

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM50235282 (CHEMBL4077967) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Reversible competitive inhibition of PKCbeta1 (unknown origin) | Bioorg Med Chem Lett 27: 781-786 (2017) Article DOI: 10.1016/j.bmcl.2017.01.038 BindingDB Entry DOI: 10.7270/Q2930WF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50235282 (CHEMBL4077967) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Reversible competitive inhibition of PKCalpha (unknown origin) | Bioorg Med Chem Lett 27: 781-786 (2017) Article DOI: 10.1016/j.bmcl.2017.01.038 BindingDB Entry DOI: 10.7270/Q2930WF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324297 (4-(1-Piperazin-1-yl[2,6]naphthyridin-3-yl)pyridin-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50235288 (CHEMBL4072879) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKCalpha (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay | Bioorg Med Chem Lett 27: 781-786 (2017) Article DOI: 10.1016/j.bmcl.2017.01.038 BindingDB Entry DOI: 10.7270/Q2930WF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50235283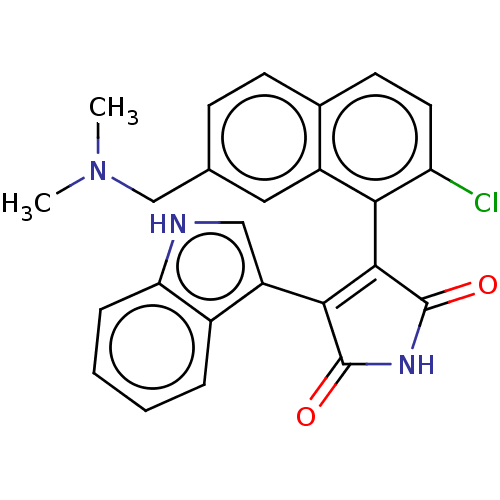 (CHEMBL4102228) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKCalpha (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay | Bioorg Med Chem Lett 27: 781-786 (2017) Article DOI: 10.1016/j.bmcl.2017.01.038 BindingDB Entry DOI: 10.7270/Q2930WF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM211959 (US9290481, 3.1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9290481 (2016) BindingDB Entry DOI: 10.7270/Q2G15ZPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324315 (CHEMBL1214998 | Cyclohexyl-[4-(1-piperazin-1-yl[2,...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM222932 (US9315500, 2.2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9315500 (2016) BindingDB Entry DOI: 10.7270/Q2V986WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324315 (CHEMBL1214998 | Cyclohexyl-[4-(1-piperazin-1-yl[2,...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM50235283 (CHEMBL4102228) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKCbeta1 (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay | Bioorg Med Chem Lett 27: 781-786 (2017) Article DOI: 10.1016/j.bmcl.2017.01.038 BindingDB Entry DOI: 10.7270/Q2930WF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM211951 (US9290481, 1.2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9290481 (2016) BindingDB Entry DOI: 10.7270/Q2G15ZPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50013165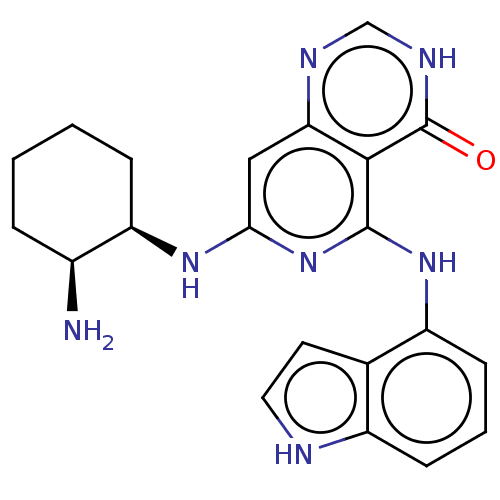 (CHEMBL3262616) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of Syk (unknown origin) using 4 uM peptide assessed as product formation after 60 mins incubation by microfluidic mobility shift assay | Bioorg Med Chem Lett 24: 2278-82 (2014) Article DOI: 10.1016/j.bmcl.2014.03.075 BindingDB Entry DOI: 10.7270/Q2PN976C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM211960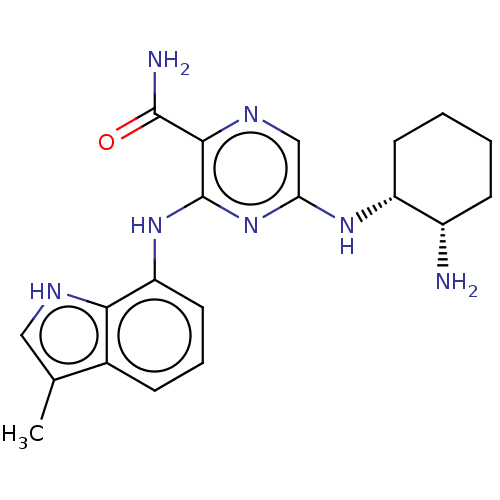 (US9290481, 4.2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9290481 (2016) BindingDB Entry DOI: 10.7270/Q2G15ZPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50013170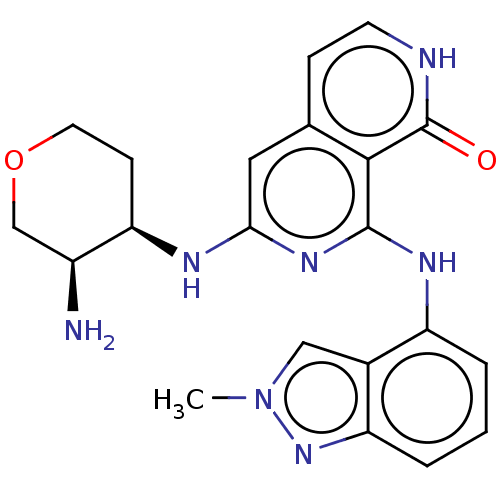 (CHEMBL3262622) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of Syk (unknown origin) using 4 uM peptide assessed as product formation after 60 mins incubation by microfluidic mobility shift assay | Bioorg Med Chem Lett 24: 2278-82 (2014) Article DOI: 10.1016/j.bmcl.2014.03.075 BindingDB Entry DOI: 10.7270/Q2PN976C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycystin-2 (Homo sapiens (Human)) | BDBM50324346 (CHEMBL1215151 | Cyclohexyl-[6-piperazin-1-yl-4-(1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD2 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM33971 (AEB071 | Sotrastaurin | med.21724, Compound 190) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKCtheta (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay | Bioorg Med Chem Lett 27: 781-786 (2017) Article DOI: 10.1016/j.bmcl.2017.01.038 BindingDB Entry DOI: 10.7270/Q2930WF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324322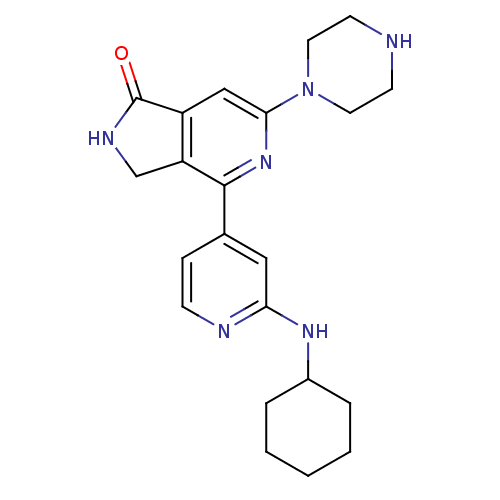 (4-(2-cyclohexylaminopyridin-4-yl)-6-(piperazin-1-y...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50076187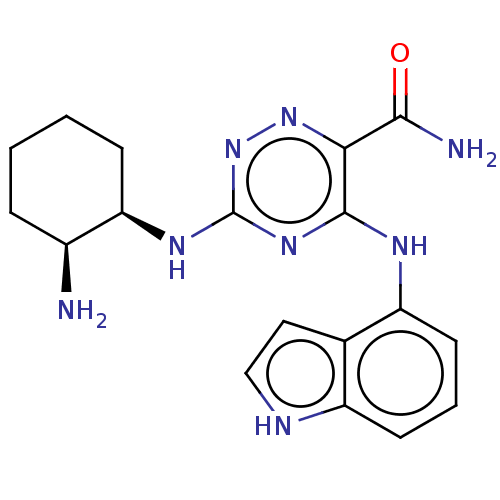 (CHEMBL3416027 | US9290481, 1.4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of Syk (unknown origin) using 5-Fluo-Ahx-GAPDYENLQELNKK-Amide as substrate after 60 mins by microfluidic mobility shift assay | J Med Chem 58: 1950-63 (2015) Article DOI: 10.1021/jm5018863 BindingDB Entry DOI: 10.7270/Q2G44S0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50076190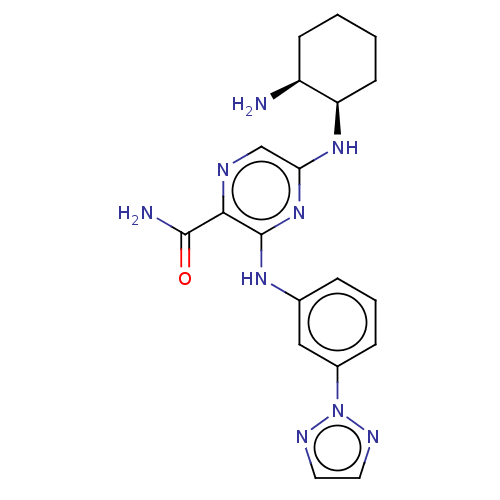 (CHEMBL3416023) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of Syk (unknown origin) using 5-Fluo-Ahx-GAPDYENLQELNKK-Amide as substrate after 60 mins by microfluidic mobility shift assay | J Med Chem 58: 1950-63 (2015) Article DOI: 10.1021/jm5018863 BindingDB Entry DOI: 10.7270/Q2G44S0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycystin-2 (Homo sapiens (Human)) | BDBM50324324 (2'-Phenylamino-6-piperazin-1-yl[2,4']bipyridinyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD2 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324306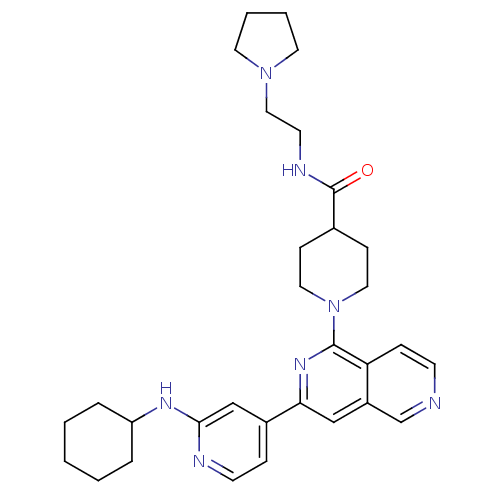 (1-[3-(2-Cyclohexylaminopyridin-4-yl)[2,6]naphthyri...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324305 (CHEMBL1214711 | N-(2-(pyrrolidin-1-yl)ethyl)-1-(3-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324296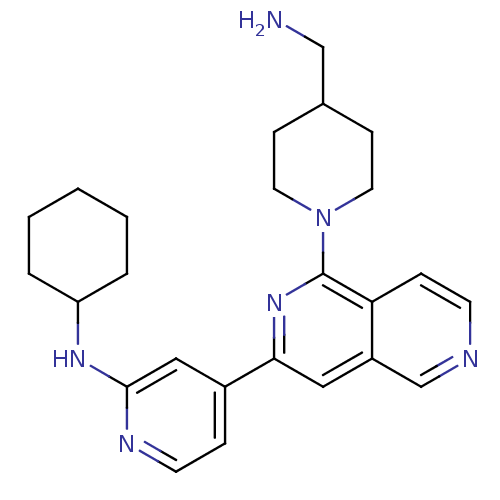 (1-[3-(2-Cyclohexylaminopyridin-4-yl)[2,6]naphthyri...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324298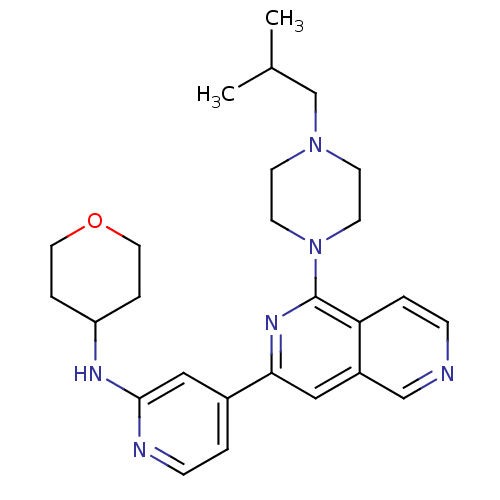 (CHEMBL1215712 | {4-[1-(4-Isobutylpiperazin-1-yl)[2...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324314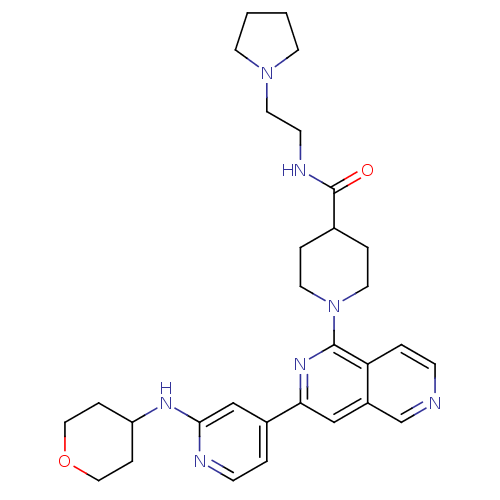 (1-{3-[2-(Tetrahydropyran-4-ylamino)pyridin-4-yl][2...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324325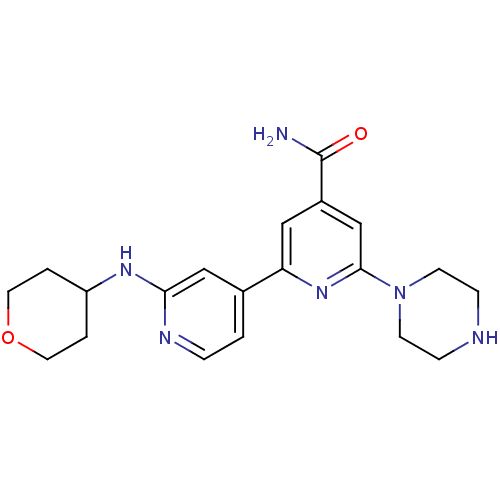 (6-Piperazin-1-yl-2'-(tetrahydropyran-4-ylamino)[2,...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324326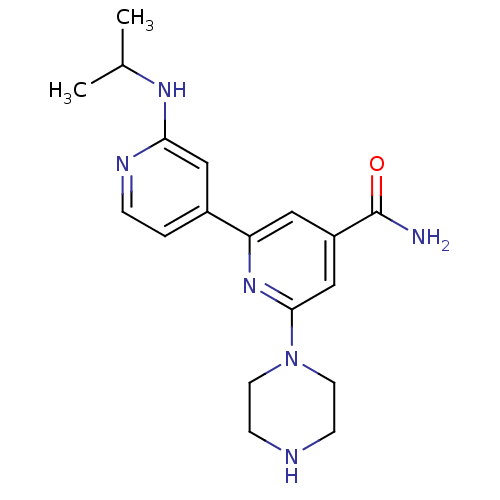 (4'-tert-Butylcarbamoyl-2''-isopropylamino-3,4,5,6-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324324 (2'-Phenylamino-6-piperazin-1-yl[2,4']bipyridinyl-4...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324327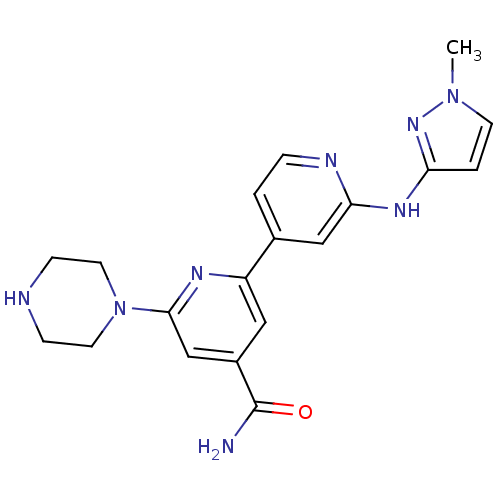 (2'-(1-Methyl-1H-pyrazol-3-ylamino)-6-piperazin-1-y...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324328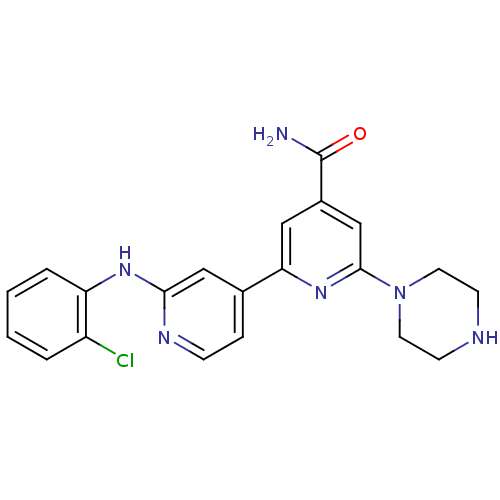 (2'-(2-Chlorophenylamino)-6-piperazin-1-yl[2,4']bip...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324323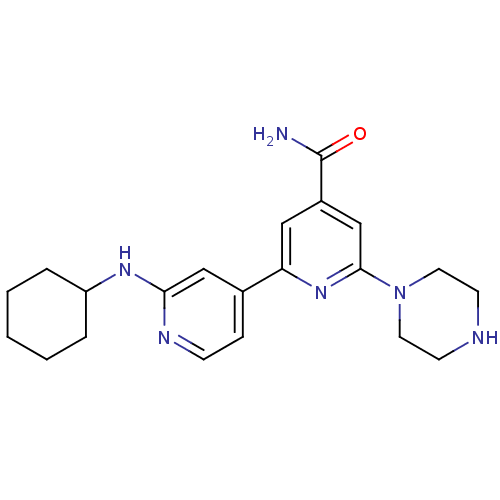 (2'-Cyclohexylamino-6-piperazin-1-yl[2,4']bipyridin...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324348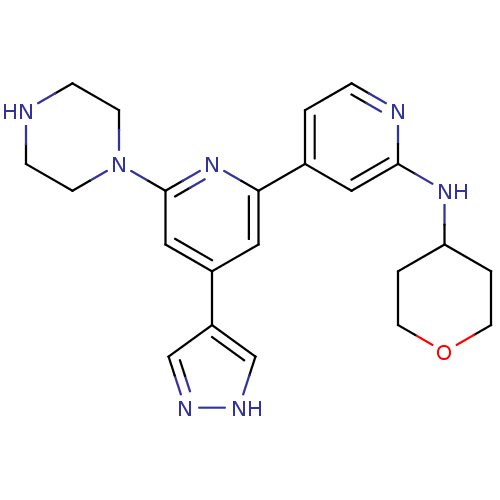 (6-(piperazin-1-yl)-4-(4H-pyrazol-4-yl)-N-(tetrahyd...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324347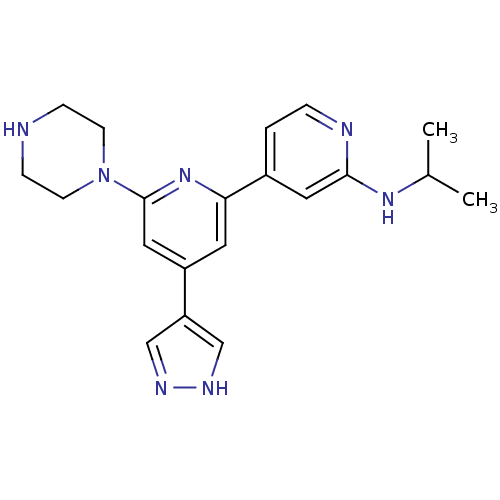 (CHEMBL1215153 | Isopropyl-[6-piperazin-1-yl-4-(1H-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324346 (CHEMBL1215151 | Cyclohexyl-[6-piperazin-1-yl-4-(1H...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM50076187 (CHEMBL3416027 | US9290481, 1.4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9290481 (2016) BindingDB Entry DOI: 10.7270/Q2G15ZPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50013169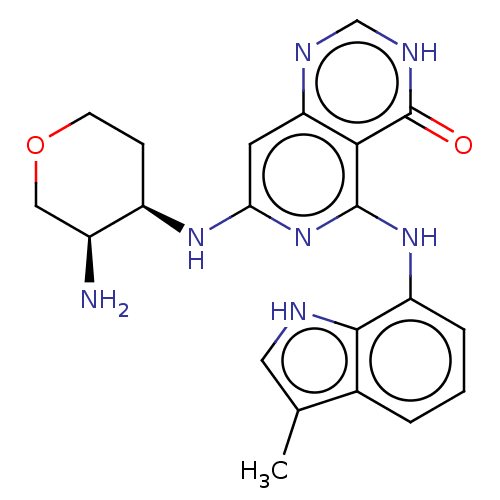 (CHEMBL3262620) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of Syk (unknown origin) using 4 uM peptide assessed as product formation after 60 mins incubation by microfluidic mobility shift assay | Bioorg Med Chem Lett 24: 2278-82 (2014) Article DOI: 10.1016/j.bmcl.2014.03.075 BindingDB Entry DOI: 10.7270/Q2PN976C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM222933 (US9315500, 2.3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9315500 (2016) BindingDB Entry DOI: 10.7270/Q2V986WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM222934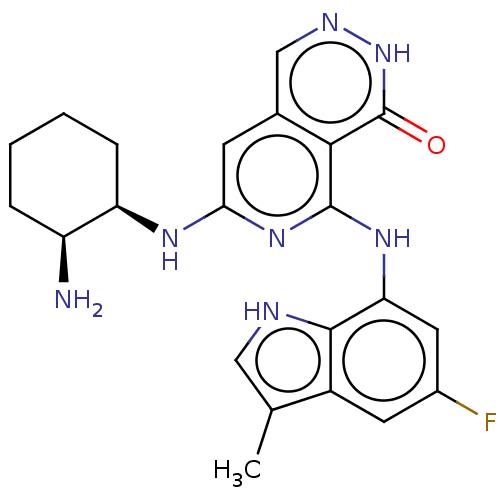 (US9315500, 2.4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9315500 (2016) BindingDB Entry DOI: 10.7270/Q2V986WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM33971 (AEB071 | Sotrastaurin | med.21724, Compound 190) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKCdelta (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay | Bioorg Med Chem Lett 27: 781-786 (2017) Article DOI: 10.1016/j.bmcl.2017.01.038 BindingDB Entry DOI: 10.7270/Q2930WF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM50235288 (CHEMBL4072879) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKCbeta1 (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay | Bioorg Med Chem Lett 27: 781-786 (2017) Article DOI: 10.1016/j.bmcl.2017.01.038 BindingDB Entry DOI: 10.7270/Q2930WF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM222935 (US9315500, 2.5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9315500 (2016) BindingDB Entry DOI: 10.7270/Q2V986WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM222931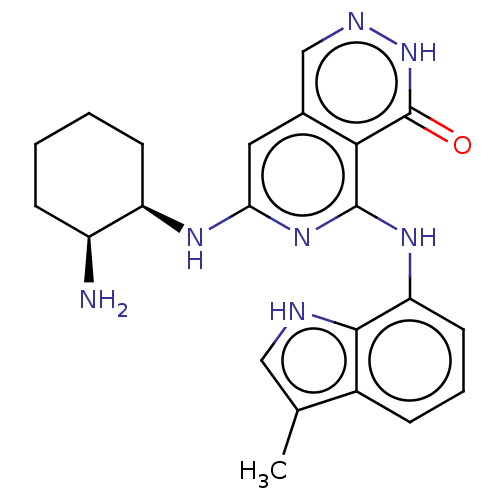 (US9315500, 2.1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9315500 (2016) BindingDB Entry DOI: 10.7270/Q2V986WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50235292 (CHEMBL4085837) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKCalpha (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay | Bioorg Med Chem Lett 27: 781-786 (2017) Article DOI: 10.1016/j.bmcl.2017.01.038 BindingDB Entry DOI: 10.7270/Q2930WF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM222952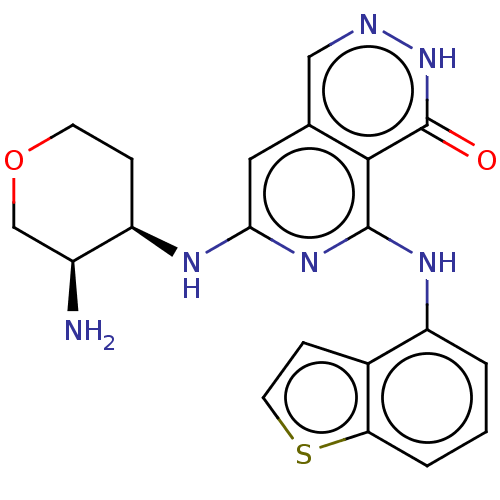 (US9315500, 3.2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9315500 (2016) BindingDB Entry DOI: 10.7270/Q2V986WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50013162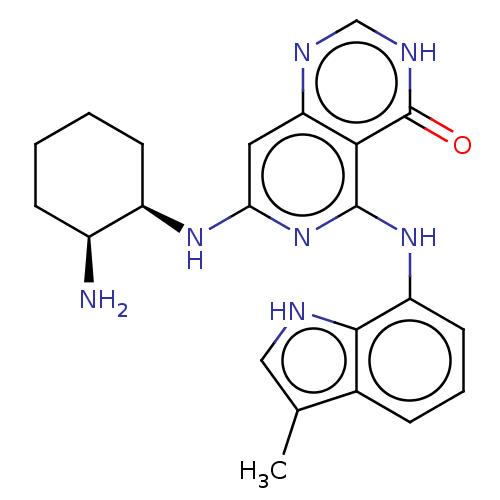 (CHEMBL3262357) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of Syk (unknown origin) using 4 uM peptide assessed as product formation after 60 mins incubation by microfluidic mobility shift assay | Bioorg Med Chem Lett 24: 2278-82 (2014) Article DOI: 10.1016/j.bmcl.2014.03.075 BindingDB Entry DOI: 10.7270/Q2PN976C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM222953 (US9315500, 3.3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9315500 (2016) BindingDB Entry DOI: 10.7270/Q2V986WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM222936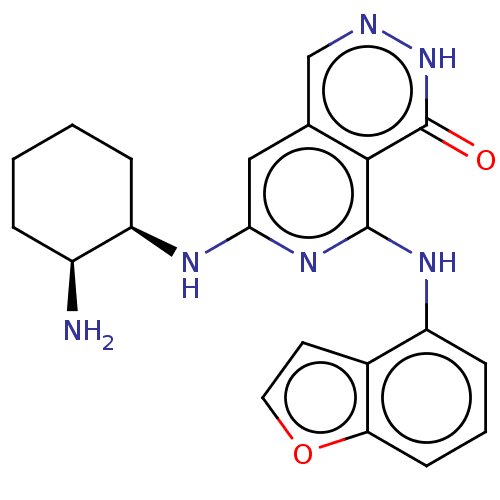 (US9315500, 2.6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9315500 (2016) BindingDB Entry DOI: 10.7270/Q2V986WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM222955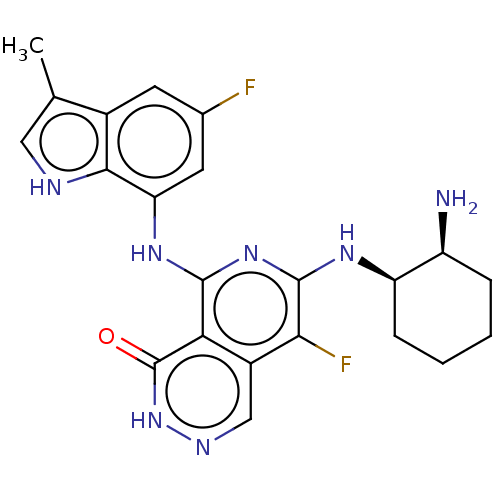 (US9315500, 1.1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9315500 (2016) BindingDB Entry DOI: 10.7270/Q2V986WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324335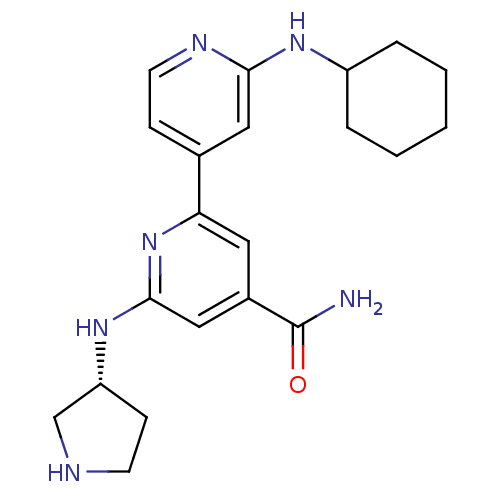 (2'-Cyclohexylamino-6-((R)-pyrrolidin-3-ylamino)[2,...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324312 (1-{3-[2-(Tetrahydropyran-4-ylamino)pyridin-4-yl][2...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 715 total ) | Next | Last >> |