

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-2A adrenergic receptor (BOVINE) | BDBM50018739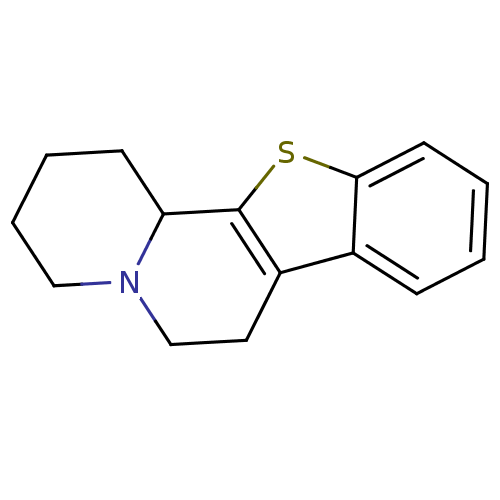 (1,3,4,5,6,11b-Hexahydro-2H-11-thia-4a-aza-benzo[a]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Affinity to alpha-2 adrenergic receptor by the displacement of [3H]-clonidine from calf cerebral cortex membranes | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (BOVINE) | BDBM50018739 (1,3,4,5,6,11b-Hexahydro-2H-11-thia-4a-aza-benzo[a]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Affinity to alpha-2 adrenergic receptor by the displacement of [3H]-clonidine from calf cerebral cortex membranes | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50018739 (1,3,4,5,6,11b-Hexahydro-2H-11-thia-4a-aza-benzo[a]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Affinity to alpha-1 adrenergic receptor by the displacement of [3H]-prazosin from calf cerebral cortex membranes | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (BOVINE) | BDBM50018736 (1,3,4,5,6,11b-Hexahydro-2H-11-oxa-4a-aza-benzo[a]f...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Affinity to alpha-2 adrenergic receptor by the displacement of [3H]-clonidine from calf cerebral cortex membranes | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50018739 (1,3,4,5,6,11b-Hexahydro-2H-11-thia-4a-aza-benzo[a]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for log 1/Ki at alpha-2 adrenergic receptor | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (BOVINE) | BDBM50018736 (1,3,4,5,6,11b-Hexahydro-2H-11-oxa-4a-aza-benzo[a]f...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Affinity to alpha-1 adrenergic receptor by the displacement of [3H]-prazosin from calf cerebral cortex membranes | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50018737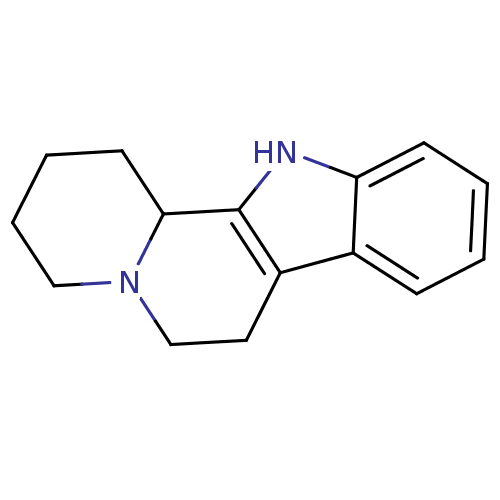 (1,2,3,4,6,7,12,12b-Octahydro-indolo[2,3-a]quinoliz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Affinity to alpha-1 adrenergic receptor by the displacement of [3H]-prazosin from calf cerebral cortex membranes | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50018737 (1,2,3,4,6,7,12,12b-Octahydro-indolo[2,3-a]quinoliz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Affinity to alpha-2 adrenergic receptor by the displacement of [3H]-clonidine from calf cerebral cortex membranes | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (BOVINE) | BDBM50018737 (1,2,3,4,6,7,12,12b-Octahydro-indolo[2,3-a]quinoliz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Affinity to alpha-1 adrenergic receptor by the displacement of [3H]-prazosin from calf cerebral cortex membranes | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (BOVINE) | BDBM50018737 (1,2,3,4,6,7,12,12b-Octahydro-indolo[2,3-a]quinoliz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for log 1/Ki at alpha-2 adrenergic receptor | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50018736 (1,3,4,5,6,11b-Hexahydro-2H-11-oxa-4a-aza-benzo[a]f...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for log 1/Ki at alpha-1 adrenergic receptor | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50018736 (1,3,4,5,6,11b-Hexahydro-2H-11-oxa-4a-aza-benzo[a]f...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for log 1/Ki at alpha-1 adrenergic receptor | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (BOVINE) | BDBM50018741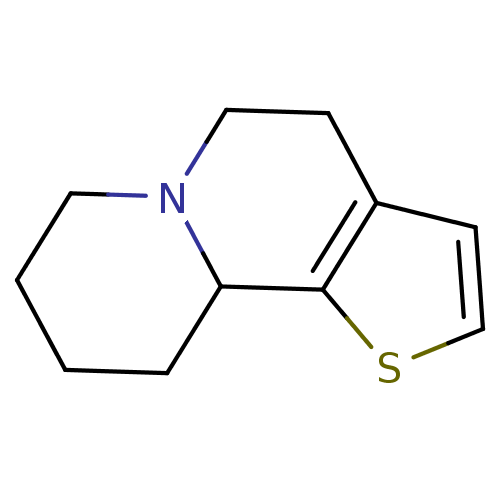 (4,7,8,9,10,10a-Hexahydro-5H-thieno[2,3-a]quinolizi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Affinity to alpha-2 adrenergic receptor by the displacement of [3H]-clonidine from calf cerebral cortex membranes | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (BOVINE) | BDBM50018741 (4,7,8,9,10,10a-Hexahydro-5H-thieno[2,3-a]quinolizi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Affinity to alpha-2 adrenergic receptor by the displacement of [3H]-clonidine from calf cerebral cortex membranes | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (BOVINE) | BDBM50018740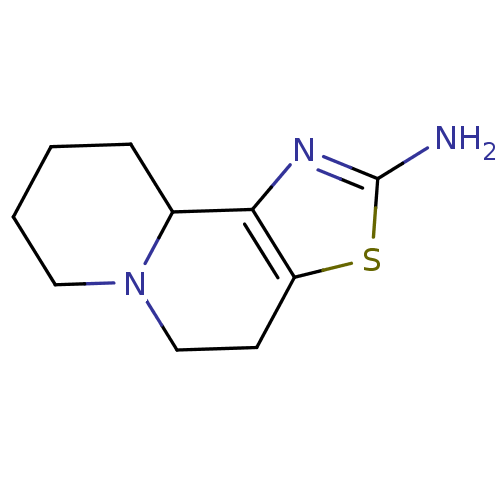 (4,7,8,9,10,10a-Hexahydro-5H-thiazolo[4,5-a]quinoli...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for log 1/Ki at alpha-2 adrenergic receptor | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (BOVINE) | BDBM50018740 (4,7,8,9,10,10a-Hexahydro-5H-thiazolo[4,5-a]quinoli...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for log 1/Ki at alpha-2 adrenergic receptor | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50018738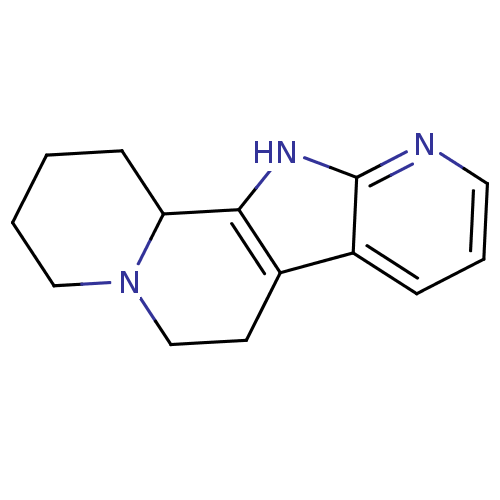 (1,2,3,4,5,6,11,11b-Octahydro-4a,10,11-triaza-benzo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 537 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for log 1/Ki at alpha-1 adrenergic receptor | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50018738 (1,2,3,4,5,6,11,11b-Octahydro-4a,10,11-triaza-benzo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for log 1/Ki at alpha-1 adrenergic receptor | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (BOVINE) | BDBM50018738 (1,2,3,4,5,6,11,11b-Octahydro-4a,10,11-triaza-benzo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Affinity to alpha-1 adrenergic receptor by the displacement of [3H]-prazosin from calf cerebral cortex membranes | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (BOVINE) | BDBM50018738 (1,2,3,4,5,6,11,11b-Octahydro-4a,10,11-triaza-benzo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 661 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for log 1/Ki at alpha-2 adrenergic receptor | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50018741 (4,7,8,9,10,10a-Hexahydro-5H-thieno[2,3-a]quinolizi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for log 1/Ki at alpha-1 adrenergic receptor | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50018741 (4,7,8,9,10,10a-Hexahydro-5H-thieno[2,3-a]quinolizi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for log 1/Ki at alpha-2 adrenergic receptor | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (BOVINE) | BDBM50018735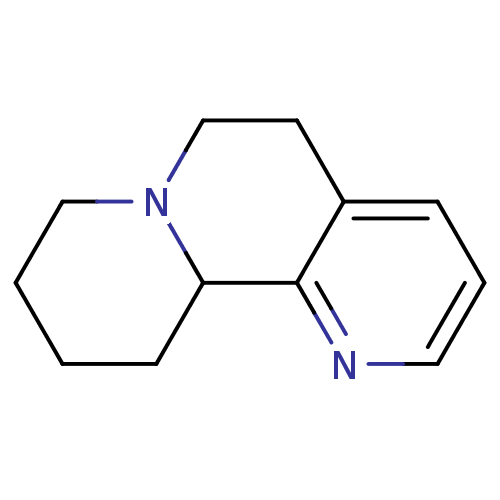 (5,6,7,8,9,10-Hexahydro-4bH-4,8a-diaza-phenanthrene...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for log 1/Ki at alpha-2 adrenergic receptor | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (BOVINE) | BDBM50018735 (5,6,7,8,9,10-Hexahydro-4bH-4,8a-diaza-phenanthrene...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for log 1/Ki at alpha-1 adrenergic receptor | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50018740 (4,7,8,9,10,10a-Hexahydro-5H-thiazolo[4,5-a]quinoli...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Affinity to alpha-1 adrenergic receptor by the displacement of [3H]-prazosin from calf cerebral cortex membranes | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50018740 (4,7,8,9,10,10a-Hexahydro-5H-thiazolo[4,5-a]quinoli...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Affinity to alpha-2 adrenergic receptor by the displacement of [3H]-clonidine from calf cerebral cortex membranes | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50018735 (5,6,7,8,9,10-Hexahydro-4bH-4,8a-diaza-phenanthrene...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for log 1/Ki at alpha-1 adrenergic receptor | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50018735 (5,6,7,8,9,10-Hexahydro-4bH-4,8a-diaza-phenanthrene...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for log 1/Ki at alpha-1 adrenergic receptor | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (BOVINE) | BDBM50018734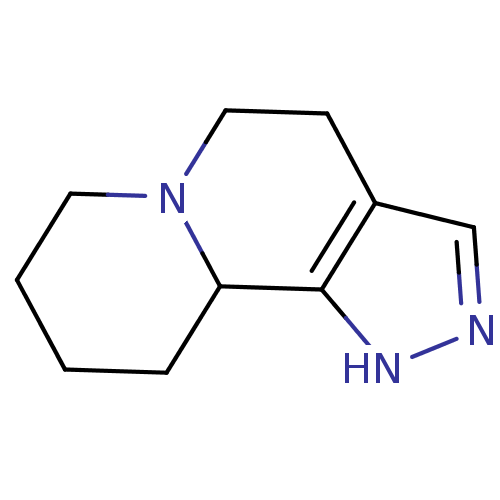 (1,4,5,7,8,9,10,10a-Octahydro-pyrazolo[3,4-a]quinol...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | 9.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for log 1/Ki at alpha-2 adrenergic receptor | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (BOVINE) | BDBM50018734 (1,4,5,7,8,9,10,10a-Octahydro-pyrazolo[3,4-a]quinol...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Affinity to alpha-1 adrenergic receptor by the displacement of [3H]-prazosin from calf cerebral cortex membranes | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50018734 (1,4,5,7,8,9,10,10a-Octahydro-pyrazolo[3,4-a]quinol...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Affinity to alpha-2 adrenergic receptor by the displacement of [3H]-clonidine from calf cerebral cortex membranes | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50018734 (1,4,5,7,8,9,10,10a-Octahydro-pyrazolo[3,4-a]quinol...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Affinity to alpha-1 adrenergic receptor by the displacement of [3H]-prazosin from calf cerebral cortex membranes | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM762 (BocPhe[CHOH(CH2)3CH=CHPhCO]IleAMBI | L-687,908 | t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 34: 1225-8 (1991) Article DOI: 10.1021/jm00107a050 BindingDB Entry DOI: 10.7270/Q2765CH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1277 (L-682, 679 analog 36 | tert-butyl N-[(2S,3S,5R)-5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Sharp and Dohme Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 34: 2852-7 (1991) Article DOI: 10.1021/jm00113a025 BindingDB Entry DOI: 10.7270/Q2M906TJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9291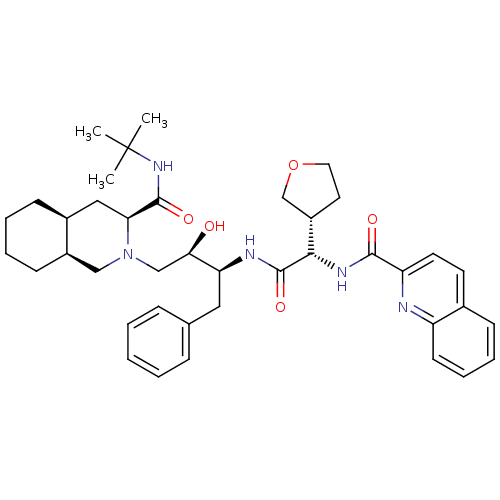 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease was determined in vitro | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM383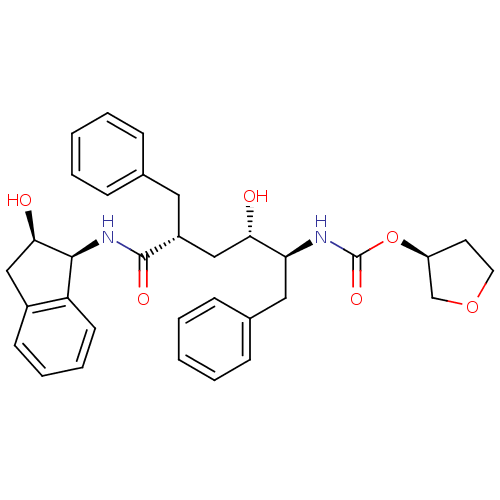 ((3S)-oxolan-3-yl N-[(2S,3S,5R)-5-benzyl-3-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease was determined in vitro | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM761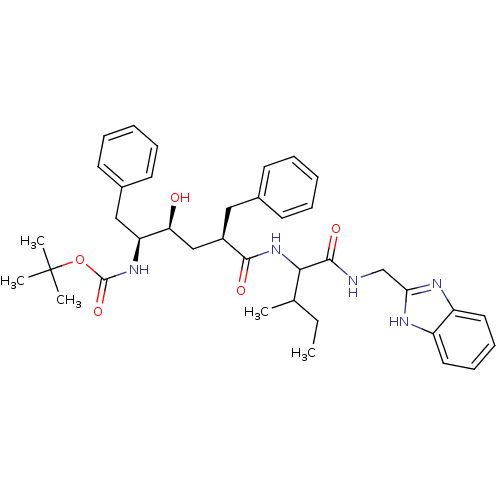 (Hydroxyethylene deriv. 12 | tert-butyl N-[(2S,3S,5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 34: 1225-8 (1991) Article DOI: 10.1021/jm00107a050 BindingDB Entry DOI: 10.7270/Q2765CH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM842 (Benzocycloalkyl Amines deriv. 12 | CHEMBL419923 | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50130373 (4-[1-Amino-1-(3-methyl-3H-imidazol-4-yl)-ethyl]-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required to inhibit recombinant human farnesyltransferase (FTase) catalyzed incorporation of [3H]FPP into recombinant Ras-CVIM. | J Med Chem 46: 2973-84 (2003) Article DOI: 10.1021/jm020587n BindingDB Entry DOI: 10.7270/Q26Q1Z0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50369371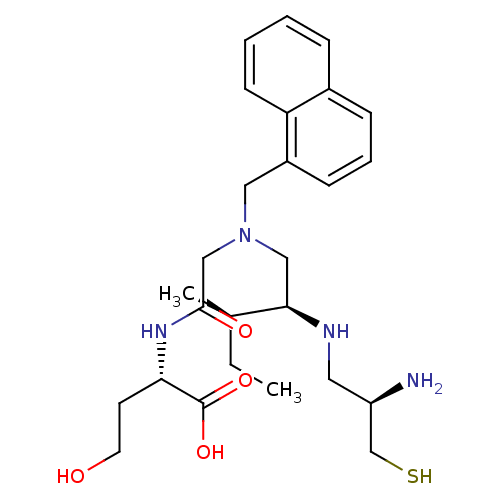 (CHEMBL1790750) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.123 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]FPP incorporation into recombinant human Ha-Ras by Farnesyltransferase | J Med Chem 41: 2651-6 (1998) Article DOI: 10.1021/jm9800907 BindingDB Entry DOI: 10.7270/Q2WW7JCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50072639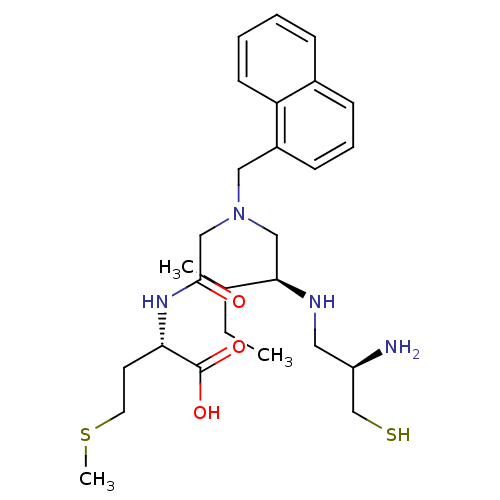 ((S)-2-(2-{[(S)-2-((R)-2-Amino-3-mercapto-propylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.123 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]- FPP incorporation into recombinant Ha-Ras by farnesyl transferase at 10 pM | Bioorg Med Chem Lett 8: 3311-6 (1999) BindingDB Entry DOI: 10.7270/Q2G44QSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1276 (L-682, 679 analog 35 | tert-butyl N-[(2S,3S,5R)-5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Sharp and Dohme Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 34: 2852-7 (1991) Article DOI: 10.1021/jm00113a025 BindingDB Entry DOI: 10.7270/Q2M906TJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM760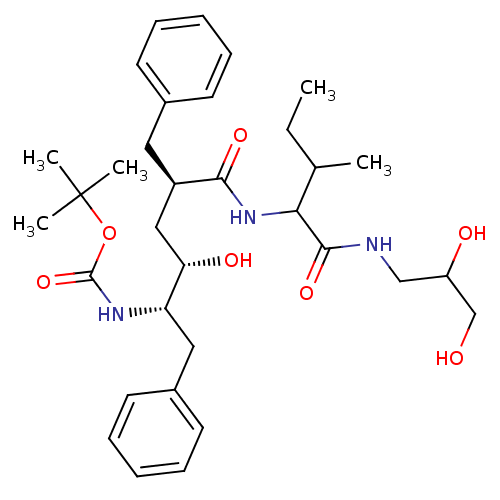 (Hydroxyethylene deriv. 11 | tert-butyl N-[(2S,3S,5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 34: 1225-8 (1991) Article DOI: 10.1021/jm00107a050 BindingDB Entry DOI: 10.7270/Q2765CH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM753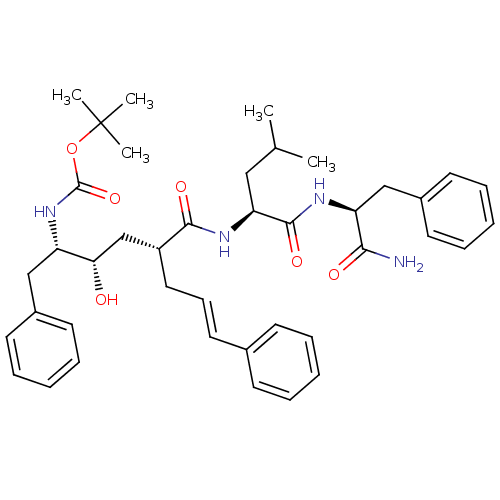 (Hydroxyethylene deriv. 4 | Hydroxyethylene dipepti...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 34: 1225-8 (1991) Article DOI: 10.1021/jm00107a050 BindingDB Entry DOI: 10.7270/Q2765CH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1046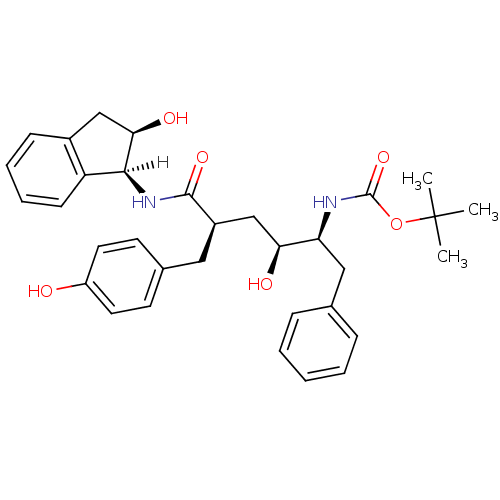 (CHEMBL298316 | L-685,927 | tert-butyl N-[(2S,3S,5R...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1039 (CHEMBL297620 | Hydroxyethylene dipeptide isostere ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50130374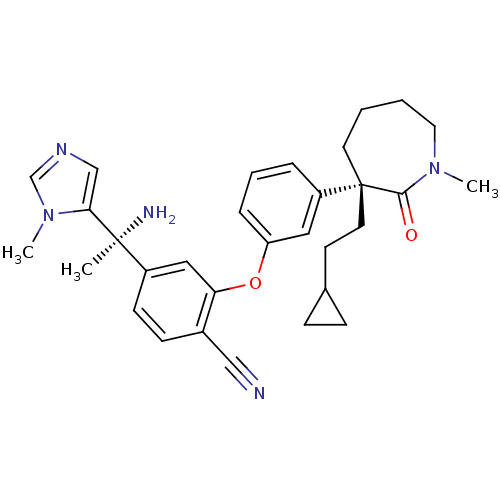 (4-[1-Amino-1-(3-methyl-3H-imidazol-4-yl)-ethyl]-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required to inhibit recombinant human farnesyltransferase (FTase) catalyzed incorporation of [3H]FPP into recombinant Ras-CVIM. | J Med Chem 46: 2973-84 (2003) Article DOI: 10.1021/jm020587n BindingDB Entry DOI: 10.7270/Q26Q1Z0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50035633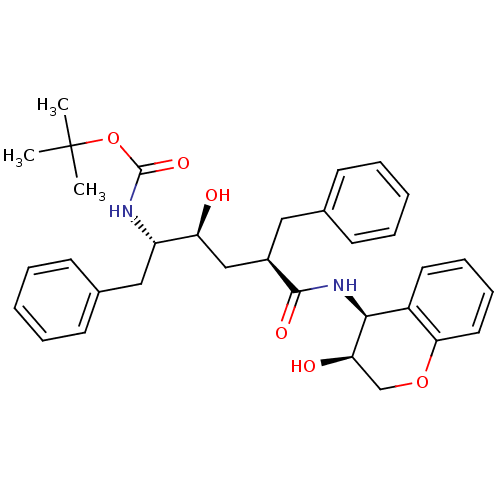 (CHEMBL111143 | [(1S,2S,4R)-1-Benzyl-2-hydroxy-4-((...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50035635 (CHEMBL322984 | [(1S,2S,4R)-1-Benzyl-4-((1S,2S,3R)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50130381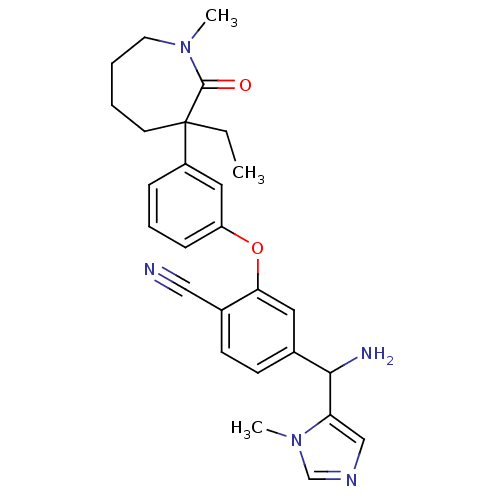 (4-[Amino-(3-methyl-3H-imidazol-4-yl)-methyl]-2-[3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required to inhibit recombinant human farnesyltransferase (FTase) catalyzed incorporation of [3H]FPP into recombinant Ras-CVIM. | J Med Chem 46: 2973-84 (2003) Article DOI: 10.1021/jm020587n BindingDB Entry DOI: 10.7270/Q26Q1Z0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 386 total ) | Next | Last >> |