM50327912

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of aurora C |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12107

(1,4,5,6-Tetrahydropyrrolo[3,4-c]pyrazole 15 | 4-(4...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2CN(Cc12)C(=O)Cc1cccs1 Show InChI InChI=1S/C23H26N6O2S/c1-27-8-10-28(11-9-27)17-6-4-16(5-7-17)23(31)24-22-19-14-29(15-20(19)25-26-22)21(30)13-18-3-2-12-32-18/h2-7,12H,8-11,13-15H2,1H3,(H2,24,25,26,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 49: 7247-51 (2006)
Article DOI: 10.1021/jm060897w
BindingDB Entry DOI: 10.7270/Q2NS0S4R |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327915

(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES C[C@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C26H28N6O2S/c1-17(18-6-4-3-5-7-18)27-26(34)22-16-21-23(35-22)24(30-29-21)28-25(33)19-8-10-20(11-9-19)32-14-12-31(2)13-15-32/h3-11,16-17H,12-15H2,1-2H3,(H,27,34)(H2,28,29,30,33)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM12985

(5-Amido-pyrrolopyrazole 9d | CHEMBL402548 | N-{5-[...)Show SMILES CO[C@@H](C(=O)N1Cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2C1)c1ccccc1 |r| Show InChI InChI=1S/C26H30N6O3/c1-30-12-14-31(15-13-30)20-10-8-19(9-11-20)25(33)27-24-21-16-32(17-22(21)28-29-24)26(34)23(35-2)18-6-4-3-5-7-18/h3-11,23H,12-17H2,1-2H3,(H2,27,28,29,33)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 49: 7247-51 (2006)
Article DOI: 10.1021/jm060897w
BindingDB Entry DOI: 10.7270/Q2NS0S4R |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327914
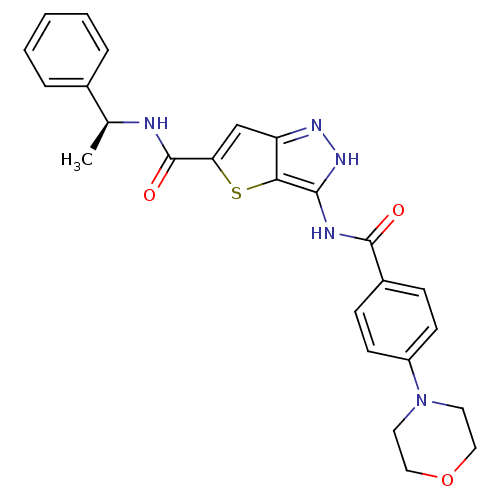
(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES C[C@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C25H25N5O3S/c1-16(17-5-3-2-4-6-17)26-25(32)21-15-20-22(34-21)23(29-28-20)27-24(31)18-7-9-19(10-8-18)30-11-13-33-14-12-30/h2-10,15-16H,11-14H2,1H3,(H,26,32)(H2,27,28,29,31)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM12984

(4-(4-methylpiperazin-1-yl)-N-{5-[(2R)-2-phenylprop...)Show SMILES C[C@@H](C(=O)N1Cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2C1)c1ccccc1 |r| Show InChI InChI=1S/C26H30N6O2/c1-18(19-6-4-3-5-7-19)26(34)32-16-22-23(17-32)28-29-24(22)27-25(33)20-8-10-21(11-9-20)31-14-12-30(2)13-15-31/h3-11,18H,12-17H2,1-2H3,(H2,27,28,29,33)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 49: 7247-51 (2006)
Article DOI: 10.1021/jm060897w
BindingDB Entry DOI: 10.7270/Q2NS0S4R |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327913
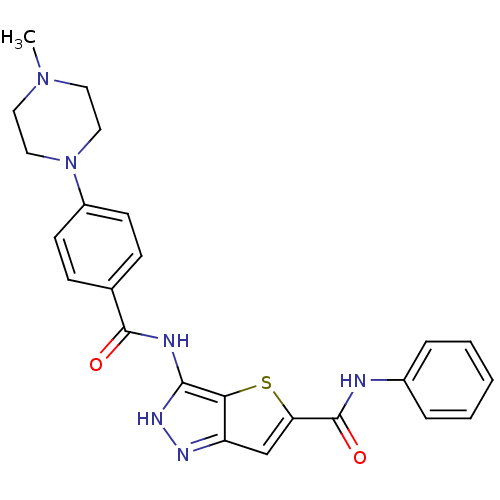
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2cc(sc12)C(=O)Nc1ccccc1 Show InChI InChI=1S/C24H24N6O2S/c1-29-11-13-30(14-12-29)18-9-7-16(8-10-18)23(31)26-22-21-19(27-28-22)15-20(33-21)24(32)25-17-5-3-2-4-6-17/h2-10,15H,11-14H2,1H3,(H,25,32)(H2,26,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50327912

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of RET |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12109

(1,4,5,6-Tetrahydropyrrolo[3,4-c]pyrazole 17 | 4-(4...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2CN(Cc12)C(=O)Cc1ccccc1 Show InChI InChI=1S/C25H28N6O2/c1-29-11-13-30(14-12-29)20-9-7-19(8-10-20)25(33)26-24-21-16-31(17-22(21)27-28-24)23(32)15-18-5-3-2-4-6-18/h2-10H,11-17H2,1H3,(H2,26,27,28,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 49: 7247-51 (2006)
Article DOI: 10.1021/jm060897w
BindingDB Entry DOI: 10.7270/Q2NS0S4R |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50327912

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50327912

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 181 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of TRKA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM12982

(5-Amido-pyrrolopyrazole 9a | CHEMBL385266 | N-{5-[...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2CN(Cc12)C(=O)[C@H](F)c1ccccc1 |r| Show InChI InChI=1S/C25H27FN6O2/c1-30-11-13-31(14-12-30)19-9-7-18(8-10-19)24(33)27-23-20-15-32(16-21(20)28-29-23)25(34)22(26)17-5-3-2-4-6-17/h2-10,22H,11-16H2,1H3,(H2,27,28,29,33)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 49: 7247-51 (2006)
Article DOI: 10.1021/jm060897w
BindingDB Entry DOI: 10.7270/Q2NS0S4R |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50327912

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 332 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of PKAalpha |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12987
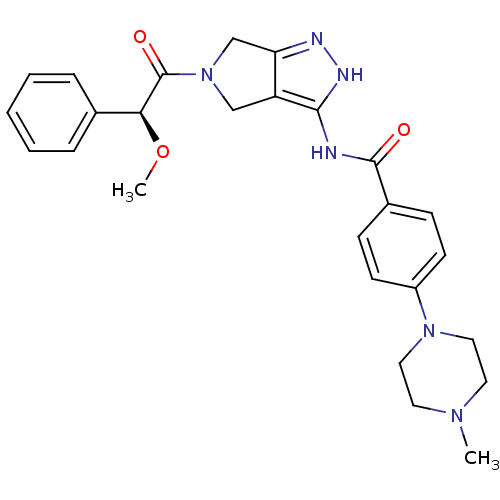
(5-Amido-pyrrolopyrazole 9f | CHEMBL384575 | N-{5-[...)Show SMILES CO[C@H](C(=O)N1Cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2C1)c1ccccc1 |r| Show InChI InChI=1S/C26H30N6O3/c1-30-12-14-31(15-13-30)20-10-8-19(9-11-20)25(33)27-24-21-16-32(17-22(21)28-29-24)26(34)23(35-2)18-6-4-3-5-7-18/h3-11,23H,12-17H2,1-2H3,(H2,27,28,29,33)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 354 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 49: 7247-51 (2006)
Article DOI: 10.1021/jm060897w
BindingDB Entry DOI: 10.7270/Q2NS0S4R |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 26
(Homo sapiens (Human)) | BDBM50327912

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 412 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of MST4 |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12986
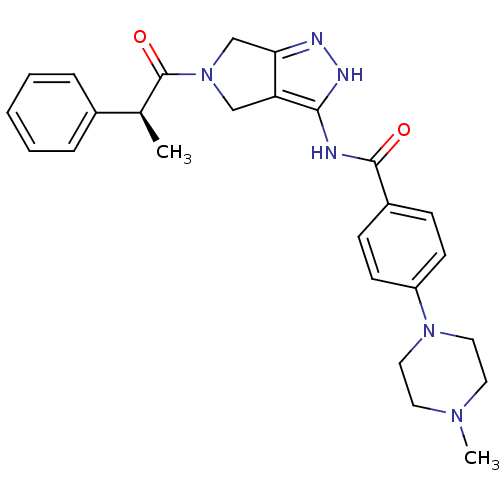
(4-(4-methylpiperazin-1-yl)-N-{5-[(2S)-2-phenylprop...)Show SMILES C[C@H](C(=O)N1Cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2C1)c1ccccc1 |r| Show InChI InChI=1S/C26H30N6O2/c1-18(19-6-4-3-5-7-19)26(34)32-16-22-23(17-32)28-29-24(22)27-25(33)20-8-10-21(11-9-20)31-14-12-30(2)13-15-31/h3-11,18H,12-17H2,1-2H3,(H2,27,28,29,33)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 452 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 49: 7247-51 (2006)
Article DOI: 10.1021/jm060897w
BindingDB Entry DOI: 10.7270/Q2NS0S4R |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50327912

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 455 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of PAK4 |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM12987

(5-Amido-pyrrolopyrazole 9f | CHEMBL384575 | N-{5-[...)Show SMILES CO[C@H](C(=O)N1Cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2C1)c1ccccc1 |r| Show InChI InChI=1S/C26H30N6O3/c1-30-12-14-31(15-13-30)20-10-8-19(9-11-20)25(33)27-24-21-16-32(17-22(21)28-29-24)26(34)23(35-2)18-6-4-3-5-7-18/h3-11,23H,12-17H2,1-2H3,(H2,27,28,29,33)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 49: 7247-51 (2006)
Article DOI: 10.1021/jm060897w
BindingDB Entry DOI: 10.7270/Q2NS0S4R |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50327912

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 762 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of PDK1 |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM12986

(4-(4-methylpiperazin-1-yl)-N-{5-[(2S)-2-phenylprop...)Show SMILES C[C@H](C(=O)N1Cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2C1)c1ccccc1 |r| Show InChI InChI=1S/C26H30N6O2/c1-18(19-6-4-3-5-7-19)26(34)32-16-22-23(17-32)28-29-24(22)27-25(33)20-8-10-21(11-9-20)31-14-12-30(2)13-15-31/h3-11,18H,12-17H2,1-2H3,(H2,27,28,29,33)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 49: 7247-51 (2006)
Article DOI: 10.1021/jm060897w
BindingDB Entry DOI: 10.7270/Q2NS0S4R |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50327912

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50327912

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data