 Found 47 hits for monomerid = 50107004,50157741,50284944,50436752,50436754
Found 47 hits for monomerid = 50107004,50157741,50284944,50436752,50436754 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin B
(Homo sapiens (Human)) | BDBM50284944
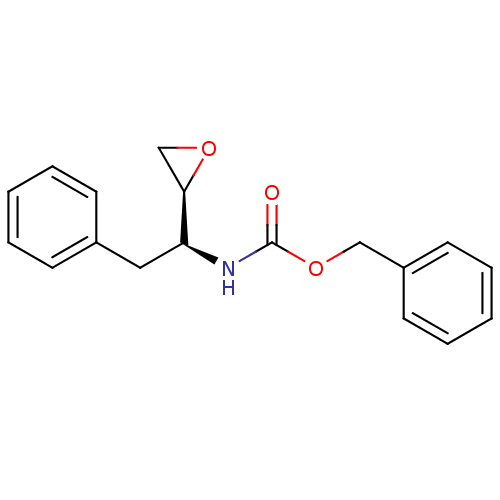
(((S)-1-(S)-Oxiranyl-2-phenyl-ethyl)-carbamic acid ...)Show InChI InChI=1S/C18H19NO3/c20-18(22-12-15-9-5-2-6-10-15)19-16(17-13-21-17)11-14-7-3-1-4-8-14/h1-10,16-17H,11-13H2,(H,19,20)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Kinetic parameter (Ki 1/min) was evaluated for the inactivation of cathepsin B |
Bioorg Med Chem Lett 5: 1767-1772 (1995)
Article DOI: 10.1016/0960-894X(95)00312-H
BindingDB Entry DOI: 10.7270/Q2MW2H4X |
More data for this
Ligand-Target Pair | |
Papain
(Carica papaya) | BDBM50284944

(((S)-1-(S)-Oxiranyl-2-phenyl-ethyl)-carbamic acid ...)Show InChI InChI=1S/C18H19NO3/c20-18(22-12-15-9-5-2-6-10-15)19-16(17-13-21-17)11-14-7-3-1-4-8-14/h1-10,16-17H,11-13H2,(H,19,20)/t16-,17+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| 0.208 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Kinetic parameter (Ki 1/min) was evaluated for the inactivation of papain |
Bioorg Med Chem Lett 5: 1767-1772 (1995)
Article DOI: 10.1016/0960-894X(95)00312-H
BindingDB Entry DOI: 10.7270/Q2MW2H4X |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L using Cbz-Phe-Arg-AMC as substrate after 10 mins by fluorescence assay |
J Med Chem 60: 6911-6923 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00405
BindingDB Entry DOI: 10.7270/Q2FJ2K73 |
More data for this
Ligand-Target Pair | |
Cysteine protease
(Trypanosoma brucei rhodesiense) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... |
J Med Chem 60: 6911-6923 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00405
BindingDB Entry DOI: 10.7270/Q2FJ2K73 |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant falcipain-2 |
J Med Chem 51: 988-96 (2008)
Article DOI: 10.1021/jm701141u
BindingDB Entry DOI: 10.7270/Q2SX6F2M |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Messina
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant falcipain-2 after 10 min |
Bioorg Med Chem 18: 4928-38 (2010)
Article DOI: 10.1016/j.bmc.2010.06.010
BindingDB Entry DOI: 10.7270/Q2NK3G1R |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Sus scrofa (pig)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of pig full length Cal1 after using SucLLVYAMC as substrate by FRET assay |
J Med Chem 56: 6054-68 (2013)
Article DOI: 10.1021/jm4006719
BindingDB Entry DOI: 10.7270/Q2VT1TG7 |
More data for this
Ligand-Target Pair | |
Papain
(Carica papaya) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of papain after using SucLLVYAMC as substrate by FRET assay |
J Med Chem 56: 6054-68 (2013)
Article DOI: 10.1021/jm4006719
BindingDB Entry DOI: 10.7270/Q2VT1TG7 |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum Falcipain-2 after 10 to 15 mins |
Eur J Med Chem 45: 3228-33 (2010)
Article DOI: 10.1016/j.ejmech.2010.04.003
BindingDB Entry DOI: 10.7270/Q2SN094T |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50284944

(((S)-1-(S)-Oxiranyl-2-phenyl-ethyl)-carbamic acid ...)Show InChI InChI=1S/C18H19NO3/c20-18(22-12-15-9-5-2-6-10-15)19-16(17-13-21-17)11-14-7-3-1-4-8-14/h1-10,16-17H,11-13H2,(H,19,20)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| 3.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Kinetic parameter of the compound (Ki mM) was evaluated for the inactivation of cathepsin B |
Bioorg Med Chem Lett 5: 1767-1772 (1995)
Article DOI: 10.1016/0960-894X(95)00312-H
BindingDB Entry DOI: 10.7270/Q2MW2H4X |
More data for this
Ligand-Target Pair | |
Papain
(Carica papaya) | BDBM50284944

(((S)-1-(S)-Oxiranyl-2-phenyl-ethyl)-carbamic acid ...)Show InChI InChI=1S/C18H19NO3/c20-18(22-12-15-9-5-2-6-10-15)19-16(17-13-21-17)11-14-7-3-1-4-8-14/h1-10,16-17H,11-13H2,(H,19,20)/t16-,17+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| 5.74E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Kinetic parameter of the compound (Ki mM) was evaluated for the inactivation of papain |
Bioorg Med Chem Lett 5: 1767-1772 (1995)
Article DOI: 10.1016/0960-894X(95)00312-H
BindingDB Entry DOI: 10.7270/Q2MW2H4X |
More data for this
Ligand-Target Pair | |
Falcipain-2
(Plasmodium falciparum) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum falcipain 2 incubated with compound for 10 mins before addition of 25 uM Z-Leu-Arg-AMC substrate by fluorescence ... |
Eur J Med Chem 46: 927-33 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.008
BindingDB Entry DOI: 10.7270/Q2ZC8345 |
More data for this
Ligand-Target Pair | |
Cysteine proteinase falcipain 3
(Plasmodium falciparum) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 482 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum falcipain 3 incubated with compound for 10 mins before addition of 25 uM Z-Leu-Arg-AMC substrate by fluorescence ... |
Eur J Med Chem 46: 927-33 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.008
BindingDB Entry DOI: 10.7270/Q2ZC8345 |
More data for this
Ligand-Target Pair | |
Papain
(Carica papaya) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of papaya papain using Z-Leu-Leu-Arg-AMC as substrate after 30 mins by spectrofluorometry |
Bioorg Med Chem 19: 7635-42 (2011)
Article DOI: 10.1016/j.bmc.2011.10.018
BindingDB Entry DOI: 10.7270/Q290247Z |
More data for this
Ligand-Target Pair | |
Papain
(Carica papaya) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon. Av. Prof. Gama Pinto
Curated by ChEMBL
| Assay Description
Inhibition of papaya papain using Z-Leu-Leu-Arg-AMC as substrate after 30 mins by fluorescence microplate reader analysis |
Eur J Med Chem 69: 365-72 (2013)
Article DOI: 10.1016/j.ejmech.2013.08.037
BindingDB Entry DOI: 10.7270/Q2CN75BS |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50436752
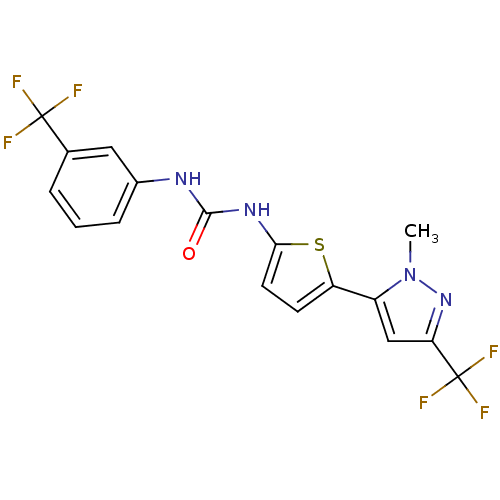
(CHEMBL2402102)Show SMILES Cn1nc(cc1-c1ccc(NC(=O)Nc2cccc(c2)C(F)(F)F)s1)C(F)(F)F Show InChI InChI=1S/C17H12F6N4OS/c1-27-11(8-13(26-27)17(21,22)23)12-5-6-14(29-12)25-15(28)24-10-4-2-3-9(7-10)16(18,19)20/h2-8H,1H3,(H2,24,25,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi cruzain |
J Med Chem 56: 5637-58 (2014)
Article DOI: 10.1021/jm301424d
BindingDB Entry DOI: 10.7270/Q24T6KR9 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50436754
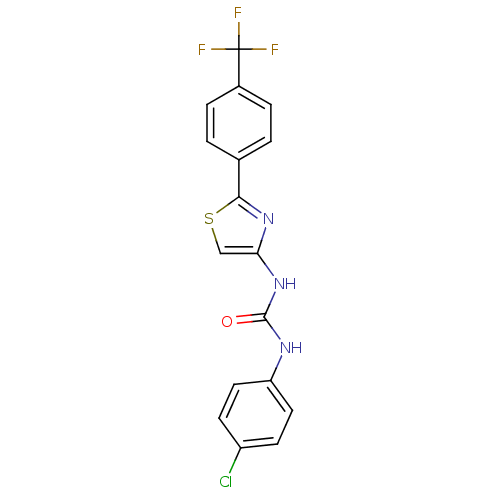
(CHEMBL2402103)Show SMILES FC(F)(F)c1ccc(cc1)-c1nc(NC(=O)Nc2ccc(Cl)cc2)cs1 Show InChI InChI=1S/C17H11ClF3N3OS/c18-12-5-7-13(8-6-12)22-16(25)24-14-9-26-15(23-14)10-1-3-11(4-2-10)17(19,20)21/h1-9H,(H2,22,24,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi cruzain |
J Med Chem 56: 5637-58 (2014)
Article DOI: 10.1021/jm301424d
BindingDB Entry DOI: 10.7270/Q24T6KR9 |
More data for this
Ligand-Target Pair | |
Papain
(Carica papaya) | BDBM50436754

(CHEMBL2402103)Show SMILES FC(F)(F)c1ccc(cc1)-c1nc(NC(=O)Nc2ccc(Cl)cc2)cs1 Show InChI InChI=1S/C17H11ClF3N3OS/c18-12-5-7-13(8-6-12)22-16(25)24-14-9-26-15(23-14)10-1-3-11(4-2-10)17(19,20)21/h1-9H,(H2,22,24,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Inhibition of papaya papain |
J Med Chem 56: 5637-58 (2014)
Article DOI: 10.1021/jm301424d
BindingDB Entry DOI: 10.7270/Q24T6KR9 |
More data for this
Ligand-Target Pair | |
Papain
(Carica papaya) | BDBM50436752

(CHEMBL2402102)Show SMILES Cn1nc(cc1-c1ccc(NC(=O)Nc2cccc(c2)C(F)(F)F)s1)C(F)(F)F Show InChI InChI=1S/C17H12F6N4OS/c1-27-11(8-13(26-27)17(21,22)23)12-5-6-14(29-12)25-15(28)24-10-4-2-3-9(7-10)16(18,19)20/h2-8H,1H3,(H2,24,25,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Inhibition of papaya papain |
J Med Chem 56: 5637-58 (2014)
Article DOI: 10.1021/jm301424d
BindingDB Entry DOI: 10.7270/Q24T6KR9 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00316j
BindingDB Entry DOI: 10.7270/Q23200VF |
More data for this
Ligand-Target Pair | |
Cysteine protease falcipain-3 [5-492]
(Plasmodium falciparum) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of falcipain3 |
J Med Chem 49: 1576-84 (2006)
Article DOI: 10.1021/jm0505765
BindingDB Entry DOI: 10.7270/Q2B27W35 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase using [14C]GS7340 substrate |
Antimicrob Agents Chemother 51: 543-50 (2007)
Article DOI: 10.1128/AAC.00968-06
BindingDB Entry DOI: 10.7270/Q2ZG6RW5 |
More data for this
Ligand-Target Pair | |
Lysosomal protective protein
(Homo sapiens (Human)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CatA using [14C]GS7340 substrate |
Antimicrob Agents Chemother 51: 543-50 (2007)
Article DOI: 10.1128/AAC.00968-06
BindingDB Entry DOI: 10.7270/Q2ZG6RW5 |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase
(Sus scrofa) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of porcine liver carboxylesterase using [14C]GS7340 substrate |
Antimicrob Agents Chemother 51: 543-50 (2007)
Article DOI: 10.1128/AAC.00968-06
BindingDB Entry DOI: 10.7270/Q2ZG6RW5 |
More data for this
Ligand-Target Pair | |
Cysteine protease falcipain-3 [5-492]
(Plasmodium falciparum) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant falcipain-3 |
J Med Chem 47: 6609-15 (2004)
Article DOI: 10.1021/jm0493717
BindingDB Entry DOI: 10.7270/Q2GT5P0D |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant falcipain-2 |
J Med Chem 47: 6609-15 (2004)
Article DOI: 10.1021/jm0493717
BindingDB Entry DOI: 10.7270/Q2GT5P0D |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin C after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Papain
(Carica papaya) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas
Curated by ChEMBL
| Assay Description
Inhibition of Carica papaya papain by spectrofluorimetry |
Eur J Med Chem 44: 1230-9 (2009)
Article DOI: 10.1016/j.ejmech.2008.09.018
BindingDB Entry DOI: 10.7270/Q26974HM |
More data for this
Ligand-Target Pair | |
Falcipain-2
(Plasmodium falciparum) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum falcipain-2 |
Bioorg Med Chem Lett 20: 942-4 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.062
BindingDB Entry DOI: 10.7270/Q26M383G |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 13.7 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cathepsin B was determined |
Bioorg Med Chem Lett 9: 3397-402 (1999)
BindingDB Entry DOI: 10.7270/Q2G44SGN |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Plasmodium falciparum falcipain-2 expressed in Escherichia coli M15(pREP4) using Cbz-Phe-Arg-AMC as substrate by fluorescen... |
J Med Chem 60: 6911-6923 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00405
BindingDB Entry DOI: 10.7270/Q2FJ2K73 |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Plasmodium falciparum recombinant falcipain 2 expressed in Escherichia coli using Z-Leu-Arg-AMC as substrate incubated for 30 min prior... |
Citation and Details
Article DOI: 10.1007/s00044-011-9854-3
BindingDB Entry DOI: 10.7270/Q2MC92XB |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant Trypanosoma cruzi cruzain using Z-FR-AMC as substrate preincubated for 10 mins followed by substrate addition measured for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116213
BindingDB Entry DOI: 10.7270/Q2183B90 |
More data for this
Ligand-Target Pair | |
Cysteine protease
(Trypanosoma brucei rhodesiense) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant Trypanosoma brucei rhodesain using Z-FR-AMC as substrate preincubated for 10 mins followed by substrate addition measured f... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116213
BindingDB Entry DOI: 10.7270/Q2183B90 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00316j
BindingDB Entry DOI: 10.7270/Q23200VF |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alagoas
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi cruzain using Z-Phe-Arg-aminomethylcoumarin as substrate measured after 5 mins by spectrofluorimetry |
Bioorg Med Chem 24: 4228-4240 (2016)
Article DOI: 10.1016/j.bmc.2016.07.013
BindingDB Entry DOI: 10.7270/Q2CZ393W |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L (unknown origin) using fluorometric Z-Phe-Arg-AMC as substrate by spectrofluorimetry |
J Nat Prod 77: 2418-22 (2014)
Article DOI: 10.1021/np500453x
BindingDB Entry DOI: 10.7270/Q2TT4SJJ |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B (unknown origin) using fluorometric Z-Phe-Arg-AMC as substrate by spectrofluorimetry |
J Nat Prod 77: 2418-22 (2014)
Article DOI: 10.1021/np500453x
BindingDB Entry DOI: 10.7270/Q2TT4SJJ |
More data for this
Ligand-Target Pair | |
Papain
(Carica papaya) | BDBM50107004
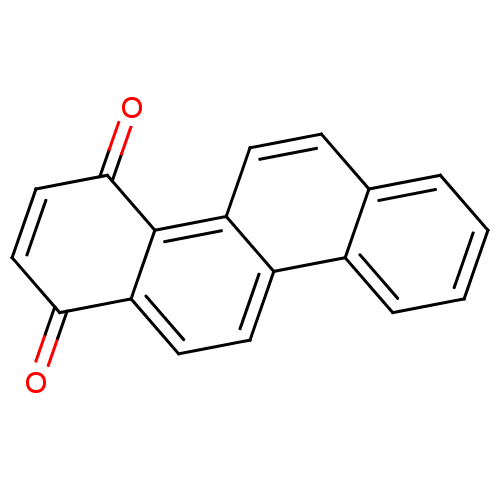
(CHEMBL421215 | Chrysene-1,4-dione)Show InChI InChI=1S/C18H10O2/c19-16-9-10-17(20)18-14-6-5-11-3-1-2-4-12(11)13(14)7-8-15(16)18/h1-10H | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against papain using HPLC assay |
Bioorg Med Chem Lett 11: 3143-6 (2001)
BindingDB Entry DOI: 10.7270/Q2T43SDG |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Pro-cathepsin H
(Homo sapiens (Human)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin H after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin B |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50107004

(CHEMBL421215 | Chrysene-1,4-dione)Show InChI InChI=1S/C18H10O2/c19-16-9-10-17(20)18-14-6-5-11-3-1-2-4-12(11)13(14)7-8-15(16)18/h1-10H | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HRV 3Cpro using HPLC assay |
Bioorg Med Chem Lett 11: 3143-6 (2001)
BindingDB Entry DOI: 10.7270/Q2T43SDG |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of falcipain2 |
J Med Chem 49: 1576-84 (2006)
Article DOI: 10.1021/jm0505765
BindingDB Entry DOI: 10.7270/Q2B27W35 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
 Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase