

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019696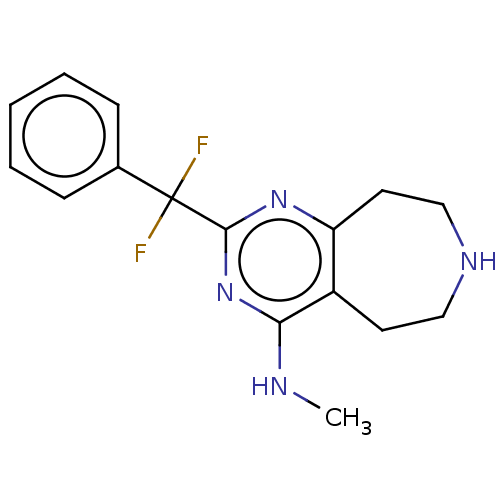 (CHEMBL3286557) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019685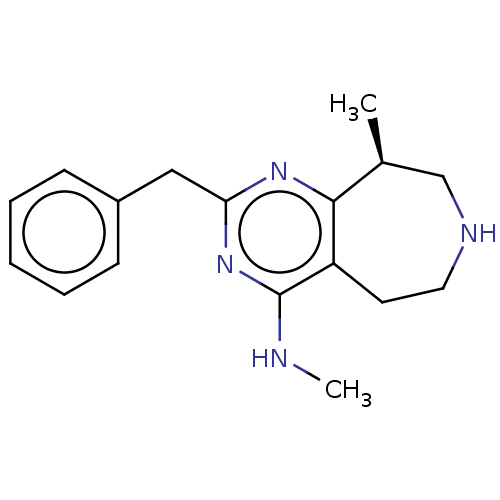 (CHEMBL3286556) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019682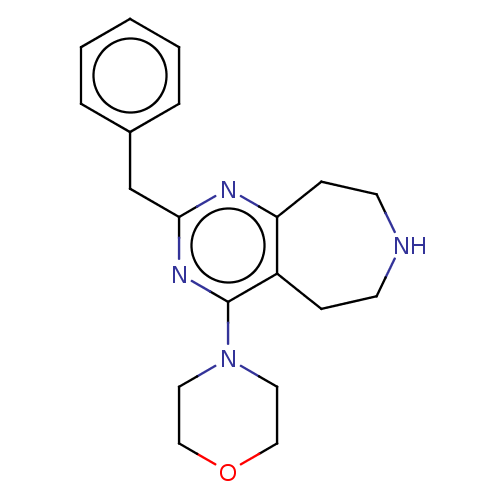 (CHEMBL3286564) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019664 (CHEMBL3286562) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019691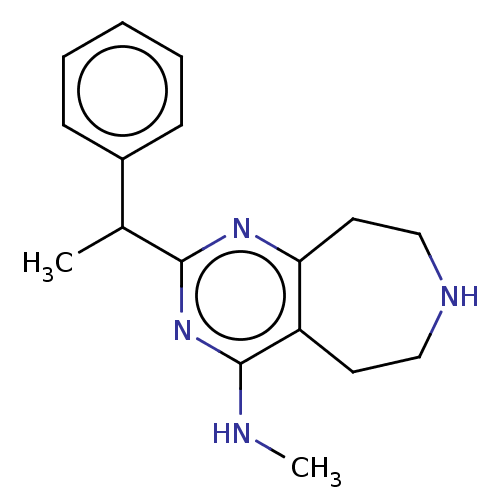 (CHEMBL3286565) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019663 (CHEMBL3286561) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019662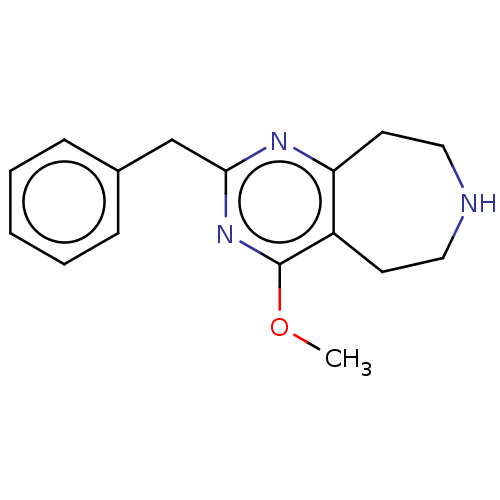 (CHEMBL3286560) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019691 (CHEMBL3286565) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019692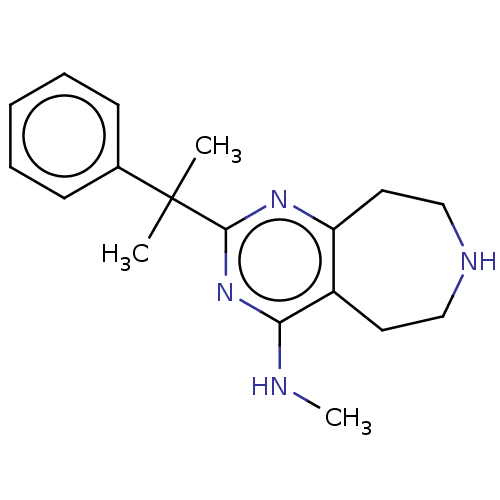 (CHEMBL3286566) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019695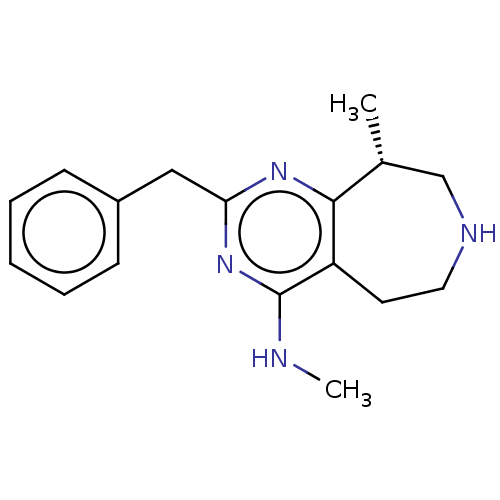 (CHEMBL3286555) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019694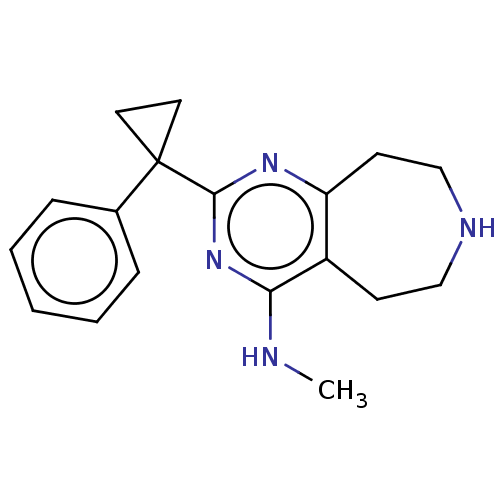 (CHEMBL3286554) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50342538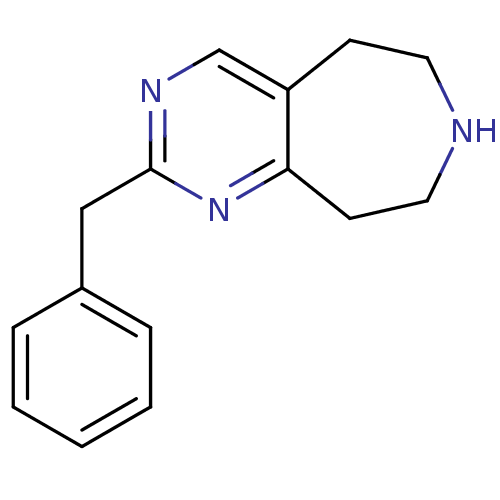 (2-benzyl-6,7,8,9-tetrahydro-5H-pyrimido[5,4-d]azep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019674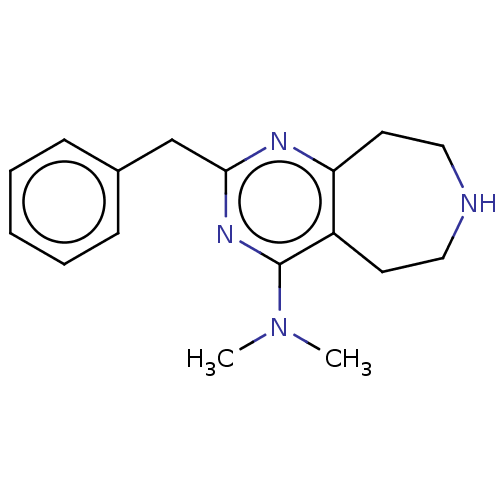 (CHEMBL3286563) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 233 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019661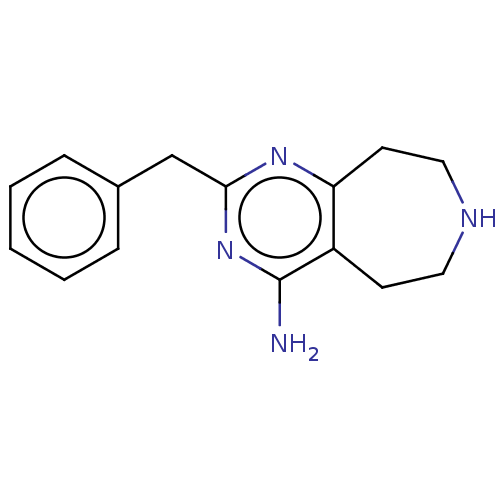 (CHEMBL3286559) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 958 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM82186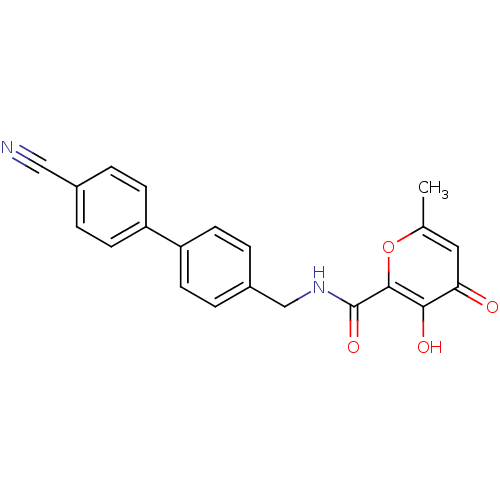 (AM-5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego | Assay Description Inhibition assay using matrix metalloproteinases. | Chem Biol Drug Des 78: 191-8 (2011) Article DOI: 10.1111/j.1747-0285.2011.01148.x BindingDB Entry DOI: 10.7270/Q2KD1WDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50264772 (3-Hydroxy-6-methyl-4-oxo-4H-pyran-2-carboxylic aci...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego | Assay Description Inhibition assay using matrix metalloproteinases. | Chem Biol Drug Des 78: 191-8 (2011) Article DOI: 10.1111/j.1747-0285.2011.01148.x BindingDB Entry DOI: 10.7270/Q2KD1WDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50019685 (CHEMBL3286556) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to 5HT2B receptor (unknown origin) | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50019696 (CHEMBL3286557) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to 5HT2B receptor (unknown origin) | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50019696 (CHEMBL3286557) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to 5HT2A receptor (unknown origin) | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50241504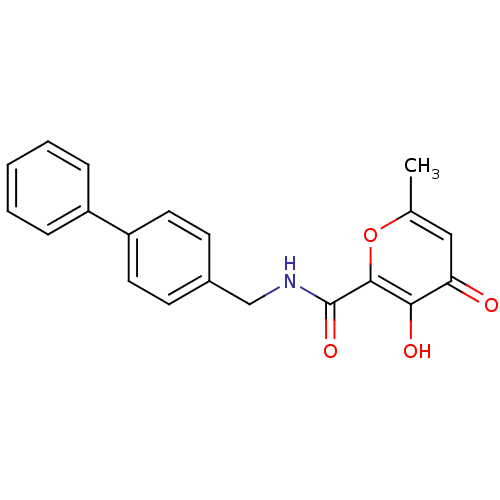 (3-hydroxy-6-methyl-4-oxo-4H-pyran-2-carboxylic aci...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego | Assay Description Inhibition assay using matrix metalloproteinases. | Chem Biol Drug Des 78: 191-8 (2011) Article DOI: 10.1111/j.1747-0285.2011.01148.x BindingDB Entry DOI: 10.7270/Q2KD1WDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50019685 (CHEMBL3286556) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to 5HT6 receptor (unknown origin) | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50019685 (CHEMBL3286556) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to 5HT2A receptor (unknown origin) | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50019685 (CHEMBL3286556) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to 5HT1A receptor (unknown origin) | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50019696 (CHEMBL3286557) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to 5HT1B receptor (unknown origin) | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM82186 (AM-5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego | Assay Description Inhibition assay using matrix metalloproteinases. | Chem Biol Drug Des 78: 191-8 (2011) Article DOI: 10.1111/j.1747-0285.2011.01148.x BindingDB Entry DOI: 10.7270/Q2KD1WDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50019696 (CHEMBL3286557) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to adrenergic beta-2 receptor (unknown origin) | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM27872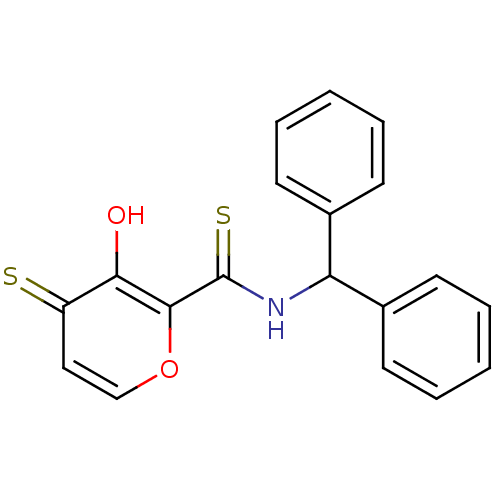 (N-(diphenylmethyl)-3-hydroxy-4-sulfanylidene-4H-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Howard Hughes Medical Institute | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained fluorogenic peptide substrate, LF, and small-molecule i... | J Med Chem 52: 1063-74 (2009) Article DOI: 10.1021/jm8013212 BindingDB Entry DOI: 10.7270/Q2DJ5CZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM27874 (3-hydroxy-N-[(3-methoxyphenyl)methyl]-4-sulfanylid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Howard Hughes Medical Institute | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained fluorogenic peptide substrate, LF, and small-molecule i... | J Med Chem 52: 1063-74 (2009) Article DOI: 10.1021/jm8013212 BindingDB Entry DOI: 10.7270/Q2DJ5CZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM27875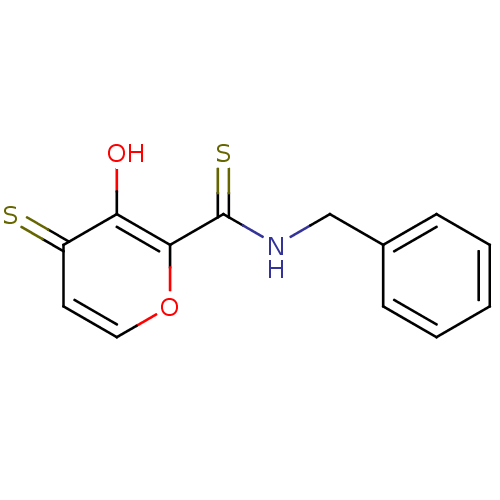 (N-benzyl-3-hydroxy-4-sulfanylidene-4H-pyran-2-carb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Howard Hughes Medical Institute | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained fluorogenic peptide substrate, LF, and small-molecule i... | J Med Chem 52: 1063-74 (2009) Article DOI: 10.1021/jm8013212 BindingDB Entry DOI: 10.7270/Q2DJ5CZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM27869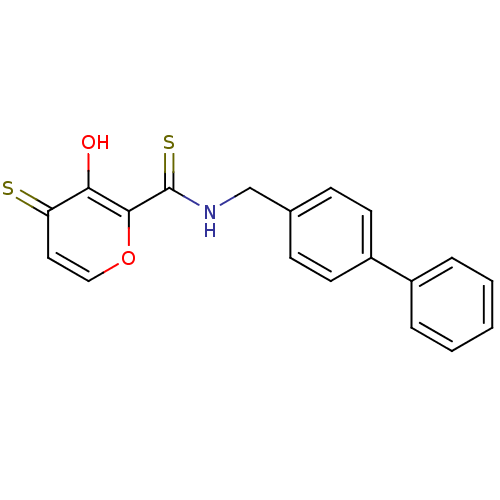 (3-hydroxy-N-[(4-phenylphenyl)methyl]-4-sulfanylide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Howard Hughes Medical Institute | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained fluorogenic peptide substrate, LF, and small-molecule i... | J Med Chem 52: 1063-74 (2009) Article DOI: 10.1021/jm8013212 BindingDB Entry DOI: 10.7270/Q2DJ5CZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM27873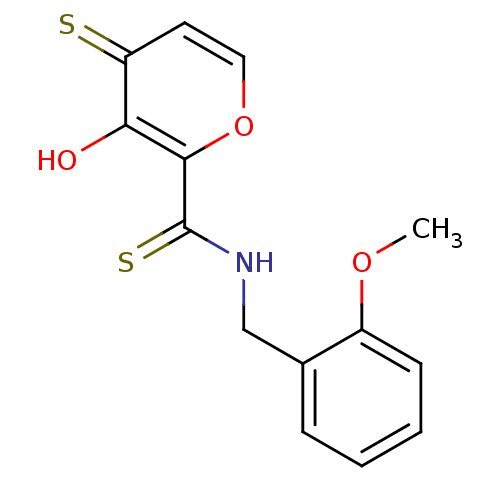 (3-hydroxy-N-[(2-methoxyphenyl)methyl]-4-sulfanylid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Howard Hughes Medical Institute | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained fluorogenic peptide substrate, LF, and small-molecule i... | J Med Chem 52: 1063-74 (2009) Article DOI: 10.1021/jm8013212 BindingDB Entry DOI: 10.7270/Q2DJ5CZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM27870 (N-[(4-fluorophenyl)methyl]-3-hydroxy-4-sulfanylide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Howard Hughes Medical Institute | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained fluorogenic peptide substrate, LF, and small-molecule i... | J Med Chem 52: 1063-74 (2009) Article DOI: 10.1021/jm8013212 BindingDB Entry DOI: 10.7270/Q2DJ5CZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50241504 (3-hydroxy-6-methyl-4-oxo-4H-pyran-2-carboxylic aci...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego | Assay Description Inhibition assay using matrix metalloproteinases. | Chem Biol Drug Des 78: 191-8 (2011) Article DOI: 10.1111/j.1747-0285.2011.01148.x BindingDB Entry DOI: 10.7270/Q2KD1WDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM27871 (3-hydroxy-N-[(4-methoxyphenyl)methyl]-4-sulfanylid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Howard Hughes Medical Institute | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained fluorogenic peptide substrate, LF, and small-molecule i... | J Med Chem 52: 1063-74 (2009) Article DOI: 10.1021/jm8013212 BindingDB Entry DOI: 10.7270/Q2DJ5CZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM27876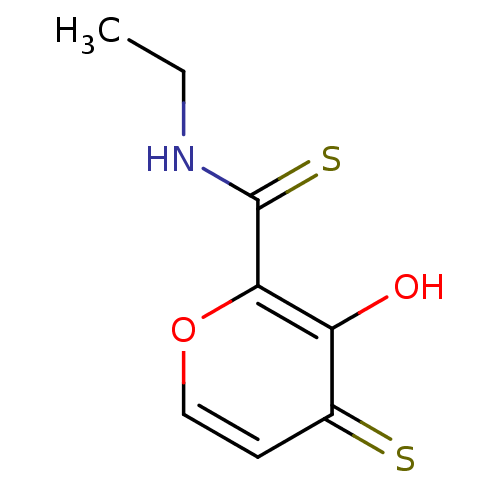 (N-ethyl-3-hydroxy-4-sulfanylidene-4H-pyran-2-carbo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Howard Hughes Medical Institute | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained fluorogenic peptide substrate, LF, and small-molecule i... | J Med Chem 52: 1063-74 (2009) Article DOI: 10.1021/jm8013212 BindingDB Entry DOI: 10.7270/Q2DJ5CZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM27859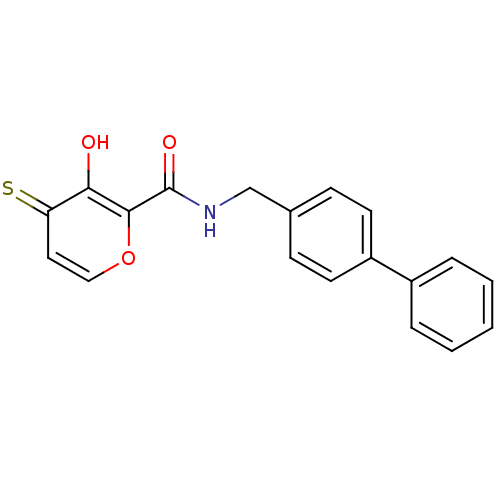 (3-hydroxy-N-[(4-phenylphenyl)methyl]-4-sulfanylide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Howard Hughes Medical Institute | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained fluorogenic peptide substrate, LF, and small-molecule i... | J Med Chem 52: 1063-74 (2009) Article DOI: 10.1021/jm8013212 BindingDB Entry DOI: 10.7270/Q2DJ5CZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM27862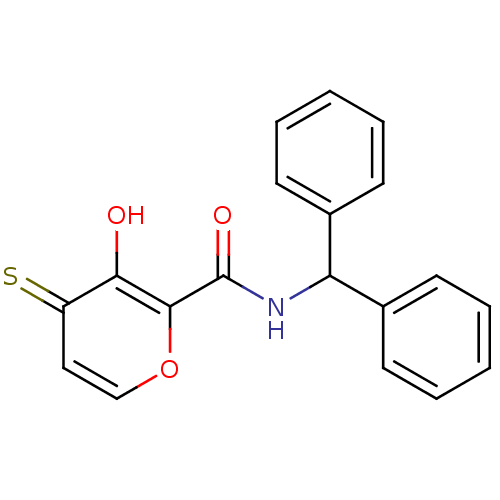 (N-(diphenylmethyl)-3-hydroxy-4-sulfanylidene-4H-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Howard Hughes Medical Institute | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained fluorogenic peptide substrate, LF, and small-molecule i... | J Med Chem 52: 1063-74 (2009) Article DOI: 10.1021/jm8013212 BindingDB Entry DOI: 10.7270/Q2DJ5CZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM27860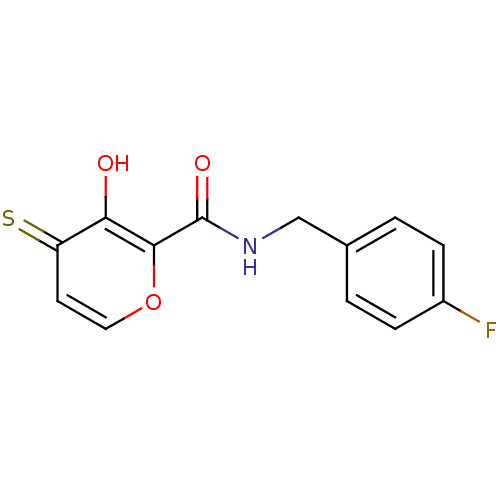 (N-[(4-fluorophenyl)methyl]-3-hydroxy-4-sulfanylide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.92E+4 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Howard Hughes Medical Institute | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained fluorogenic peptide substrate, LF, and small-molecule i... | J Med Chem 52: 1063-74 (2009) Article DOI: 10.1021/jm8013212 BindingDB Entry DOI: 10.7270/Q2DJ5CZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM27863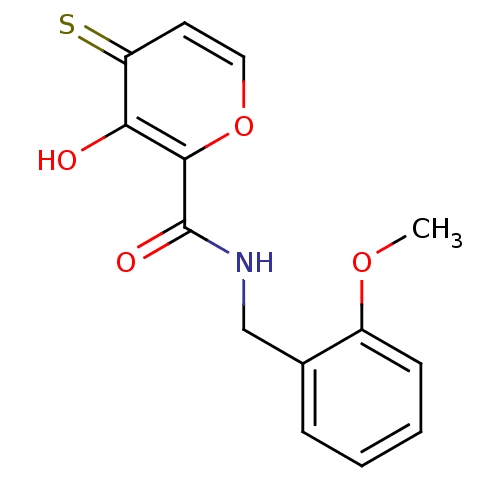 (3-hydroxy-N-[(2-methoxyphenyl)methyl]-4-sulfanylid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.35E+4 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Howard Hughes Medical Institute | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained fluorogenic peptide substrate, LF, and small-molecule i... | J Med Chem 52: 1063-74 (2009) Article DOI: 10.1021/jm8013212 BindingDB Entry DOI: 10.7270/Q2DJ5CZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM27864 (3-hydroxy-N-[(3-methoxyphenyl)methyl]-4-sulfanylid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.74E+4 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Howard Hughes Medical Institute | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained fluorogenic peptide substrate, LF, and small-molecule i... | J Med Chem 52: 1063-74 (2009) Article DOI: 10.1021/jm8013212 BindingDB Entry DOI: 10.7270/Q2DJ5CZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM27867 (1-hydroxy-N-[(4-phenylphenyl)methyl]-6-sulfanylide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Howard Hughes Medical Institute | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained fluorogenic peptide substrate, LF, and small-molecule i... | J Med Chem 52: 1063-74 (2009) Article DOI: 10.1021/jm8013212 BindingDB Entry DOI: 10.7270/Q2DJ5CZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50264772 (3-Hydroxy-6-methyl-4-oxo-4H-pyran-2-carboxylic aci...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego | Assay Description Inhibition assay using matrix metalloproteinases. | Chem Biol Drug Des 78: 191-8 (2011) Article DOI: 10.1111/j.1747-0285.2011.01148.x BindingDB Entry DOI: 10.7270/Q2KD1WDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM27861 (3-hydroxy-N-[(4-methoxyphenyl)methyl]-4-sulfanylid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.04E+4 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Howard Hughes Medical Institute | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained fluorogenic peptide substrate, LF, and small-molecule i... | J Med Chem 52: 1063-74 (2009) Article DOI: 10.1021/jm8013212 BindingDB Entry DOI: 10.7270/Q2DJ5CZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM27866 (N-ethyl-3-hydroxy-4-sulfanylidene-4H-pyran-2-carbo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.25E+4 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Howard Hughes Medical Institute | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained fluorogenic peptide substrate, LF, and small-molecule i... | J Med Chem 52: 1063-74 (2009) Article DOI: 10.1021/jm8013212 BindingDB Entry DOI: 10.7270/Q2DJ5CZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM27850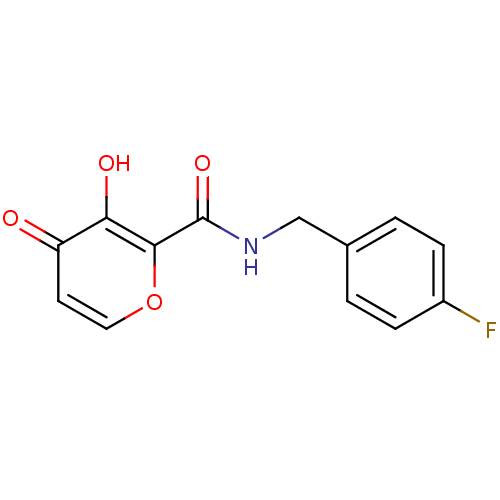 (N-[(4-fluorophenyl)methyl]-3-hydroxy-4-oxo-4H-pyra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Howard Hughes Medical Institute | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained fluorogenic peptide substrate, LF, and small-molecule i... | J Med Chem 52: 1063-74 (2009) Article DOI: 10.1021/jm8013212 BindingDB Entry DOI: 10.7270/Q2DJ5CZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM27849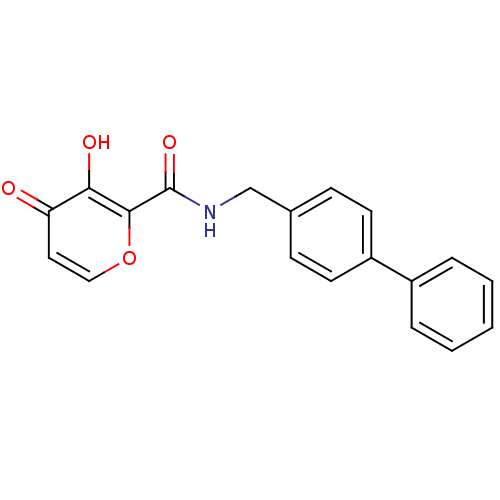 (3-hydroxy-4-oxo-N-[(4-phenylphenyl)methyl]-4H-pyra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Howard Hughes Medical Institute | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained fluorogenic peptide substrate, LF, and small-molecule i... | J Med Chem 52: 1063-74 (2009) Article DOI: 10.1021/jm8013212 BindingDB Entry DOI: 10.7270/Q2DJ5CZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM27851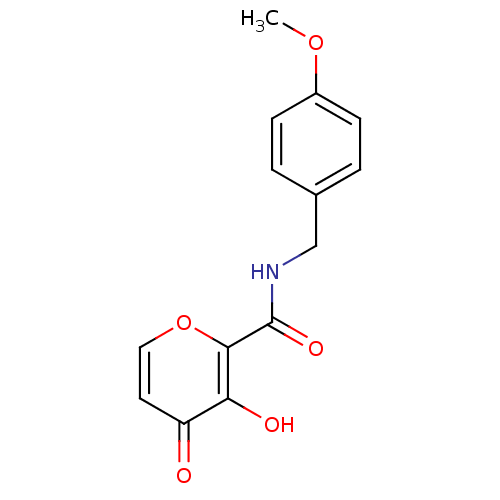 (3-hydroxy-N-[(4-methoxyphenyl)methyl]-4-oxo-4H-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Howard Hughes Medical Institute | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained fluorogenic peptide substrate, LF, and small-molecule i... | J Med Chem 52: 1063-74 (2009) Article DOI: 10.1021/jm8013212 BindingDB Entry DOI: 10.7270/Q2DJ5CZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM27852 (N-(diphenylmethyl)-3-hydroxy-4-oxo-4H-pyran-2-carb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Howard Hughes Medical Institute | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained fluorogenic peptide substrate, LF, and small-molecule i... | J Med Chem 52: 1063-74 (2009) Article DOI: 10.1021/jm8013212 BindingDB Entry DOI: 10.7270/Q2DJ5CZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM27853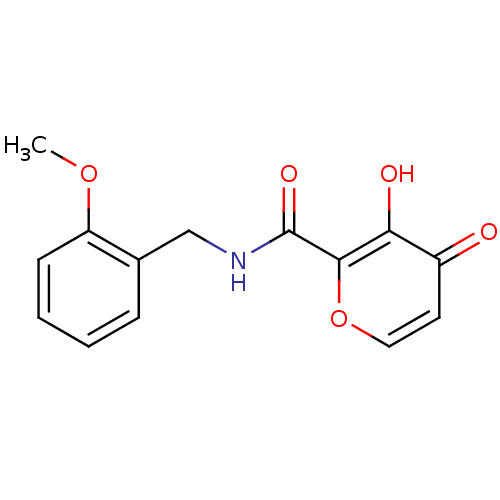 (3-hydroxy-N-[(2-methoxyphenyl)methyl]-4-oxo-4H-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Howard Hughes Medical Institute | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained fluorogenic peptide substrate, LF, and small-molecule i... | J Med Chem 52: 1063-74 (2009) Article DOI: 10.1021/jm8013212 BindingDB Entry DOI: 10.7270/Q2DJ5CZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM27854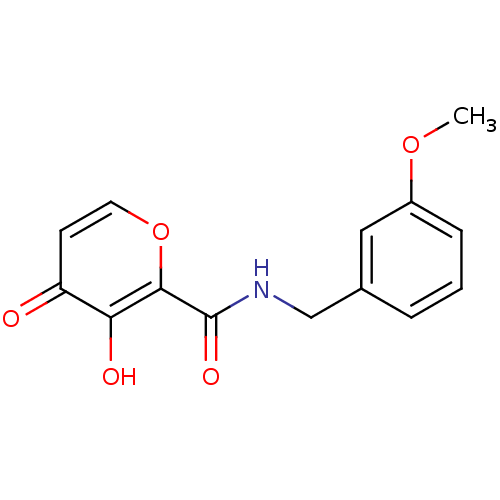 (3-hydroxy-N-[(3-methoxyphenyl)methyl]-4-oxo-4H-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Howard Hughes Medical Institute | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained fluorogenic peptide substrate, LF, and small-molecule i... | J Med Chem 52: 1063-74 (2009) Article DOI: 10.1021/jm8013212 BindingDB Entry DOI: 10.7270/Q2DJ5CZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 84 total ) | Next | Last >> |