 Found 238 hits with Last Name = 'van der meer' and Initial = 't'
Found 238 hits with Last Name = 'van der meer' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527524

(CHEMBL4566742)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CCC1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C27H32N4O6/c1-36-21-8-7-17(15-22(21)37-2)26-19-5-3-4-6-20(19)27(35)31(28-26)18-11-13-29(14-12-18)25(34)16-30-23(32)9-10-24(30)33/h3-4,7-8,15,18-20H,5-6,9-14,16H2,1-2H3/t19-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0617 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527552
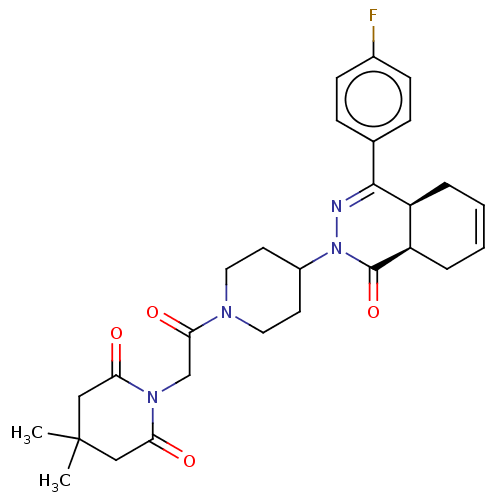
(CHEMBL4435111)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CC(C)(C)CC1=O)C2=O)c1ccc(F)cc1 |r,c:3,9| Show InChI InChI=1S/C28H33FN4O4/c1-28(2)15-23(34)32(24(35)16-28)17-25(36)31-13-11-20(12-14-31)33-27(37)22-6-4-3-5-21(22)26(30-33)18-7-9-19(29)10-8-18/h3-4,7-10,20-22H,5-6,11-17H2,1-2H3/t21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527532
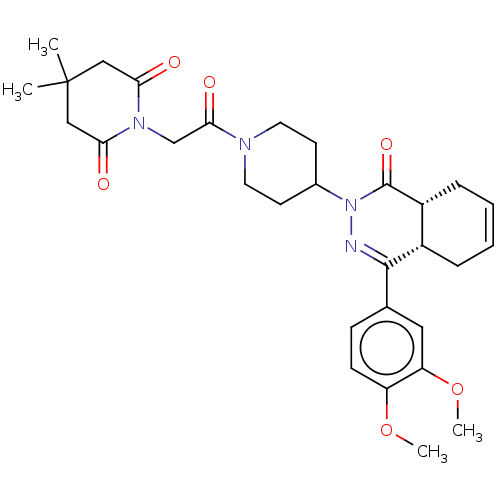
(CHEMBL4441748)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CC(C)(C)CC1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C30H38N4O6/c1-30(2)16-25(35)33(26(36)17-30)18-27(37)32-13-11-20(12-14-32)34-29(38)22-8-6-5-7-21(22)28(31-34)19-9-10-23(39-3)24(15-19)40-4/h5-6,9-10,15,20-22H,7-8,11-14,16-18H2,1-4H3/t21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527537
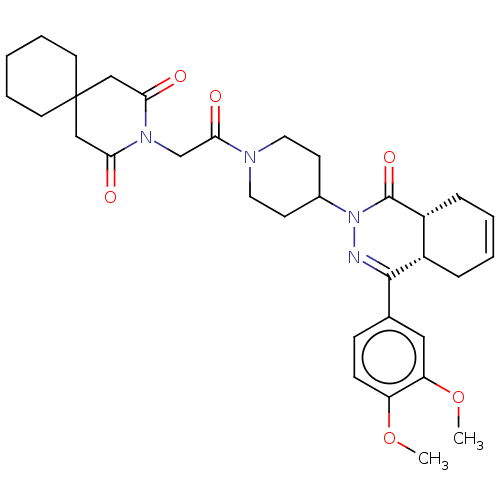
(CHEMBL4453005)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CC3(CCCCC3)CC1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C33H42N4O6/c1-42-26-11-10-22(18-27(26)43-2)31-24-8-4-5-9-25(24)32(41)37(34-31)23-12-16-35(17-13-23)30(40)21-36-28(38)19-33(20-29(36)39)14-6-3-7-15-33/h4-5,10-11,18,23-25H,3,6-9,12-17,19-21H2,1-2H3/t24-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527531
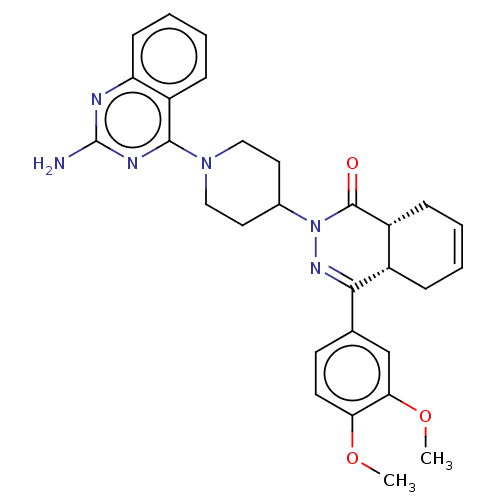
(CHEMBL4524402)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1nc(N)nc3ccccc13)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C29H32N6O3/c1-37-24-12-11-18(17-25(24)38-2)26-20-7-3-4-8-21(20)28(36)35(33-26)19-13-15-34(16-14-19)27-22-9-5-6-10-23(22)31-29(30)32-27/h3-6,9-12,17,19-21H,7-8,13-16H2,1-2H3,(H2,30,31,32)/t20-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527528
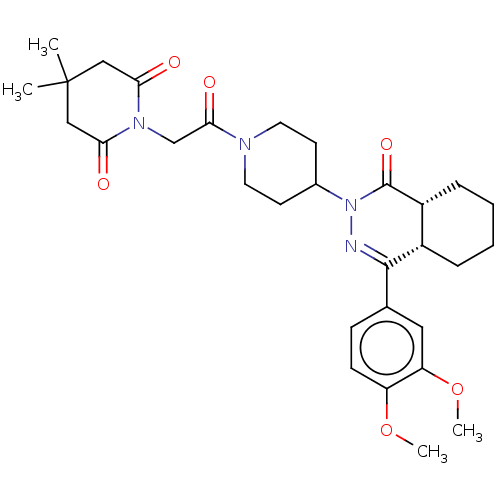
(CHEMBL4441871)Show SMILES [H][C@@]12CCCC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CC(C)(C)CC1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:9| Show InChI InChI=1S/C30H40N4O6/c1-30(2)16-25(35)33(26(36)17-30)18-27(37)32-13-11-20(12-14-32)34-29(38)22-8-6-5-7-21(22)28(31-34)19-9-10-23(39-3)24(15-19)40-4/h9-10,15,20-22H,5-8,11-14,16-18H2,1-4H3/t21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527550
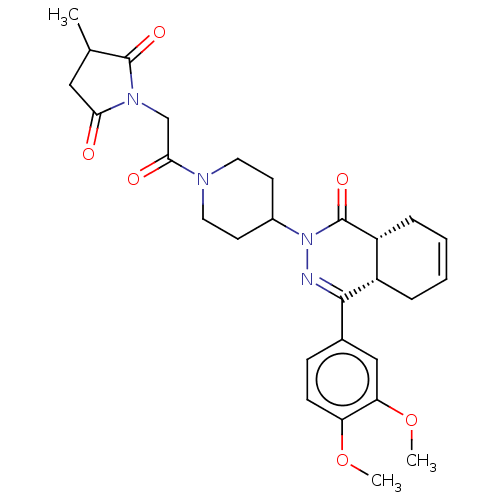
(CHEMBL4461456)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CC(C)C1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C28H34N4O6/c1-17-14-24(33)31(27(17)35)16-25(34)30-12-10-19(11-13-30)32-28(36)21-7-5-4-6-20(21)26(29-32)18-8-9-22(37-2)23(15-18)38-3/h4-5,8-9,15,17,19-21H,6-7,10-14,16H2,1-3H3/t17?,20-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527535
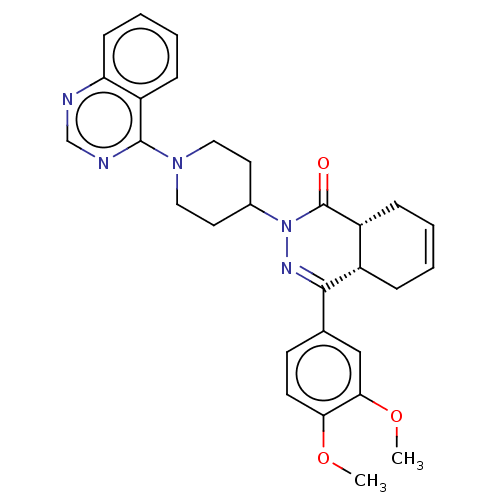
(CHEMBL4589927)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1ncnc3ccccc13)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C29H31N5O3/c1-36-25-12-11-19(17-26(25)37-2)27-21-7-3-4-8-22(21)29(35)34(32-27)20-13-15-33(16-14-20)28-23-9-5-6-10-24(23)30-18-31-28/h3-6,9-12,17-18,20-22H,7-8,13-16H2,1-2H3/t21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527533
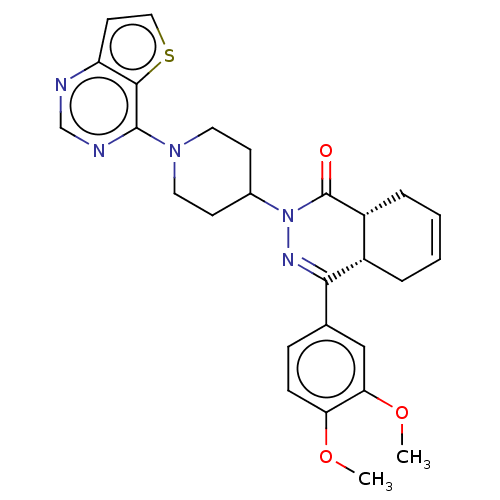
(CHEMBL4592553)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1ncnc3ccsc13)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C27H29N5O3S/c1-34-22-8-7-17(15-23(22)35-2)24-19-5-3-4-6-20(19)27(33)32(30-24)18-9-12-31(13-10-18)26-25-21(11-14-36-25)28-16-29-26/h3-4,7-8,11,14-16,18-20H,5-6,9-10,12-13H2,1-2H3/t19-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527555
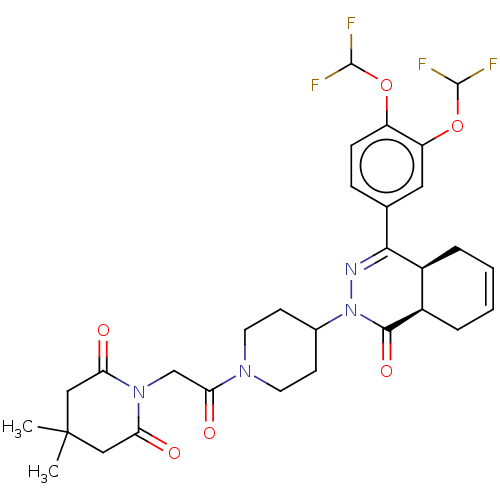
(CHEMBL4530777)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CC(C)(C)CC1=O)C2=O)c1ccc(OC(F)F)c(OC(F)F)c1 |r,c:3,9| Show InChI InChI=1S/C30H34F4N4O6/c1-30(2)14-23(39)37(24(40)15-30)16-25(41)36-11-9-18(10-12-36)38-27(42)20-6-4-3-5-19(20)26(35-38)17-7-8-21(43-28(31)32)22(13-17)44-29(33)34/h3-4,7-8,13,18-20,28-29H,5-6,9-12,14-16H2,1-2H3/t19-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527567
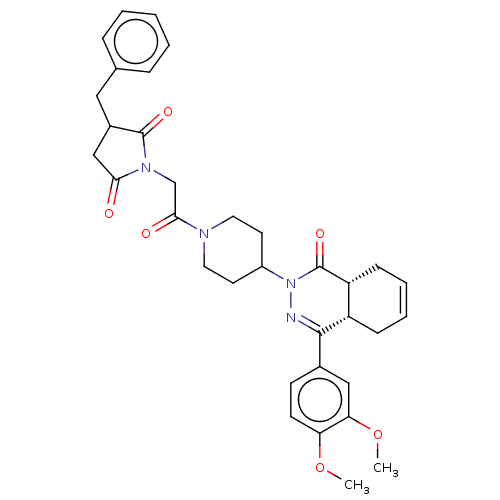
(CHEMBL4473426)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CC(Cc3ccccc3)C1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C34H38N4O6/c1-43-28-13-12-23(19-29(28)44-2)32-26-10-6-7-11-27(26)34(42)38(35-32)25-14-16-36(17-15-25)31(40)21-37-30(39)20-24(33(37)41)18-22-8-4-3-5-9-22/h3-9,12-13,19,24-27H,10-11,14-18,20-21H2,1-2H3/t24?,26-,27+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527560
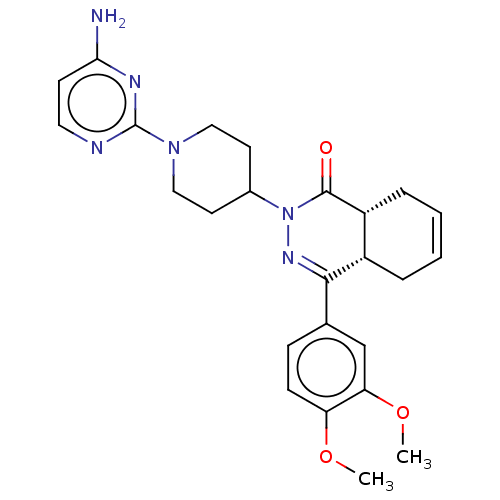
(CHEMBL4444578)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1nccc(N)n1)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C25H30N6O3/c1-33-20-8-7-16(15-21(20)34-2)23-18-5-3-4-6-19(18)24(32)31(29-23)17-10-13-30(14-11-17)25-27-12-9-22(26)28-25/h3-4,7-9,12,15,17-19H,5-6,10-11,13-14H2,1-2H3,(H2,26,27,28)/t18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527554
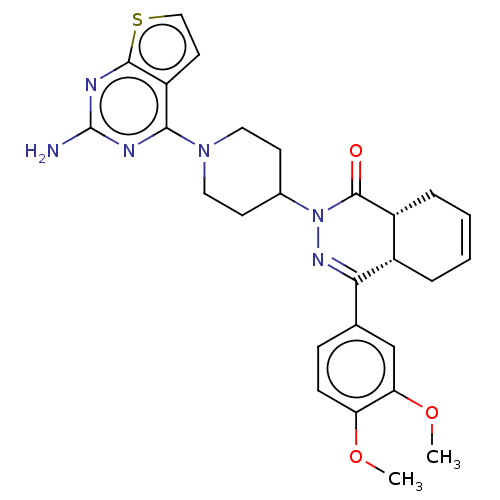
(CHEMBL4460623)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1nc(N)nc3sccc13)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C27H30N6O3S/c1-35-21-8-7-16(15-22(21)36-2)23-18-5-3-4-6-19(18)26(34)33(31-23)17-9-12-32(13-10-17)24-20-11-14-37-25(20)30-27(28)29-24/h3-4,7-8,11,14-15,17-19H,5-6,9-10,12-13H2,1-2H3,(H2,28,29,30)/t18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527559
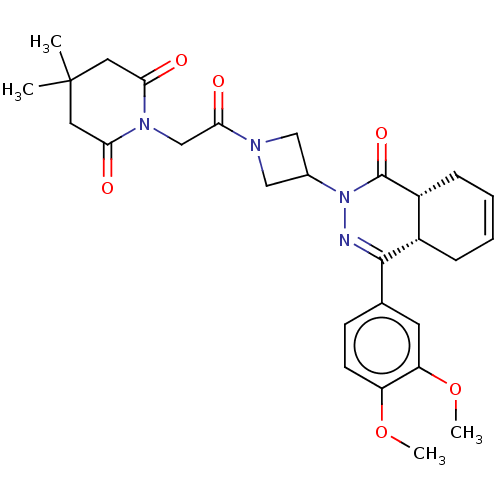
(CHEMBL4591859)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CN(C1)C(=O)CN1C(=O)CC(C)(C)CC1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C28H34N4O6/c1-28(2)12-23(33)31(24(34)13-28)16-25(35)30-14-18(15-30)32-27(36)20-8-6-5-7-19(20)26(29-32)17-9-10-21(37-3)22(11-17)38-4/h5-6,9-11,18-20H,7-8,12-16H2,1-4H3/t19-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.389 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527525
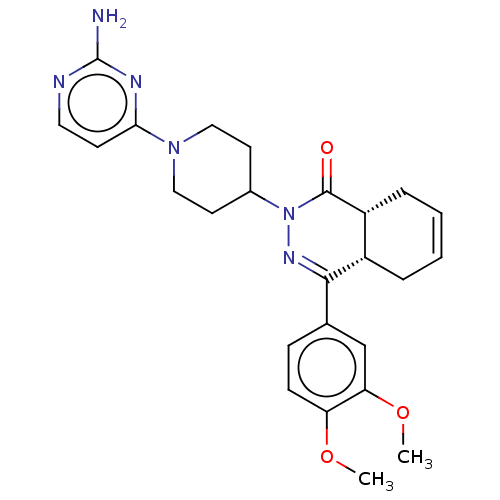
(CHEMBL4522249)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1ccnc(N)n1)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C25H30N6O3/c1-33-20-8-7-16(15-21(20)34-2)23-18-5-3-4-6-19(18)24(32)31(29-23)17-10-13-30(14-11-17)22-9-12-27-25(26)28-22/h3-4,7-9,12,15,17-19H,5-6,10-11,13-14H2,1-2H3,(H2,26,27,28)/t18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527529
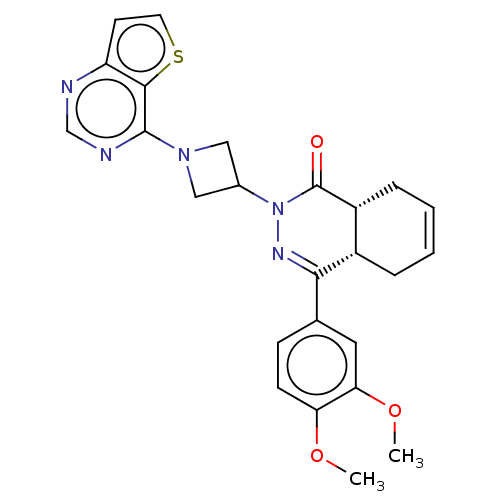
(CHEMBL4454600)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CN(C1)c1ncnc3ccsc13)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C25H25N5O3S/c1-32-20-8-7-15(11-21(20)33-2)22-17-5-3-4-6-18(17)25(31)30(28-22)16-12-29(13-16)24-23-19(9-10-34-23)26-14-27-24/h3-4,7-11,14,16-18H,5-6,12-13H2,1-2H3/t17-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527527
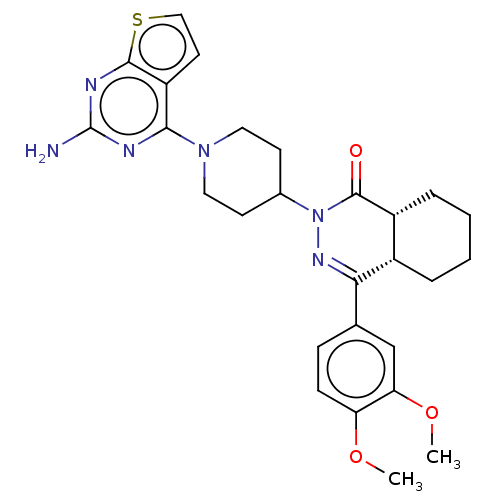
(CHEMBL4466777)Show SMILES [H][C@@]12CCCC[C@]1([H])C(=NN(C1CCN(CC1)c1nc(N)nc3sccc13)C2=O)c1ccc(OC)c(OC)c1 |r,c:9| Show InChI InChI=1S/C27H32N6O3S/c1-35-21-8-7-16(15-22(21)36-2)23-18-5-3-4-6-19(18)26(34)33(31-23)17-9-12-32(13-10-17)24-20-11-14-37-25(20)30-27(28)29-24/h7-8,11,14-15,17-19H,3-6,9-10,12-13H2,1-2H3,(H2,28,29,30)/t18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.661 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527530
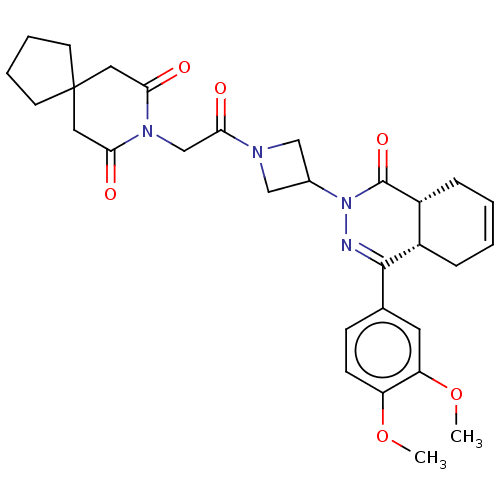
(CHEMBL4461705)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CN(C1)C(=O)CN1C(=O)CC3(CCCC3)CC1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C30H36N4O6/c1-39-23-10-9-19(13-24(23)40-2)28-21-7-3-4-8-22(21)29(38)34(31-28)20-16-32(17-20)27(37)18-33-25(35)14-30(15-26(33)36)11-5-6-12-30/h3-4,9-10,13,20-22H,5-8,11-12,14-18H2,1-2H3/t21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527534
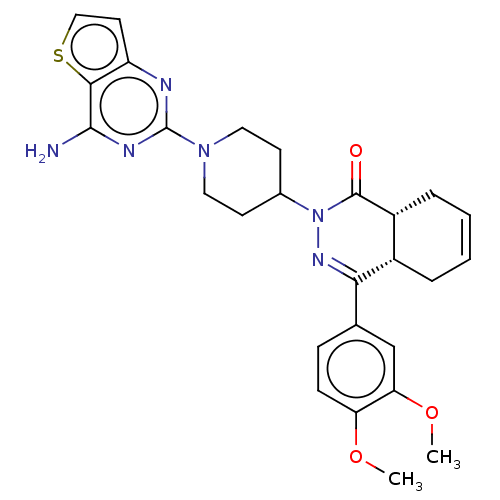
(CHEMBL4472307)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1nc(N)c3sccc3n1)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C27H30N6O3S/c1-35-21-8-7-16(15-22(21)36-2)23-18-5-3-4-6-19(18)26(34)33(31-23)17-9-12-32(13-10-17)27-29-20-11-14-37-24(20)25(28)30-27/h3-4,7-8,11,14-15,17-19H,5-6,9-10,12-13H2,1-2H3,(H2,28,29,30)/t18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527548
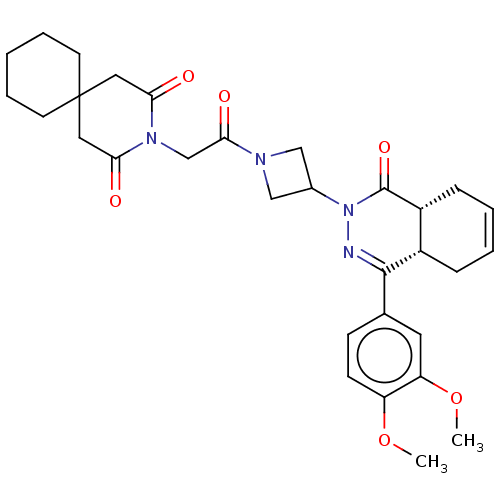
(CHEMBL4465097)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CN(C1)C(=O)CN1C(=O)CC3(CCCCC3)CC1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C31H38N4O6/c1-40-24-11-10-20(14-25(24)41-2)29-22-8-4-5-9-23(22)30(39)35(32-29)21-17-33(18-21)28(38)19-34-26(36)15-31(16-27(34)37)12-6-3-7-13-31/h4-5,10-11,14,21-23H,3,6-9,12-13,15-19H2,1-2H3/t22-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527549
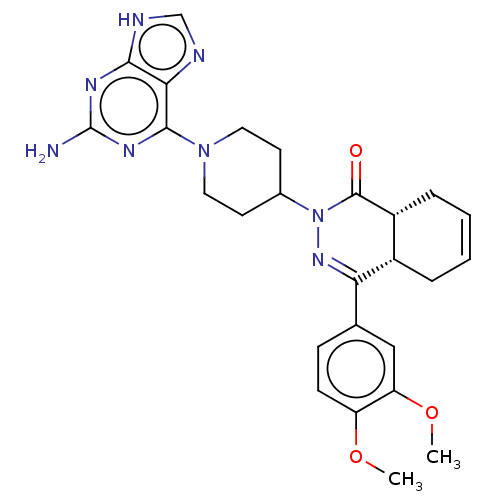
(CHEMBL4452707)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1nc(N)nc3[nH]cnc13)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C26H30N8O3/c1-36-19-8-7-15(13-20(19)37-2)21-17-5-3-4-6-18(17)25(35)34(32-21)16-9-11-33(12-10-16)24-22-23(29-14-28-22)30-26(27)31-24/h3-4,7-8,13-14,16-18H,5-6,9-12H2,1-2H3,(H3,27,28,29,30,31)/t17-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527523

(CHEMBL4461658)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CN(C1)c1nc(N)nc3sccc13)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C25H26N6O3S/c1-33-19-8-7-14(11-20(19)34-2)21-16-5-3-4-6-17(16)24(32)31(29-21)15-12-30(13-15)22-18-9-10-35-23(18)28-25(26)27-22/h3-4,7-11,15-17H,5-6,12-13H2,1-2H3,(H2,26,27,28)/t16-,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527557
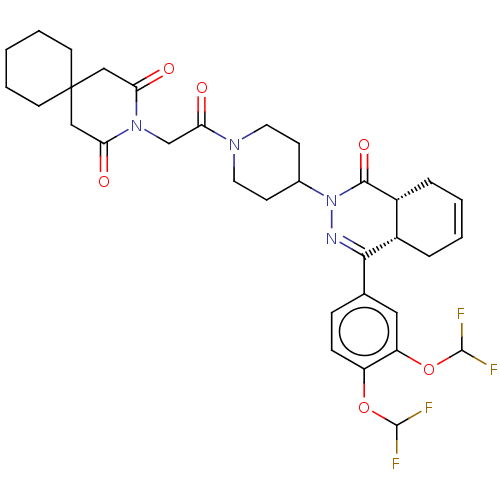
(CHEMBL4533993)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CC3(CCCCC3)CC1=O)C2=O)c1ccc(OC(F)F)c(OC(F)F)c1 |r,c:3,9| Show InChI InChI=1S/C33H38F4N4O6/c34-31(35)46-24-9-8-20(16-25(24)47-32(36)37)29-22-6-2-3-7-23(22)30(45)41(38-29)21-10-14-39(15-11-21)28(44)19-40-26(42)17-33(18-27(40)43)12-4-1-5-13-33/h2-3,8-9,16,21-23,31-32H,1,4-7,10-15,17-19H2/t22-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527566
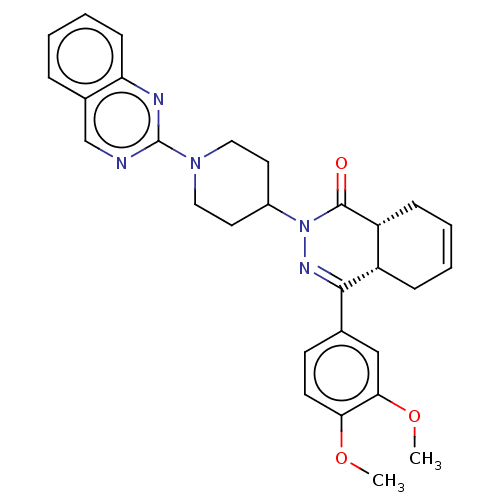
(CHEMBL4472329)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1ncc3ccccc3n1)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C29H31N5O3/c1-36-25-12-11-19(17-26(25)37-2)27-22-8-4-5-9-23(22)28(35)34(32-27)21-13-15-33(16-14-21)29-30-18-20-7-3-6-10-24(20)31-29/h3-7,10-12,17-18,21-23H,8-9,13-16H2,1-2H3/t22-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527551
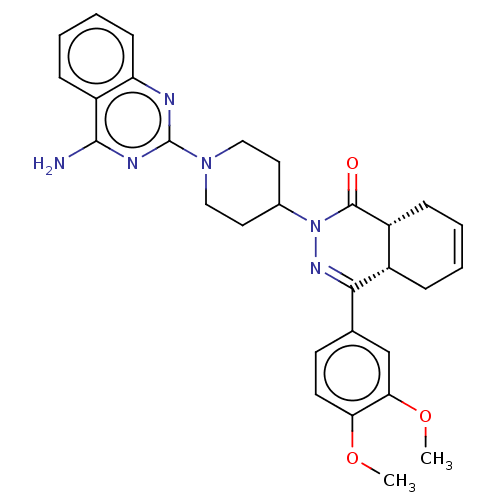
(CHEMBL4575003)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1nc(N)c3ccccc3n1)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C29H32N6O3/c1-37-24-12-11-18(17-25(24)38-2)26-20-7-3-4-8-21(20)28(36)35(33-26)19-13-15-34(16-14-19)29-31-23-10-6-5-9-22(23)27(30)32-29/h3-6,9-12,17,19-21H,7-8,13-16H2,1-2H3,(H2,30,31,32)/t20-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527536
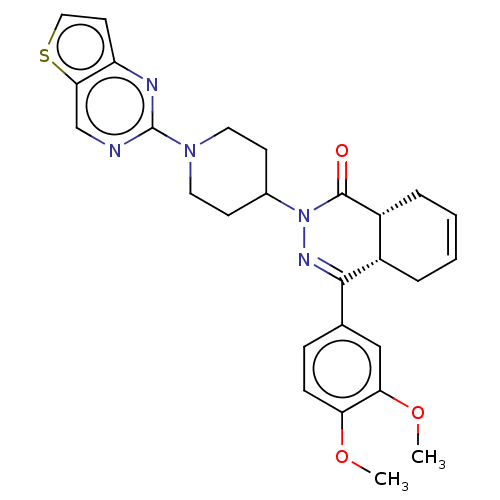
(CHEMBL4466905)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1ncc3sccc3n1)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C27H29N5O3S/c1-34-22-8-7-17(15-23(22)35-2)25-19-5-3-4-6-20(19)26(33)32(30-25)18-9-12-31(13-10-18)27-28-16-24-21(29-27)11-14-36-24/h3-4,7-8,11,14-16,18-20H,5-6,9-10,12-13H2,1-2H3/t19-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50361010
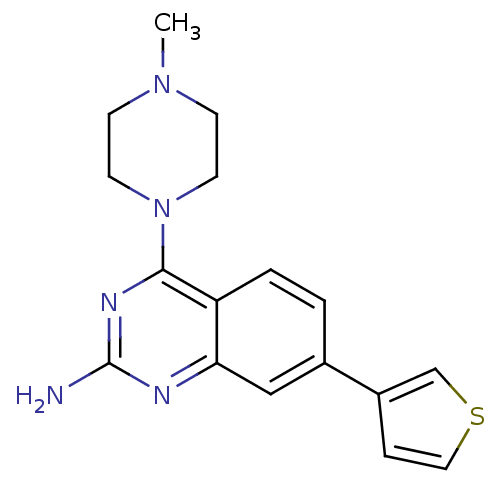
(CHEMBL1935572)Show InChI InChI=1S/C17H19N5S/c1-21-5-7-22(8-6-21)16-14-3-2-12(13-4-9-23-11-13)10-15(14)19-17(18)20-16/h2-4,9-11H,5-8H2,1H3,(H2,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV
Curated by ChEMBL
| Assay Description
Antagonist activity at human H4 receptor expressed in HEK293T cells assessed as inhibition of histamine-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 22: 461-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.104
BindingDB Entry DOI: 10.7270/Q2D79BT2 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human H4 receptor expressed in HEK cell membranes |
Bioorg Med Chem Lett 22: 461-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.104
BindingDB Entry DOI: 10.7270/Q2D79BT2 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50361035
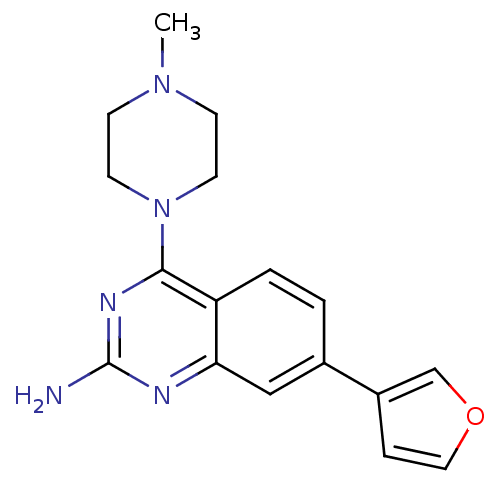
(CHEMBL1935571)Show InChI InChI=1S/C17H19N5O/c1-21-5-7-22(8-6-21)16-14-3-2-12(13-4-9-23-11-13)10-15(14)19-17(18)20-16/h2-4,9-11H,5-8H2,1H3,(H2,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV
Curated by ChEMBL
| Assay Description
Antagonist activity at human H4 receptor expressed in HEK293T cells assessed as inhibition of histamine-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 22: 461-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.104
BindingDB Entry DOI: 10.7270/Q2D79BT2 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50361015

(CHEMBL592379)Show SMILES CN1CCN(CC1)c1nc(NCCS(=O)(=O)Nc2ccccc2)c2cc(Cl)ccc2n1 Show InChI InChI=1S/C21H25ClN6O2S/c1-27-10-12-28(13-11-27)21-24-19-8-7-16(22)15-18(19)20(25-21)23-9-14-31(29,30)26-17-5-3-2-4-6-17/h2-8,15,26H,9-14H2,1H3,(H,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human H4 receptor expressed in HEK cell membranes |
Bioorg Med Chem Lett 22: 461-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.104
BindingDB Entry DOI: 10.7270/Q2D79BT2 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50361010

(CHEMBL1935572)Show InChI InChI=1S/C17H19N5S/c1-21-5-7-22(8-6-21)16-14-3-2-12(13-4-9-23-11-13)10-15(14)19-17(18)20-16/h2-4,9-11H,5-8H2,1H3,(H2,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human H4 receptor expressed in HEK cell membranes |
Bioorg Med Chem Lett 22: 461-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.104
BindingDB Entry DOI: 10.7270/Q2D79BT2 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50361011
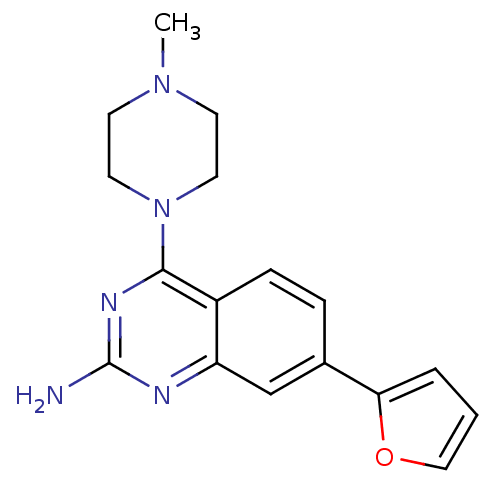
(CHEMBL1935573)Show InChI InChI=1S/C17H19N5O/c1-21-6-8-22(9-7-21)16-13-5-4-12(15-3-2-10-23-15)11-14(13)19-17(18)20-16/h2-5,10-11H,6-9H2,1H3,(H2,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV
Curated by ChEMBL
| Assay Description
Antagonist activity at human H4 receptor expressed in HEK293T cells assessed as inhibition of histamine-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 22: 461-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.104
BindingDB Entry DOI: 10.7270/Q2D79BT2 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM26396
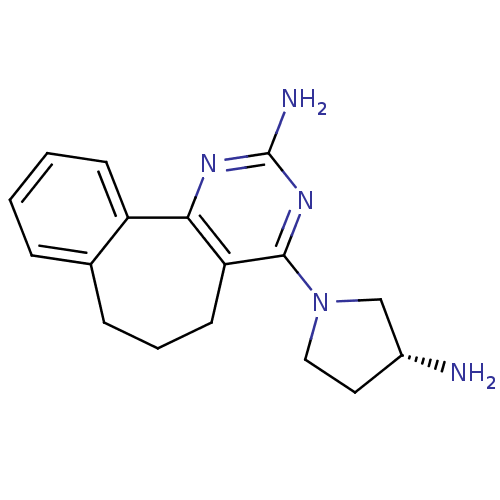
(2,4-diamino-5,6-disubstituted pyrimidine, 10 | 6-[...)Show SMILES N[C@@H]1CCN(C1)c1nc(N)nc-2c1CCCc1ccccc-21 |r| Show InChI InChI=1S/C17H21N5/c18-12-8-9-22(10-12)16-14-7-3-5-11-4-1-2-6-13(11)15(14)20-17(19)21-16/h1-2,4,6,12H,3,5,7-10,18H2,(H2,19,20,21)/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human H4 receptor expressed in HEK cell membranes |
Bioorg Med Chem Lett 22: 461-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.104
BindingDB Entry DOI: 10.7270/Q2D79BT2 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50361011

(CHEMBL1935573)Show InChI InChI=1S/C17H19N5O/c1-21-6-8-22(9-7-21)16-13-5-4-12(15-3-2-10-23-15)11-14(13)19-17(18)20-16/h2-5,10-11H,6-9H2,1H3,(H2,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human H4 receptor expressed in HEK cell membranes |
Bioorg Med Chem Lett 22: 461-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.104
BindingDB Entry DOI: 10.7270/Q2D79BT2 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50361014
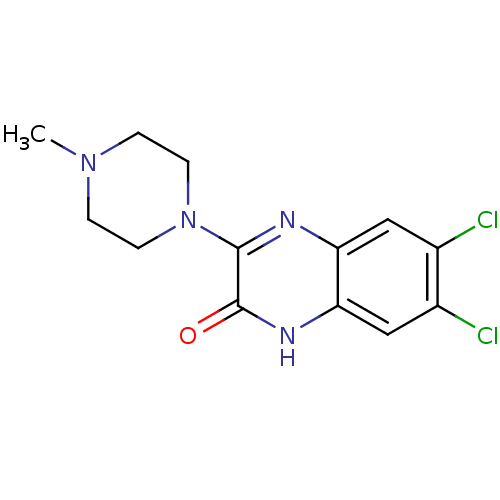
(CHEMBL260549 | VUF-10214)Show InChI InChI=1S/C13H14Cl2N4O/c1-18-2-4-19(5-3-18)12-13(20)17-11-7-9(15)8(14)6-10(11)16-12/h6-7H,2-5H2,1H3,(H,17,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human H4 receptor expressed in HEK cell membranes |
Bioorg Med Chem Lett 22: 461-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.104
BindingDB Entry DOI: 10.7270/Q2D79BT2 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50361013
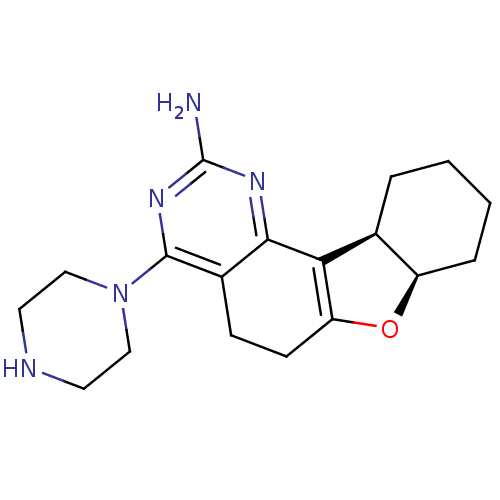
(CHEMBL1935450)Show SMILES Nc1nc(N2CCNCC2)c2CCC3=C([C@H]4CCCC[C@H]4O3)c2n1 |r,t:14| Show InChI InChI=1S/C18H25N5O/c19-18-21-16-12(17(22-18)23-9-7-20-8-10-23)5-6-14-15(16)11-3-1-2-4-13(11)24-14/h11,13,20H,1-10H2,(H2,19,21,22)/t11-,13+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human H4 receptor expressed in HEK cell membranes |
Bioorg Med Chem Lett 22: 461-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.104
BindingDB Entry DOI: 10.7270/Q2D79BT2 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Rattus norvegicus (rat)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV
Curated by ChEMBL
| Assay Description
Binding affinity to rat histamine H4 receptor |
Bioorg Med Chem Lett 22: 461-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.104
BindingDB Entry DOI: 10.7270/Q2D79BT2 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H4 receptor |
Bioorg Med Chem Lett 22: 461-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.104
BindingDB Entry DOI: 10.7270/Q2D79BT2 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50191289
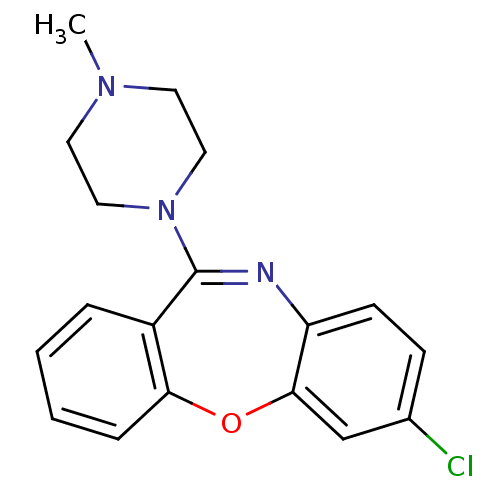
(7-chloro-11-(4-methyl-piperazin-1-yl)-dibenzo[b,f]...)Show SMILES CN1CCN(CC1)C1=Nc2ccc(Cl)cc2Oc2ccccc12 |t:8| Show InChI InChI=1S/C18H18ClN3O/c1-21-8-10-22(11-9-21)18-14-4-2-3-5-16(14)23-17-12-13(19)6-7-15(17)20-18/h2-7,12H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human H4 receptor expressed in HEK cell membranes |
Bioorg Med Chem Lett 22: 461-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.104
BindingDB Entry DOI: 10.7270/Q2D79BT2 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50361035

(CHEMBL1935571)Show InChI InChI=1S/C17H19N5O/c1-21-5-7-22(8-6-21)16-14-3-2-12(13-4-9-23-11-13)10-15(14)19-17(18)20-16/h2-4,9-11H,5-8H2,1H3,(H2,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human H4 receptor expressed in HEK cell membranes |
Bioorg Med Chem Lett 22: 461-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.104
BindingDB Entry DOI: 10.7270/Q2D79BT2 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50361012
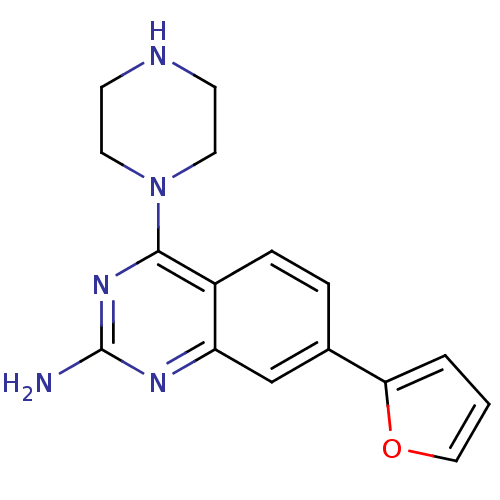
(CHEMBL1935574)Show InChI InChI=1S/C16H17N5O/c17-16-19-13-10-11(14-2-1-9-22-14)3-4-12(13)15(20-16)21-7-5-18-6-8-21/h1-4,9-10,18H,5-8H2,(H2,17,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV
Curated by ChEMBL
| Assay Description
Antagonist activity at human H4 receptor expressed in HEK293T cells assessed as inhibition of histamine-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 22: 461-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.104
BindingDB Entry DOI: 10.7270/Q2D79BT2 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human H4 receptor expressed in HEK cell membranes |
Bioorg Med Chem Lett 22: 461-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.104
BindingDB Entry DOI: 10.7270/Q2D79BT2 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Mus musculus (mouse)) | BDBM50433359
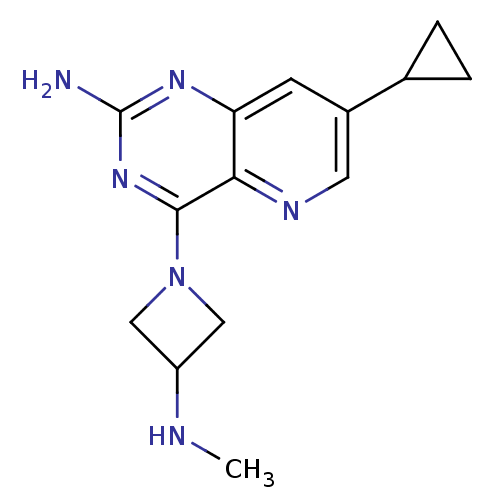
(CHEMBL2376804)Show InChI InChI=1S/C14H18N6/c1-16-10-6-20(7-10)13-12-11(18-14(15)19-13)4-9(5-17-12)8-2-3-8/h4-5,8,10,16H,2-3,6-7H2,1H3,(H2,15,18,19) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV
Curated by ChEMBL
| Assay Description
Binding affinity to mouse histamine H4 receptor |
Bioorg Med Chem Lett 23: 2663-70 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.091
BindingDB Entry DOI: 10.7270/Q2K35W11 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50361034
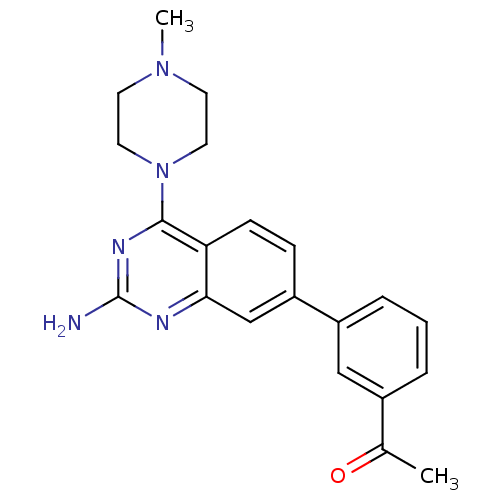
(CHEMBL1935570)Show SMILES CN1CCN(CC1)c1nc(N)nc2cc(ccc12)-c1cccc(c1)C(C)=O Show InChI InChI=1S/C21H23N5O/c1-14(27)15-4-3-5-16(12-15)17-6-7-18-19(13-17)23-21(22)24-20(18)26-10-8-25(2)9-11-26/h3-7,12-13H,8-11H2,1-2H3,(H2,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human H4 receptor expressed in HEK cell membranes |
Bioorg Med Chem Lett 22: 461-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.104
BindingDB Entry DOI: 10.7270/Q2D79BT2 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50361012

(CHEMBL1935574)Show InChI InChI=1S/C16H17N5O/c17-16-19-13-10-11(14-2-1-9-22-14)3-4-12(13)15(20-16)21-7-5-18-6-8-21/h1-4,9-10,18H,5-8H2,(H2,17,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human H4 receptor expressed in HEK cell membranes |
Bioorg Med Chem Lett 22: 461-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.104
BindingDB Entry DOI: 10.7270/Q2D79BT2 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50361029
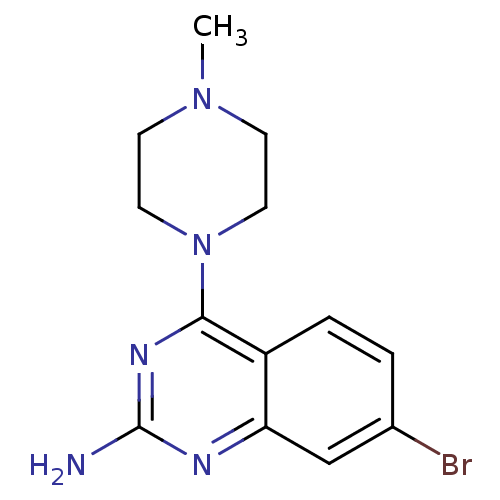
(CHEMBL1935565)Show InChI InChI=1S/C13H16BrN5/c1-18-4-6-19(7-5-18)12-10-3-2-9(14)8-11(10)16-13(15)17-12/h2-3,8H,4-7H2,1H3,(H2,15,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human H4 receptor expressed in HEK cell membranes |
Bioorg Med Chem Lett 22: 461-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.104
BindingDB Entry DOI: 10.7270/Q2D79BT2 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50361030
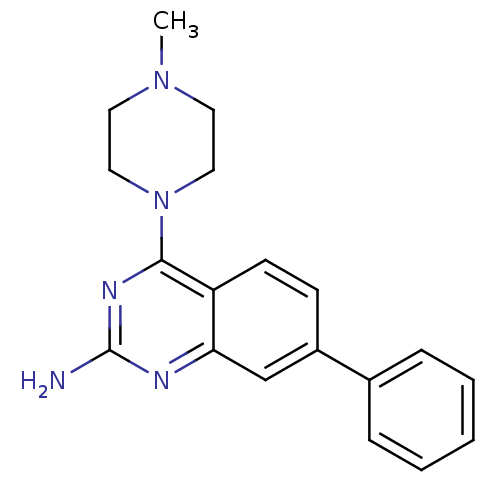
(CHEMBL1935566)Show InChI InChI=1S/C19H21N5/c1-23-9-11-24(12-10-23)18-16-8-7-15(14-5-3-2-4-6-14)13-17(16)21-19(20)22-18/h2-8,13H,9-12H2,1H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human H4 receptor expressed in HEK cell membranes |
Bioorg Med Chem Lett 22: 461-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.104
BindingDB Entry DOI: 10.7270/Q2D79BT2 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Mus musculus (mouse)) | BDBM50433358
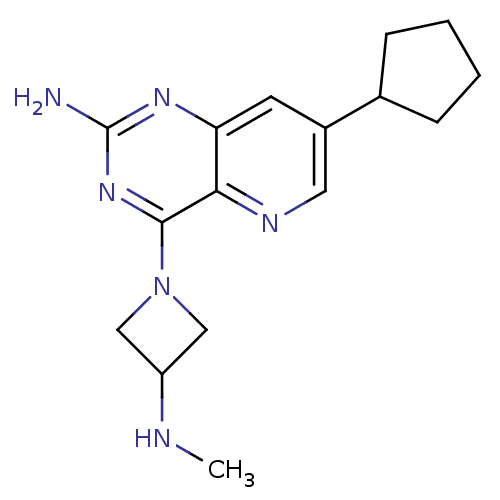
(CHEMBL2376803)Show InChI InChI=1S/C16H22N6/c1-18-12-8-22(9-12)15-14-13(20-16(17)21-15)6-11(7-19-14)10-4-2-3-5-10/h6-7,10,12,18H,2-5,8-9H2,1H3,(H2,17,20,21) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV
Curated by ChEMBL
| Assay Description
Binding affinity to mouse histamine H4 receptor |
Bioorg Med Chem Lett 23: 2663-70 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.091
BindingDB Entry DOI: 10.7270/Q2K35W11 |
More data for this
Ligand-Target Pair | |
Phosphodiesterase
(Trypanosoma brucei) | BDBM50527524

(CHEMBL4566742)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CCC1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C27H32N4O6/c1-36-21-8-7-17(15-22(21)37-2)26-19-5-3-4-6-20(19)27(35)31(28-26)18-11-13-29(14-12-18)25(34)16-30-23(32)9-10-24(30)33/h3-4,7-8,15,18-20H,5-6,9-14,16H2,1-2H3/t19-,20+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei PDEB1 (565 to 918 residues) expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP ph... |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H4 receptor
(Mus musculus (mouse)) | BDBM50361012

(CHEMBL1935574)Show InChI InChI=1S/C16H17N5O/c17-16-19-13-10-11(14-2-1-9-22-14)3-4-12(13)15(20-16)21-7-5-18-6-8-21/h1-4,9-10,18H,5-8H2,(H2,17,19,20) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from mouse H4R |
Bioorg Med Chem Lett 22: 461-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.104
BindingDB Entry DOI: 10.7270/Q2D79BT2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data