 Found 455 hits with Last Name = 'de jong' and Initial = 'r'
Found 455 hits with Last Name = 'de jong' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50204291
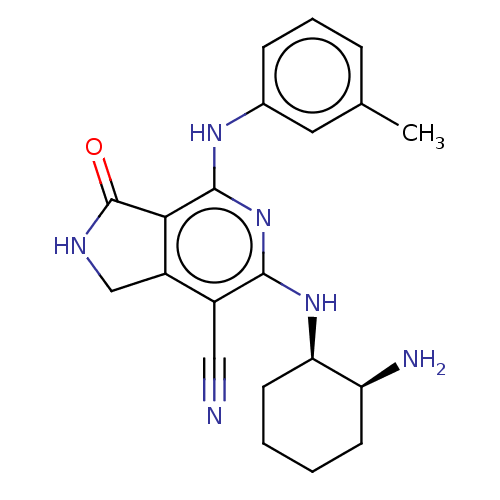
(CHEMBL3953104 | US20230295171, Example 76)Show SMILES Cc1cccc(Nc2nc(N[C@@H]3CCCC[C@@H]3N)c(C#N)c3CNC(=O)c23)c1 |r| Show InChI InChI=1S/C21H24N6O/c1-12-5-4-6-13(9-12)25-20-18-15(11-24-21(18)28)14(10-22)19(27-20)26-17-8-3-2-7-16(17)23/h4-6,9,16-17H,2-3,7-8,11,23H2,1H3,(H,24,28)(H2,25,26,27)/t16-,17+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal 6His-tagged SYK (356 to 635 residues) expressed in Sf9 insect cells using 5-carboxyfluorescein(FAM)-EEPLYWSFPAKKK-NH2 ... |
Bioorg Med Chem Lett 26: 5947-5950 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.087
BindingDB Entry DOI: 10.7270/Q29888Z2 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D]
(Homo sapiens (Human)) | BDBM16958
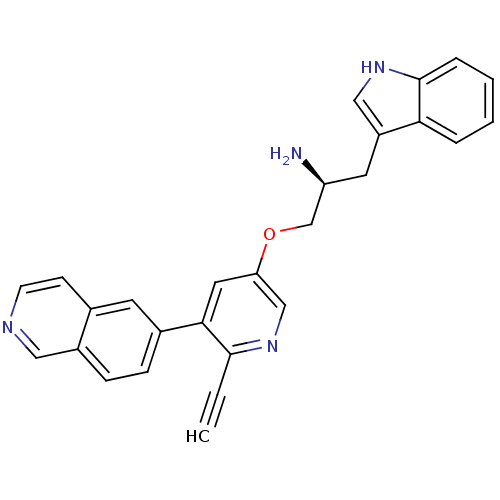
(6-{5-[(2S)-2-amino-3-(1H-indol-3-yl)propoxy]-2-eth...)Show SMILES N[C@H](COc1cnc(C#C)c(c1)-c1ccc2cnccc2c1)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C27H22N4O/c1-2-26-25(19-7-8-20-14-29-10-9-18(20)11-19)13-23(16-31-26)32-17-22(28)12-21-15-30-27-6-4-3-5-24(21)27/h1,3-11,13-16,22,30H,12,17,28H2/t22-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... |
Bioorg Med Chem Lett 16: 3150-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.041
BindingDB Entry DOI: 10.7270/Q2F769TT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50204296
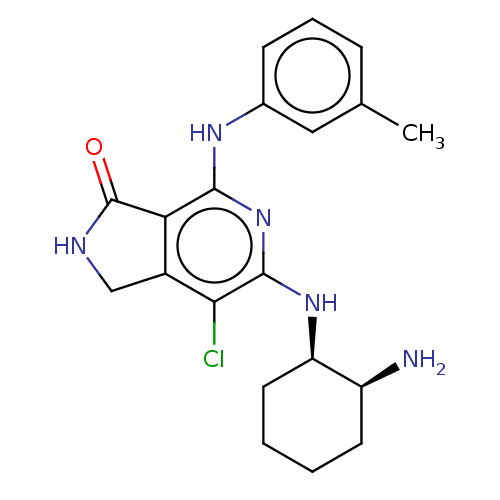
(CHEMBL3941633 | US20230295171, Example 75)Show SMILES Cc1cccc(Nc2nc(N[C@@H]3CCCC[C@@H]3N)c(Cl)c3CNC(=O)c23)c1 |r| Show InChI InChI=1S/C20H24ClN5O/c1-11-5-4-6-12(9-11)24-18-16-13(10-23-20(16)27)17(21)19(26-18)25-15-8-3-2-7-14(15)22/h4-6,9,14-15H,2-3,7-8,10,22H2,1H3,(H,23,27)(H2,24,25,26)/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal 6His-tagged SYK (356 to 635 residues) expressed in Sf9 insect cells using 5-carboxyfluorescein(FAM)-EEPLYWSFPAKKK-NH2 ... |
Bioorg Med Chem Lett 26: 5947-5950 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.087
BindingDB Entry DOI: 10.7270/Q29888Z2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM620222
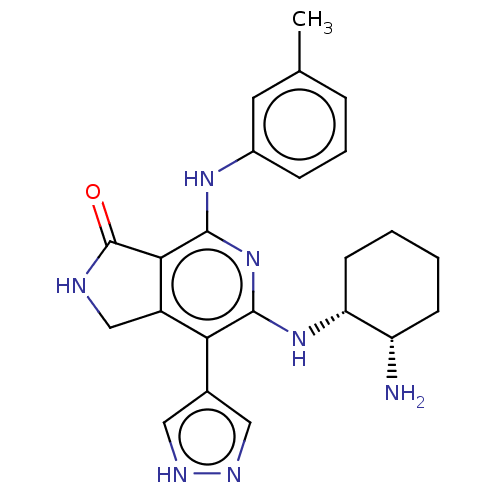
(BDBM50204292 | US20230295171, Example 82)Show SMILES Cc1cccc(Nc2nc(N[C@@H]3CCCC[C@@H]3N)c(-c3cn[nH]c3)c3CNC(=O)c23)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal 6His-tagged SYK (356 to 635 residues) expressed in Sf9 insect cells using 5-carboxyfluorescein(FAM)-EEPLYWSFPAKKK-NH2 ... |
Bioorg Med Chem Lett 26: 5947-5950 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.087
BindingDB Entry DOI: 10.7270/Q29888Z2 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Bos taurus (bovine)) | BDBM16958

(6-{5-[(2S)-2-amino-3-(1H-indol-3-yl)propoxy]-2-eth...)Show SMILES N[C@H](COc1cnc(C#C)c(c1)-c1ccc2cnccc2c1)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C27H22N4O/c1-2-26-25(19-7-8-20-14-29-10-9-18(20)11-19)13-23(16-31-26)32-17-22(28)12-21-15-30-27-6-4-3-5-24(21)27/h1,3-11,13-16,22,30H,12,17,28H2/t22-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The kinase assay uses purified recombinant enzyme and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidi... |
Bioorg Med Chem Lett 16: 3150-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.041
BindingDB Entry DOI: 10.7270/Q2F769TT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50234304

(CHEMBL4062894)Show SMILES CS(=O)(=O)N1CCC[C@@H](C1)c1csc(n1)-c1cc(ccc1NC(=O)c1ncc[nH]1)N1CCNCC1 |r| Show InChI InChI=1S/C23H29N7O3S2/c1-35(32,33)30-10-2-3-16(14-30)20-15-34-23(28-20)18-13-17(29-11-8-24-9-12-29)4-5-19(18)27-22(31)21-25-6-7-26-21/h4-7,13,15-16,24H,2-3,8-12,14H2,1H3,(H,25,26)(H,27,31)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of Axl (unknown origin) |
Bioorg Med Chem Lett 27: 1099-1104 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.024
BindingDB Entry DOI: 10.7270/Q2D50Q6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50204293
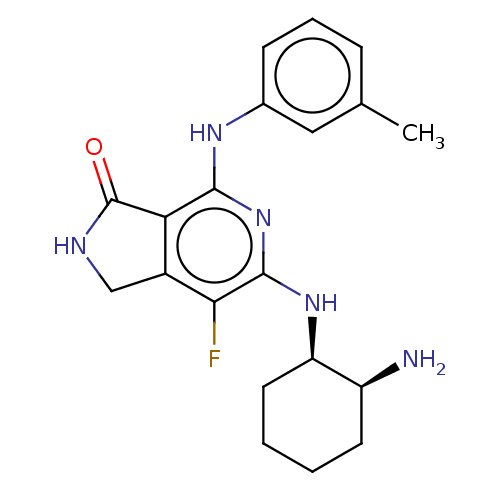
(CHEMBL3983415 | US20230295171, Example 25)Show SMILES Cc1cccc(Nc2nc(N[C@@H]3CCCC[C@@H]3N)c(F)c3CNC(=O)c23)c1 |r| Show InChI InChI=1S/C20H24FN5O/c1-11-5-4-6-12(9-11)24-18-16-13(10-23-20(16)27)17(21)19(26-18)25-15-8-3-2-7-14(15)22/h4-6,9,14-15H,2-3,7-8,10,22H2,1H3,(H,23,27)(H2,24,25,26)/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal 6His-tagged SYK (356 to 635 residues) expressed in Sf9 insect cells using 5-carboxyfluorescein(FAM)-EEPLYWSFPAKKK-NH2 ... |
Bioorg Med Chem Lett 26: 5947-5950 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.087
BindingDB Entry DOI: 10.7270/Q29888Z2 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D]
(Homo sapiens (Human)) | BDBM16957
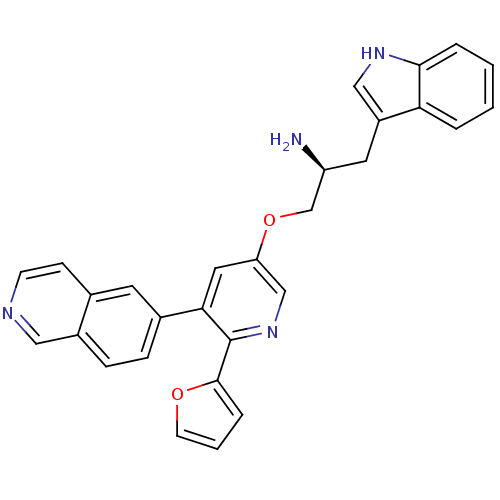
(6-{5-[(2S)-2-amino-3-(1H-indol-3-yl)propoxy]-2-(fu...)Show SMILES N[C@H](COc1cnc(-c2ccco2)c(c1)-c1ccc2cnccc2c1)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C29H24N4O2/c30-23(13-22-16-32-27-5-2-1-4-25(22)27)18-35-24-14-26(29(33-17-24)28-6-3-11-34-28)20-7-8-21-15-31-10-9-19(21)12-20/h1-12,14-17,23,32H,13,18,30H2/t23-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... |
Bioorg Med Chem Lett 16: 3150-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.041
BindingDB Entry DOI: 10.7270/Q2F769TT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50234305

(CHEMBL4090103)Show SMILES CN(C)CCOc1ccc(NC(=O)c2ncc[nH]2)c(c1)-c1nc(cs1)[C@H]1CCCN(C1)S(C)(=O)=O |r| Show InChI InChI=1S/C23H30N6O4S2/c1-28(2)11-12-33-17-6-7-19(26-22(30)21-24-8-9-25-21)18(13-17)23-27-20(15-34-23)16-5-4-10-29(14-16)35(3,31)32/h6-9,13,15-16H,4-5,10-12,14H2,1-3H3,(H,24,25)(H,26,30)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of Axl (unknown origin) |
Bioorg Med Chem Lett 27: 1099-1104 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.024
BindingDB Entry DOI: 10.7270/Q2D50Q6F |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D]
(Homo sapiens (Human)) | BDBM16954

(6-{5-[(2S)-2-amino-3-(1H-indol-3-yl)propoxy]-2-chl...)Show SMILES N[C@H](COc1cnc(Cl)c(c1)-c1ccc2cnccc2c1)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C25H21ClN4O/c26-25-23(17-5-6-18-12-28-8-7-16(18)9-17)11-21(14-30-25)31-15-20(27)10-19-13-29-24-4-2-1-3-22(19)24/h1-9,11-14,20,29H,10,15,27H2/t20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... |
Bioorg Med Chem Lett 16: 3150-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.041
BindingDB Entry DOI: 10.7270/Q2F769TT |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D]
(Homo sapiens (Human)) | BDBM16956

(6-{5-[(2S)-2-amino-3-(1H-indol-3-yl)propoxy]-2-eth...)Show SMILES N[C@H](COc1cnc(C=C)c(c1)-c1ccc2cnccc2c1)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C27H24N4O/c1-2-26-25(19-7-8-20-14-29-10-9-18(20)11-19)13-23(16-31-26)32-17-22(28)12-21-15-30-27-6-4-3-5-24(21)27/h2-11,13-16,22,30H,1,12,17,28H2/t22-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... |
Bioorg Med Chem Lett 16: 3150-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.041
BindingDB Entry DOI: 10.7270/Q2F769TT |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D]
(Homo sapiens (Human)) | BDBM15067
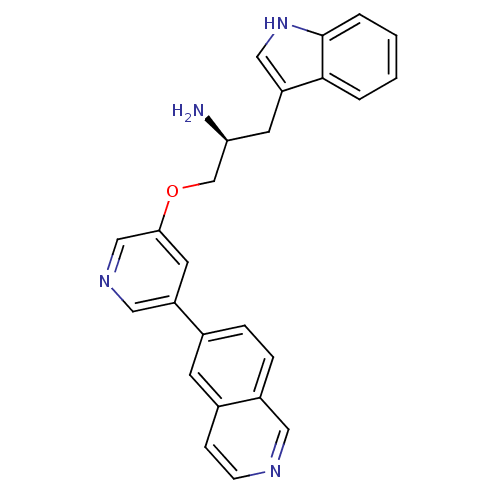
((2S)-1-(1H-indol-3-yl)-3-(5-isoquinolin-6-ylpyridi...)Show SMILES N[C@H](COc1cncc(c1)-c1ccc2cnccc2c1)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C25H22N4O/c26-22(10-21-14-29-25-4-2-1-3-24(21)25)16-30-23-11-20(13-28-15-23)17-5-6-19-12-27-8-7-18(19)9-17/h1-9,11-15,22,29H,10,16,26H2/t22-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... |
Bioorg Med Chem Lett 16: 3150-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.041
BindingDB Entry DOI: 10.7270/Q2F769TT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50204290
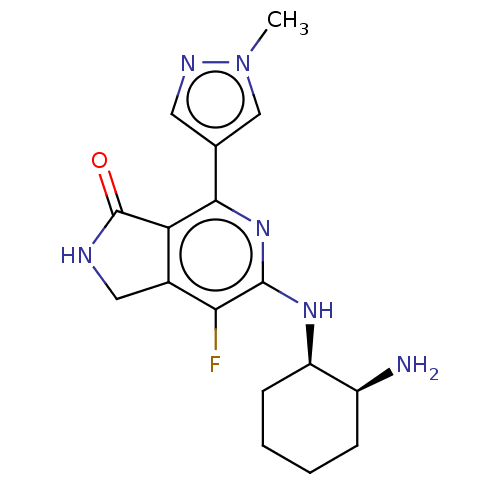
(CHEMBL3979920 | US11077111, Compound IIIa | US2023...)Show SMILES Cn1cc(cn1)-c1nc(N[C@@H]2CCCC[C@@H]2N)c(F)c2CNC(=O)c12 |r| Show InChI InChI=1S/C17H21FN6O/c1-24-8-9(6-21-24)15-13-10(7-20-17(13)25)14(18)16(23-15)22-12-5-3-2-4-11(12)19/h6,8,11-12H,2-5,7,19H2,1H3,(H,20,25)(H,22,23)/t11-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal GST-tagged SYK cytoplasmic domain expressed in baculovirus expression system by Z'-LYTE assay |
Bioorg Med Chem Lett 26: 5947-5950 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.087
BindingDB Entry DOI: 10.7270/Q29888Z2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50234306
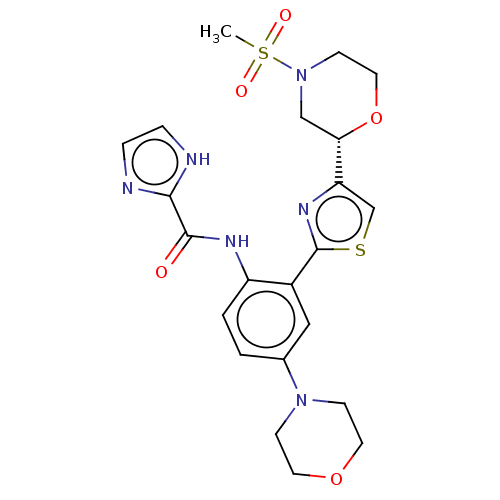
(CHEMBL4094158)Show SMILES CS(=O)(=O)N1CCO[C@@H](C1)c1csc(n1)-c1cc(ccc1NC(=O)c1ncc[nH]1)N1CCOCC1 |r| Show InChI InChI=1S/C22H26N6O5S2/c1-35(30,31)28-8-11-33-19(13-28)18-14-34-22(26-18)16-12-15(27-6-9-32-10-7-27)2-3-17(16)25-21(29)20-23-4-5-24-20/h2-5,12,14,19H,6-11,13H2,1H3,(H,23,24)(H,25,29)/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of Axl (unknown origin) |
Bioorg Med Chem Lett 27: 1099-1104 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.024
BindingDB Entry DOI: 10.7270/Q2D50Q6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50204295
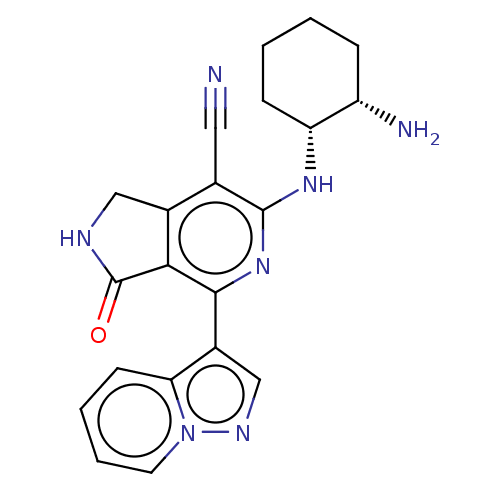
(CHEMBL3944381)Show SMILES N[C@H]1CCCC[C@H]1Nc1nc(-c2cnn3ccccc23)c2C(=O)NCc2c1C#N |r| Show InChI InChI=1S/C21H21N7O/c22-9-12-13-10-24-21(29)18(13)19(14-11-25-28-8-4-3-7-17(14)28)27-20(12)26-16-6-2-1-5-15(16)23/h3-4,7-8,11,15-16H,1-2,5-6,10,23H2,(H,24,29)(H,26,27)/t15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal 6His-tagged SYK (356 to 635 residues) expressed in Sf9 insect cells using 5-carboxyfluorescein(FAM)-EEPLYWSFPAKKK-NH2 ... |
Bioorg Med Chem Lett 26: 5947-5950 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.087
BindingDB Entry DOI: 10.7270/Q29888Z2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50204288
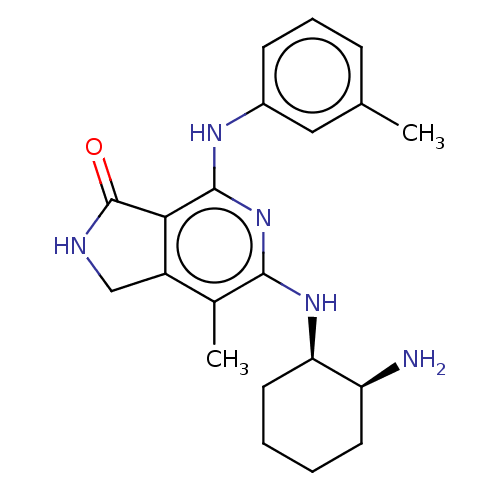
(CHEMBL3925430 | US20230295171, Example 88)Show SMILES Cc1cccc(Nc2nc(N[C@@H]3CCCC[C@@H]3N)c(C)c3CNC(=O)c23)c1 |r| Show InChI InChI=1S/C21H27N5O/c1-12-6-5-7-14(10-12)24-20-18-15(11-23-21(18)27)13(2)19(26-20)25-17-9-4-3-8-16(17)22/h5-7,10,16-17H,3-4,8-9,11,22H2,1-2H3,(H,23,27)(H2,24,25,26)/t16-,17+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal 6His-tagged SYK (356 to 635 residues) expressed in Sf9 insect cells using 5-carboxyfluorescein(FAM)-EEPLYWSFPAKKK-NH2 ... |
Bioorg Med Chem Lett 26: 5947-5950 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.087
BindingDB Entry DOI: 10.7270/Q29888Z2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50204290

(CHEMBL3979920 | US11077111, Compound IIIa | US2023...)Show SMILES Cn1cc(cn1)-c1nc(N[C@@H]2CCCC[C@@H]2N)c(F)c2CNC(=O)c12 |r| Show InChI InChI=1S/C17H21FN6O/c1-24-8-9(6-21-24)15-13-10(7-20-17(13)25)14(18)16(23-15)22-12-5-3-2-4-11(12)19/h6,8,11-12H,2-5,7,19H2,1H3,(H,20,25)(H,22,23)/t11-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal 6His-tagged SYK (356 to 635 residues) expressed in Sf9 insect cells using 5-carboxyfluorescein(FAM)-EEPLYWSFPAKKK-NH2 ... |
Bioorg Med Chem Lett 26: 5947-5950 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.087
BindingDB Entry DOI: 10.7270/Q29888Z2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50234303
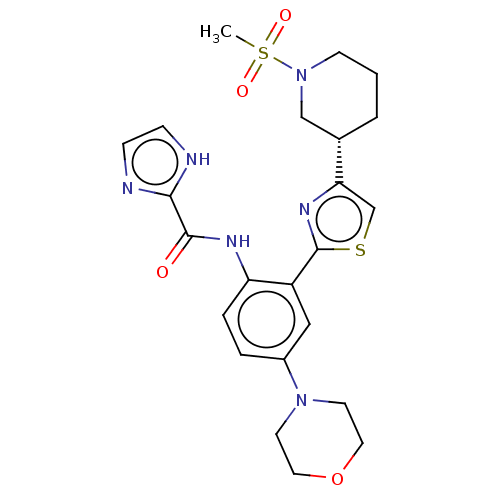
(CHEMBL4066616)Show SMILES CS(=O)(=O)N1CCC[C@@H](C1)c1csc(n1)-c1cc(ccc1NC(=O)c1ncc[nH]1)N1CCOCC1 |r| Show InChI InChI=1S/C23H28N6O4S2/c1-35(31,32)29-8-2-3-16(14-29)20-15-34-23(27-20)18-13-17(28-9-11-33-12-10-28)4-5-19(18)26-22(30)21-24-6-7-25-21/h4-7,13,15-16H,2-3,8-12,14H2,1H3,(H,24,25)(H,26,30)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of Axl (unknown origin) |
Bioorg Med Chem Lett 27: 1099-1104 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.024
BindingDB Entry DOI: 10.7270/Q2D50Q6F |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D]
(Homo sapiens (Human)) | BDBM16978
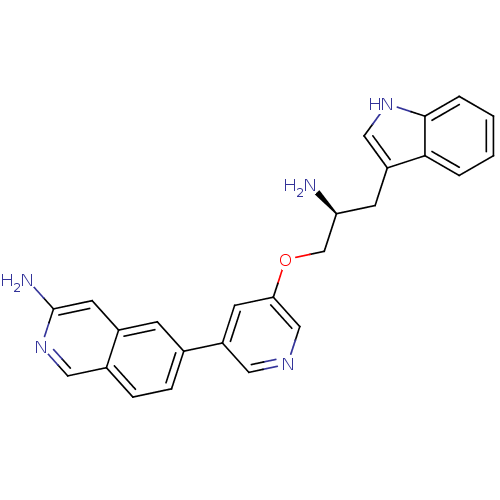
(6-{5-[(2S)-2-amino-3-(1H-indol-3-yl)propoxy]pyridi...)Show SMILES N[C@H](COc1cncc(c1)-c1ccc2cnc(N)cc2c1)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C25H23N5O/c26-21(8-20-13-29-24-4-2-1-3-23(20)24)15-31-22-9-19(11-28-14-22)16-5-6-17-12-30-25(27)10-18(17)7-16/h1-7,9-14,21,29H,8,15,26H2,(H2,27,30)/t21-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... |
Bioorg Med Chem Lett 16: 3150-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.041
BindingDB Entry DOI: 10.7270/Q2F769TT |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D]
(Homo sapiens (Human)) | BDBM16968
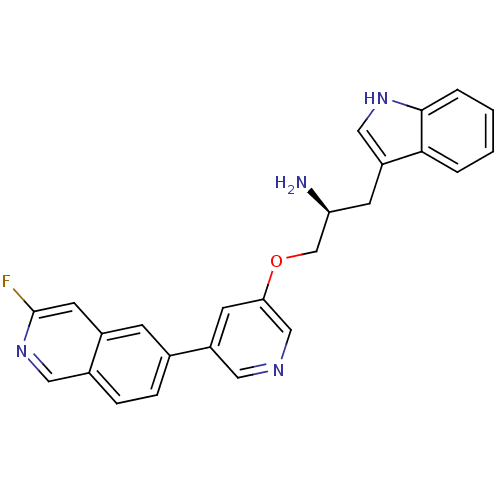
(6-{5-[(2S)-2-amino-3-(1H-indol-3-yl)propoxy]pyridi...)Show SMILES N[C@H](COc1cncc(c1)-c1ccc2cnc(F)cc2c1)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C25H21FN4O/c26-25-10-18-7-16(5-6-17(18)12-30-25)19-9-22(14-28-11-19)31-15-21(27)8-20-13-29-24-4-2-1-3-23(20)24/h1-7,9-14,21,29H,8,15,27H2/t21-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... |
Bioorg Med Chem Lett 16: 3150-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.041
BindingDB Entry DOI: 10.7270/Q2F769TT |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D]
(Homo sapiens (Human)) | BDBM16955
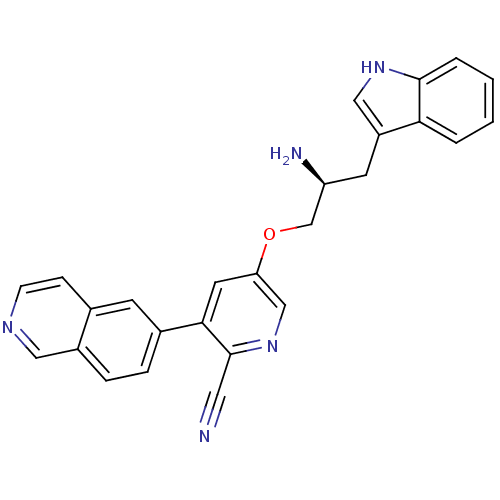
(5-[(2S)-2-amino-3-(1H-indol-3-yl)propoxy]-3-(isoqu...)Show SMILES N[C@H](COc1cnc(C#N)c(c1)-c1ccc2cnccc2c1)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C26H21N5O/c27-12-26-24(18-5-6-19-13-29-8-7-17(19)9-18)11-22(15-31-26)32-16-21(28)10-20-14-30-25-4-2-1-3-23(20)25/h1-9,11,13-15,21,30H,10,16,28H2/t21-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... |
Bioorg Med Chem Lett 16: 3150-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.041
BindingDB Entry DOI: 10.7270/Q2F769TT |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50204290

(CHEMBL3979920 | US11077111, Compound IIIa | US2023...)Show SMILES Cn1cc(cn1)-c1nc(N[C@@H]2CCCC[C@@H]2N)c(F)c2CNC(=O)c12 |r| Show InChI InChI=1S/C17H21FN6O/c1-24-8-9(6-21-24)15-13-10(7-20-17(13)25)14(18)16(23-15)22-12-5-3-2-4-11(12)19/h6,8,11-12H,2-5,7,19H2,1H3,(H,20,25)(H,22,23)/t11-,12+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal 6His-tagged FLT3 (564 to 993 residues) expressed in Sf9 insect cells using 5-carboxyfluorescein(FAM)-KKKKEEIYFFFG-NH2 ... |
Bioorg Med Chem Lett 26: 5947-5950 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.087
BindingDB Entry DOI: 10.7270/Q29888Z2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50204289
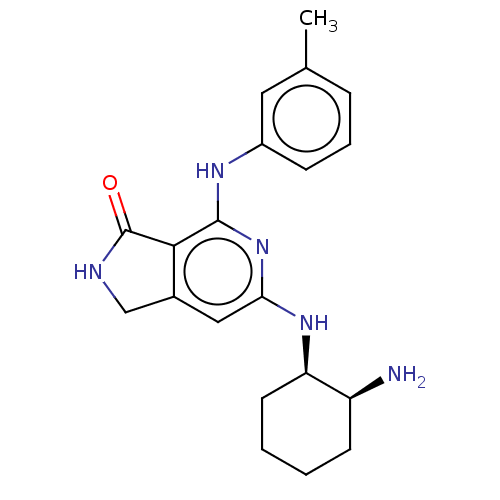
(CHEMBL3892927 | US20230295171, Example 13)Show SMILES Cc1cccc(Nc2nc(N[C@@H]3CCCC[C@@H]3N)cc3CNC(=O)c23)c1 |r| Show InChI InChI=1S/C20H25N5O/c1-12-5-4-6-14(9-12)23-19-18-13(11-22-20(18)26)10-17(25-19)24-16-8-3-2-7-15(16)21/h4-6,9-10,15-16H,2-3,7-8,11,21H2,1H3,(H,22,26)(H2,23,24,25)/t15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal 6His-tagged SYK (356 to 635 residues) expressed in Sf9 insect cells using 5-carboxyfluorescein(FAM)-EEPLYWSFPAKKK-NH2 ... |
Bioorg Med Chem Lett 26: 5947-5950 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.087
BindingDB Entry DOI: 10.7270/Q29888Z2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317947
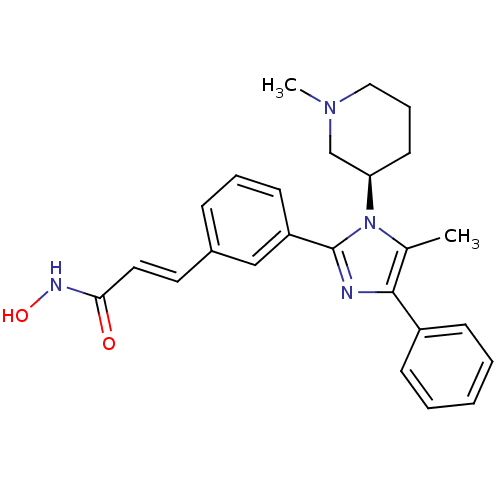
((R)-N-hydroxy-3-(3-(5-methyl-1-(1-methylpiperidin-...)Show SMILES CN1CCC[C@H](C1)n1c(C)c(nc1-c1cccc(\C=C\C(=O)NO)c1)-c1ccccc1 |r| Show InChI InChI=1S/C25H28N4O2/c1-18-24(20-9-4-3-5-10-20)26-25(29(18)22-12-7-15-28(2)17-22)21-11-6-8-19(16-21)13-14-23(30)27-31/h3-6,8-11,13-14,16,22,31H,7,12,15,17H2,1-2H3,(H,27,30)/b14-13+/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317949
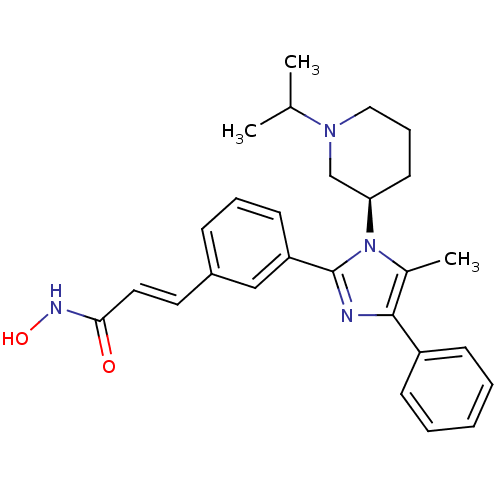
((R)-N-hydroxy-3-(3-(1-(1-isopropylpiperidin-3-yl)-...)Show SMILES CC(C)N1CCC[C@H](C1)n1c(C)c(nc1-c1cccc(\C=C\C(=O)NO)c1)-c1ccccc1 |r| Show InChI InChI=1S/C27H32N4O2/c1-19(2)30-16-8-13-24(18-30)31-20(3)26(22-10-5-4-6-11-22)28-27(31)23-12-7-9-21(17-23)14-15-25(32)29-33/h4-7,9-12,14-15,17,19,24,33H,8,13,16,18H2,1-3H3,(H,29,32)/b15-14+/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317960

(3-(3-(5-benzyl-4-methyl-1-phenethyl-1H-imidazol-2-...)Show SMILES Cc1nc(-c2cccc(\C=C\C(=O)NO)c2)n(CCc2ccccc2)c1Cc1ccccc1 Show InChI InChI=1S/C28H27N3O2/c1-21-26(20-23-11-6-3-7-12-23)31(18-17-22-9-4-2-5-10-22)28(29-21)25-14-8-13-24(19-25)15-16-27(32)30-33/h2-16,19,33H,17-18,20H2,1H3,(H,30,32)/b16-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317946
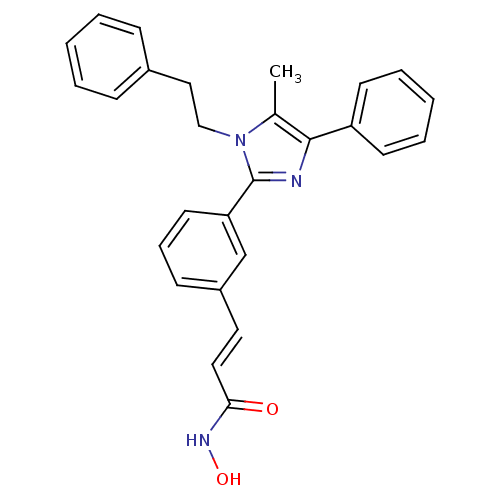
(CHEMBL1095451 | N-hydroxy-3-(3-(5-methyl-1-pheneth...)Show SMILES Cc1c(nc(-c2cccc(\C=C\C(=O)NO)c2)n1CCc1ccccc1)-c1ccccc1 Show InChI InChI=1S/C27H25N3O2/c1-20-26(23-12-6-3-7-13-23)28-27(30(20)18-17-21-9-4-2-5-10-21)24-14-8-11-22(19-24)15-16-25(31)29-32/h2-16,19,32H,17-18H2,1H3,(H,29,31)/b16-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM16958

(6-{5-[(2S)-2-amino-3-(1H-indol-3-yl)propoxy]-2-eth...)Show SMILES N[C@H](COc1cnc(C#C)c(c1)-c1ccc2cnccc2c1)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C27H22N4O/c1-2-26-25(19-7-8-20-14-29-10-9-18(20)11-19)13-23(16-31-26)32-17-22(28)12-21-15-30-27-6-4-3-5-24(21)27/h1,3-11,13-16,22,30H,12,17,28H2/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... |
Bioorg Med Chem Lett 16: 3150-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.041
BindingDB Entry DOI: 10.7270/Q2F769TT |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Bos taurus (bovine)) | BDBM16954

(6-{5-[(2S)-2-amino-3-(1H-indol-3-yl)propoxy]-2-chl...)Show SMILES N[C@H](COc1cnc(Cl)c(c1)-c1ccc2cnccc2c1)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C25H21ClN4O/c26-25-23(17-5-6-18-12-28-8-7-16(18)9-17)11-21(14-30-25)31-15-20(27)10-19-13-29-24-4-2-1-3-22(19)24/h1-9,11-14,20,29H,10,15,27H2/t20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The kinase assay uses purified recombinant enzyme and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidi... |
Bioorg Med Chem Lett 16: 3150-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.041
BindingDB Entry DOI: 10.7270/Q2F769TT |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM16954

(6-{5-[(2S)-2-amino-3-(1H-indol-3-yl)propoxy]-2-chl...)Show SMILES N[C@H](COc1cnc(Cl)c(c1)-c1ccc2cnccc2c1)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C25H21ClN4O/c26-25-23(17-5-6-18-12-28-8-7-16(18)9-17)11-21(14-30-25)31-15-20(27)10-19-13-29-24-4-2-1-3-22(19)24/h1-9,11-14,20,29H,10,15,27H2/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... |
Bioorg Med Chem Lett 16: 3150-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.041
BindingDB Entry DOI: 10.7270/Q2F769TT |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 catalytic activity after 10 mins by ELISA |
Bioorg Med Chem Lett 23: 4501-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.055
BindingDB Entry DOI: 10.7270/Q2TT4SC9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50438239
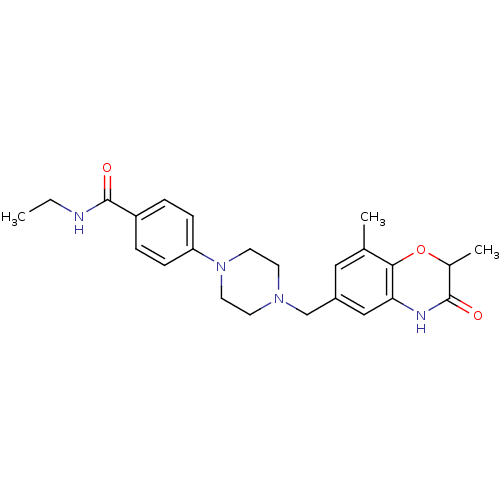
(CHEMBL2407975)Show SMILES CCNC(=O)c1ccc(cc1)N1CCN(Cc2cc(C)c3OC(C)C(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H30N4O3/c1-4-25-24(30)19-5-7-20(8-6-19)28-11-9-27(10-12-28)15-18-13-16(2)22-21(14-18)26-23(29)17(3)31-22/h5-8,13-14,17H,4,9-12,15H2,1-3H3,(H,25,30)(H,26,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 catalytic activity after 10 mins by ELISA |
Bioorg Med Chem Lett 23: 4501-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.055
BindingDB Entry DOI: 10.7270/Q2TT4SC9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50516024

(CHEMBL4540705)Show SMILES C[C@@]12COCCN1c1nc(ncc1N(CC1CC1)C2=O)-c1ccc(NC(=O)NC2CC2)cc1 |r| Show InChI InChI=1S/C24H28N6O3/c1-24-14-33-11-10-30(24)21-19(29(22(24)31)13-15-2-3-15)12-25-20(28-21)16-4-6-17(7-5-16)26-23(32)27-18-8-9-18/h4-7,12,15,18H,2-3,8-11,13-14H2,1H3,(H2,26,27,32)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of human mTOR measured after 30 mins in presence of ATP by Lanthascreen TR-FRET assay |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126659
BindingDB Entry DOI: 10.7270/Q22N55N8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317948
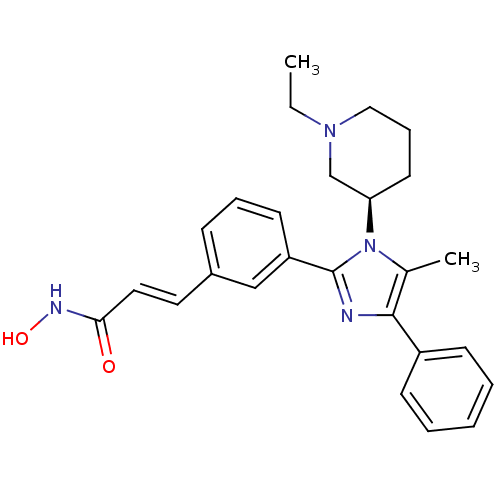
(6-(1-((R)-1-ethylpiperidin-3-yl)-5-methyl-4-phenyl...)Show SMILES CCN1CCC[C@H](C1)n1c(C)c(nc1-c1cccc(\C=C\C(=O)NO)c1)-c1ccccc1 |r| Show InChI InChI=1S/C26H30N4O2/c1-3-29-16-8-13-23(18-29)30-19(2)25(21-10-5-4-6-11-21)27-26(30)22-12-7-9-20(17-22)14-15-24(31)28-32/h4-7,9-12,14-15,17,23,32H,3,8,13,16,18H2,1-2H3,(H,28,31)/b15-14+/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317954
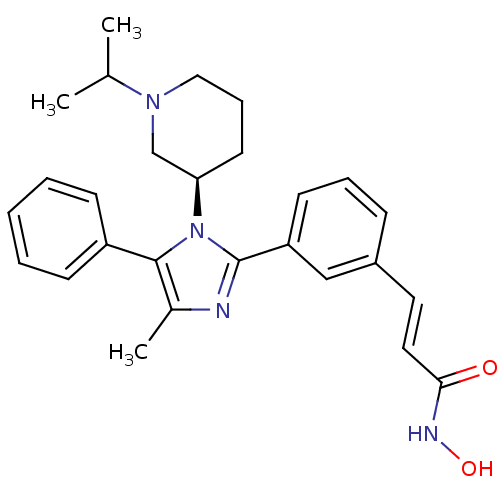
((R)-N-hydroxy-3-(3-(1-(1-isopropylpiperidin-3-yl)-...)Show SMILES CC(C)N1CCC[C@H](C1)n1c(nc(C)c1-c1ccccc1)-c1cccc(\C=C\C(=O)NO)c1 |r| Show InChI InChI=1S/C27H32N4O2/c1-19(2)30-16-8-13-24(18-30)31-26(22-10-5-4-6-11-22)20(3)28-27(31)23-12-7-9-21(17-23)14-15-25(32)29-33/h4-7,9-12,14-15,17,19,24,33H,8,13,16,18H2,1-3H3,(H,29,32)/b15-14+/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317957
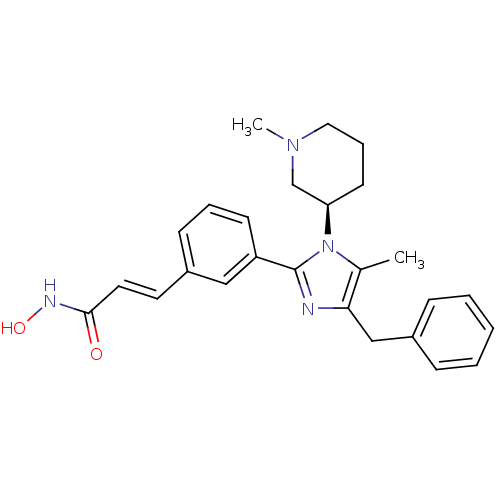
((R)-3-(3-(4-benzyl-5-methyl-1-(1-methylpiperidin-3...)Show SMILES CN1CCC[C@H](C1)n1c(C)c(Cc2ccccc2)nc1-c1cccc(\C=C\C(=O)NO)c1 |r| Show InChI InChI=1S/C26H30N4O2/c1-19-24(17-20-8-4-3-5-9-20)27-26(30(19)23-12-7-15-29(2)18-23)22-11-6-10-21(16-22)13-14-25(31)28-32/h3-6,8-11,13-14,16,23,32H,7,12,15,17-18H2,1-2H3,(H,28,31)/b14-13+/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50516023

(CHEMBL4466519)Show SMILES CNC(=O)Nc1ccc(cc1)-c1ncc2N(CC3CC3)C(=O)[C@]3(C)COCCN3c2n1 |r| Show InChI InChI=1S/C22H26N6O3/c1-22-13-31-10-9-28(22)19-17(27(20(22)29)12-14-3-4-14)11-24-18(26-19)15-5-7-16(8-6-15)25-21(30)23-2/h5-8,11,14H,3-4,9-10,12-13H2,1-2H3,(H2,23,25,30)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of human mTOR measured after 30 mins in presence of ATP by Lanthascreen TR-FRET assay |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126659
BindingDB Entry DOI: 10.7270/Q22N55N8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317951

(CHEMBL1096433 | N-hydroxy-3-(3-(4-methyl-1-pheneth...)Show SMILES Cc1nc(-c2cccc(\C=C\C(=O)NO)c2)n(CCc2ccccc2)c1-c1ccccc1 Show InChI InChI=1S/C27H25N3O2/c1-20-26(23-12-6-3-7-13-23)30(18-17-21-9-4-2-5-10-21)27(28-20)24-14-8-11-22(19-24)15-16-25(31)29-32/h2-16,19,32H,17-18H2,1H3,(H,29,31)/b16-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317953

((R)-3-(3-(1-(1-ethylpiperidin-3-yl)-4-methyl-5-phe...)Show SMILES CCN1CCC[C@H](C1)n1c(nc(C)c1-c1ccccc1)-c1cccc(\C=C\C(=O)NO)c1 |r| Show InChI InChI=1S/C26H30N4O2/c1-3-29-16-8-13-23(18-29)30-25(21-10-5-4-6-11-21)19(2)27-26(30)22-12-7-9-20(17-22)14-15-24(31)28-32/h4-7,9-12,14-15,17,23,32H,3,8,13,16,18H2,1-2H3,(H,28,31)/b15-14+/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |
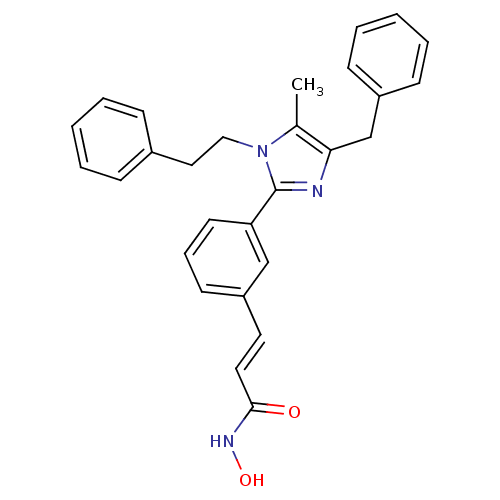
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317956
(3-(3-(4-benzyl-5-methyl-1-phenethyl-1H-imidazol-2-...)Show SMILES Cc1c(Cc2ccccc2)nc(-c2cccc(\C=C\C(=O)NO)c2)n1CCc1ccccc1 Show InChI InChI=1S/C28H27N3O2/c1-21-26(20-23-11-6-3-7-12-23)29-28(31(21)18-17-22-9-4-2-5-10-22)25-14-8-13-24(19-25)15-16-27(32)30-33/h2-16,19,33H,17-18,20H2,1H3,(H,30,32)/b16-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317962

(3-(3-(4,5-dimethyl-1-phenethyl-1H-imidazol-2-yl)ph...)Show SMILES Cc1nc(-c2cccc(\C=C\C(=O)NO)c2)n(CCc2ccccc2)c1C Show InChI InChI=1S/C22H23N3O2/c1-16-17(2)25(14-13-18-7-4-3-5-8-18)22(23-16)20-10-6-9-19(15-20)11-12-21(26)24-27/h3-12,15,27H,13-14H2,1-2H3,(H,24,26)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317965

(CHEMBL1099096 | N-hydroxy-3-(3-(1-phenethyl-1H-ben...)Show SMILES ONC(=O)\C=C\c1cccc(c1)-c1nc2ccccc2n1CCc1ccccc1 Show InChI InChI=1S/C24H21N3O2/c28-23(26-29)14-13-19-9-6-10-20(17-19)24-25-21-11-4-5-12-22(21)27(24)16-15-18-7-2-1-3-8-18/h1-14,17,29H,15-16H2,(H,26,28)/b14-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Bos taurus (bovine)) | BDBM16978

(6-{5-[(2S)-2-amino-3-(1H-indol-3-yl)propoxy]pyridi...)Show SMILES N[C@H](COc1cncc(c1)-c1ccc2cnc(N)cc2c1)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C25H23N5O/c26-21(8-20-13-29-24-4-2-1-3-23(20)24)15-31-22-9-19(11-28-14-22)16-5-6-17-12-30-25(27)10-18(17)7-16/h1-7,9-14,21,29H,8,15,26H2,(H2,27,30)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The kinase assay uses purified recombinant enzyme and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidi... |
Bioorg Med Chem Lett 16: 3150-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.041
BindingDB Entry DOI: 10.7270/Q2F769TT |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D]
(Homo sapiens (Human)) | BDBM15097

(N-[(2S)-2-amino-3-(1H-indol-3-yl)propyl]-5-[(E)-2-...)Show SMILES N[C@H](CNc1cncc(\C=C\c2ccncc2)c1)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C23H23N5/c24-20(12-19-14-28-23-4-2-1-3-22(19)23)15-27-21-11-18(13-26-16-21)6-5-17-7-9-25-10-8-17/h1-11,13-14,16,20,27-28H,12,15,24H2/b6-5+/t20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... |
Bioorg Med Chem Lett 16: 1679-85 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.017
BindingDB Entry DOI: 10.7270/Q23F4MWN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317952
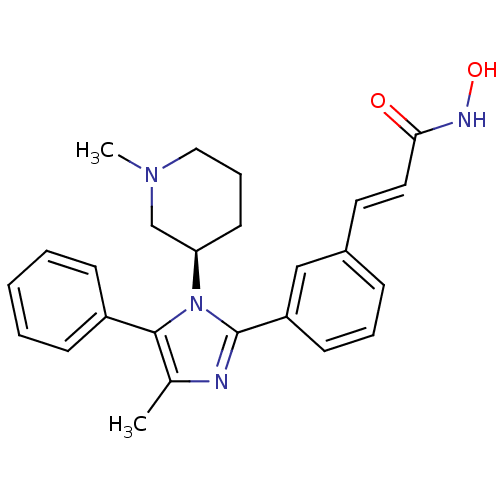
((R)-N-hydroxy-3-(3-(4-methyl-1-(1-methylpiperidin-...)Show SMILES CN1CCC[C@H](C1)n1c(nc(C)c1-c1ccccc1)-c1cccc(\C=C\C(=O)NO)c1 |r| Show InChI InChI=1S/C25H28N4O2/c1-18-24(20-9-4-3-5-10-20)29(22-12-7-15-28(2)17-22)25(26-18)21-11-6-8-19(16-21)13-14-23(30)27-31/h3-6,8-11,13-14,16,22,31H,7,12,15,17H2,1-2H3,(H,27,30)/b14-13+/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317958

((R)-3-(3-(4-benzyl-1-(1-ethylpiperidin-3-yl)-5-met...)Show SMILES CCN1CCC[C@H](C1)n1c(C)c(Cc2ccccc2)nc1-c1cccc(\C=C\C(=O)NO)c1 |r| Show InChI InChI=1S/C27H32N4O2/c1-3-30-16-8-13-24(19-30)31-20(2)25(18-21-9-5-4-6-10-21)28-27(31)23-12-7-11-22(17-23)14-15-26(32)29-33/h4-7,9-12,14-15,17,24,33H,3,8,13,16,18-19H2,1-2H3,(H,29,32)/b15-14+/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317959
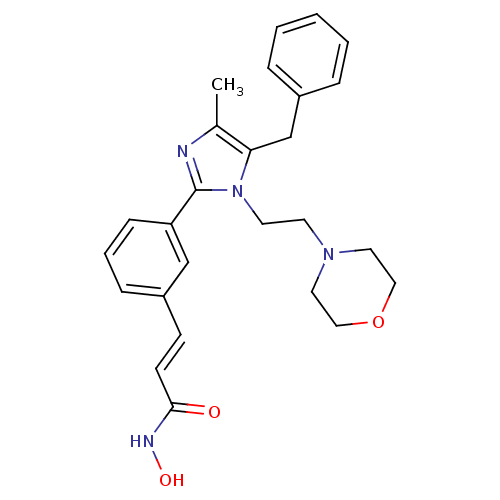
(3-(3-(5-benzyl-4-methyl-1-(2-morpholinoethyl)-1H-i...)Show SMILES Cc1nc(-c2cccc(\C=C\C(=O)NO)c2)n(CCN2CCOCC2)c1Cc1ccccc1 Show InChI InChI=1S/C26H30N4O3/c1-20-24(19-21-6-3-2-4-7-21)30(13-12-29-14-16-33-17-15-29)26(27-20)23-9-5-8-22(18-23)10-11-25(31)28-32/h2-11,18,32H,12-17,19H2,1H3,(H,28,31)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317945

(CHEMBL1095450 | N-hydroxy-3-(3-(5-methyl-1-(2-morp...)Show SMILES Cc1c(nc(-c2cccc(\C=C\C(=O)NO)c2)n1CCN1CCOCC1)-c1ccccc1 Show InChI InChI=1S/C25H28N4O3/c1-19-24(21-7-3-2-4-8-21)26-25(29(19)13-12-28-14-16-32-17-15-28)22-9-5-6-20(18-22)10-11-23(30)27-31/h2-11,18,31H,12-17H2,1H3,(H,27,30)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317973

((R)-N-hydroxy-3-(3-(1-(1-methylpiperidin-3-yl)-1H-...)Show SMILES CN1CCC[C@H](C1)n1c(nc2ccccc12)-c1cccc(\C=C\C(=O)NO)c1 |r| Show InChI InChI=1S/C22H24N4O2/c1-25-13-5-8-18(15-25)26-20-10-3-2-9-19(20)23-22(26)17-7-4-6-16(14-17)11-12-21(27)24-28/h2-4,6-7,9-12,14,18,28H,5,8,13,15H2,1H3,(H,24,27)/b12-11+/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317943

((R)-3-(3-(1-(1-ethylpiperidin-3-yl)-1H-benzo[d]imi...)Show SMILES CCN1CCC[C@H](C1)n1c(nc2ccccc12)-c1cccc(\C=C\C(=O)NO)c1 |r| Show InChI InChI=1S/C23H26N4O2/c1-2-26-14-6-9-19(16-26)27-21-11-4-3-10-20(21)24-23(27)18-8-5-7-17(15-18)12-13-22(28)25-29/h3-5,7-8,10-13,15,19,29H,2,6,9,14,16H2,1H3,(H,25,28)/b13-12+/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data