

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Wt: 306.3 BDBM50355501  Purchase Purchase | Wt: 440.4 BDBM50357312  Purchase Purchase | Wt: 476.8 BDBM31093  Purchase Purchase | Wt: 455.5 BDBM50429867 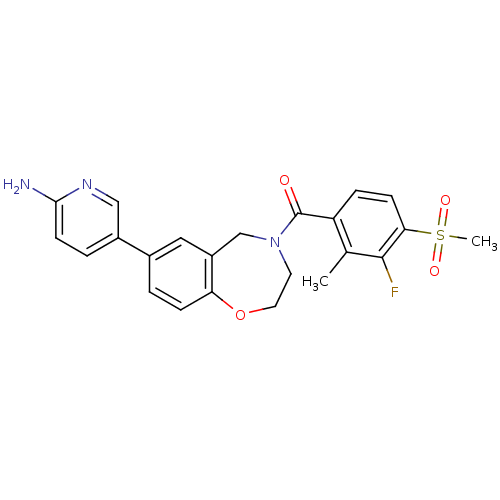 Purchase Purchase | Wt: 470.4 BDBM60665 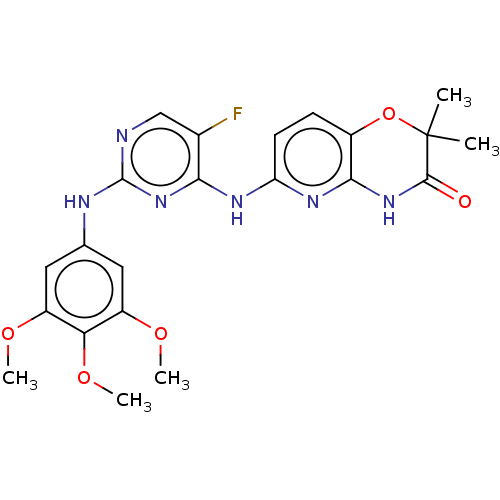 Purchase Purchase |
| Wt: 539.6 BDBM50026612  Purchase Purchase | Wt: 359.4 BDBM60589  Purchase Purchase | Wt: 466.5 BDBM31096  Purchase Purchase | Wt: 475.3 BDBM21 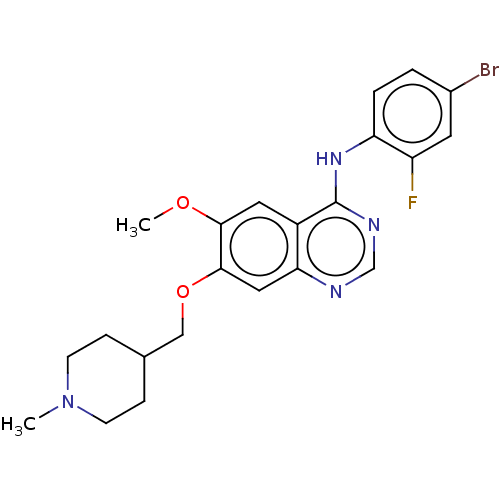 Purchase Purchase |
| << First | Previous | Displayed 76 to 84 (of 84 total ) |
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50355501 (INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of purified JAK1 incubated for 30 mins | J Med Chem 55: 10090-107 (2012) Article DOI: 10.1021/jm3012239 BindingDB Entry DOI: 10.7270/Q2Q241D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50355501 (INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human JAK2 (828-1132) expressed in baculovirus-infected Sf9 cells using EQEDEPEGDYFEWLE as substrate after 1 hr by HTRF assay | J Med Chem 56: 4521-36 (2013) Article DOI: 10.1021/jm400266t BindingDB Entry DOI: 10.7270/Q2VX0HX0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50355501 (INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of JAK2 kinase domain using N-terminal 5-carboxyfluorescein-tagged Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 m... | J Med Chem 55: 6176-93 (2012) Article DOI: 10.1021/jm300628c BindingDB Entry DOI: 10.7270/Q25Q4X6S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50355501 (INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human JAK1 (837-1142) expressed in baculovirus-infected Sf9 cells using EQEDEPEGDYFEWLE as substrate after 1 hr by HTRF assay | J Med Chem 56: 4521-36 (2013) Article DOI: 10.1021/jm400266t BindingDB Entry DOI: 10.7270/Q2VX0HX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50355501 (INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of JAK1 kinase domain using N-terminal 5-carboxyfluorescein-tagged Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 m... | J Med Chem 55: 6176-93 (2012) Article DOI: 10.1021/jm300628c BindingDB Entry DOI: 10.7270/Q25Q4X6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM21 (CHEMBL24828 | N-(4-bromo-2-fluorophenyl)-6-methoxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | Article | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 7: 1365-1370 (1997) Article DOI: 10.1016/S0960-894X(97)00165-0 BindingDB Entry DOI: 10.7270/Q21G0M7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50355501 (INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of purified JAK2 incubated for 30 mins | J Med Chem 55: 10090-107 (2012) Article DOI: 10.1021/jm3012239 BindingDB Entry DOI: 10.7270/Q2Q241D4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50355501 (INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of TYK2 kinase domain using N-terminal 5-carboxyfluorescein-tagged Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 m... | J Med Chem 55: 6176-93 (2012) Article DOI: 10.1021/jm300628c BindingDB Entry DOI: 10.7270/Q25Q4X6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50355501 (INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of purified TYK2 incubated for 30 mins | J Med Chem 55: 10090-107 (2012) Article DOI: 10.1021/jm3012239 BindingDB Entry DOI: 10.7270/Q2Q241D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50355501 (INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of JAK3 kinase domain using N-terminal 5-carboxyfluorescein-tagged Leu-Pro-Leu-Asp-Lys-Asp-Tyr-Tyr-Val-Val-Arg as substrate after 30 mins | J Med Chem 55: 6176-93 (2012) Article DOI: 10.1021/jm300628c BindingDB Entry DOI: 10.7270/Q25Q4X6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50355501 (INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human JAK3 (781-1124) expressed in baculovirus-infected Sf9 cells using EQEDEPEGDYFEWLE as substrate after 1 hr by HTRF assay | J Med Chem 56: 4521-36 (2013) Article DOI: 10.1021/jm400266t BindingDB Entry DOI: 10.7270/Q2VX0HX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50355501 (INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of purified JAK3 incubated for 30 mins | J Med Chem 55: 10090-107 (2012) Article DOI: 10.1021/jm3012239 BindingDB Entry DOI: 10.7270/Q2Q241D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50026612 (BIBF-1120 | Nintedanib | US10981896, Compound Nint...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activities of compounds of the invention against VEGFR2 (VEGFR2 Kinase Enzyme System: Promega), were evaluated by mixing the VEGFR2 pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084K3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50026612 (BIBF-1120 | Nintedanib | US10981896, Compound Nint...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay was performed in buffer containing 40 mM Tris pH 7.5, 20 mM MgCl2, 0.1 mg/mL BSA and 1 mM DTT. The inhibitory activities of compounds of the in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XP784N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology of China Curated by ChEMBL | Assay Description Reversible inhibition of full length recombinant human N-terminal His tagged BKT expressed in baculovirus infected Sf9 insect cells using poly (Glu,T... | Eur J Med Chem 131: 107-125 (2017) Article DOI: 10.1016/j.ejmech.2017.03.001 BindingDB Entry DOI: 10.7270/Q21V5HCK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50026612 (BIBF-1120 | Nintedanib | US10981896, Compound Nint...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM50026612 (BIBF-1120 | Nintedanib | US10981896, Compound Nint...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activities of compounds of the invention against FGFR3 (FGFR3 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR3 prote... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM50026612 (BIBF-1120 | Nintedanib | US10981896, Compound Nint...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay was performed in buffer containing 40 mM Tris pH 7.5, 20 mM MgCl2, 0.1 mg/mL BSA, 1 mM DTT and 2 mM MnCl2. The inhibitory activities of compoun... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XP784N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Competitive inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells in presence of ATP | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50026612 (BIBF-1120 | Nintedanib | US10981896, Compound Nint...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activities of compounds of the invention against PDGFRβ (PDGFRβ Kinase Enzyme System: Promega), were evaluated by mixing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50026612 (BIBF-1120 | Nintedanib | US10981896, Compound Nint...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay was performed in buffer containing 40 mM Tris pH 7.5, 20 mM MgCl2, 0.1 mg/mL BSA and 1 mM DTT. The inhibitory activities of compounds of the in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XP784N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50026612 (BIBF-1120 | Nintedanib | US10981896, Compound Nint...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activities of compounds of the invention against PDGFRα (PDGFRα Kinase Enzyme System: Promega), were evaluated by mixing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50026612 (BIBF-1120 | Nintedanib | US10981896, Compound Nint...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay was performed in buffer containing 40 mM Tris pH 7.5, 20 mM MgCl2, 0.1 mg/mL BSA and 1 mM DTT. The inhibitory activities of compounds of the in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XP784N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM60665 (BDBM50249542 | US9145414, R406 | US9212178, R406) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rigel, Inc. Curated by ChEMBL | Assay Description Inhibition of SYK | J Med Chem 55: 3614-43 (2012) Article DOI: 10.1021/jm201271b BindingDB Entry DOI: 10.7270/Q2NZ88RC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50026612 (BIBF-1120 | Nintedanib | US10981896, Compound Nint...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay was performed in buffer containing 40 mM Tris pH 7.5, 20 mM MgCl2, 0.1 mg/mL BSA and 1 mM DTT. The inhibitory activities of compounds of the in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XP784N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50026612 (BIBF-1120 | Nintedanib | US10981896, Compound Nint...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activities of compounds of the invention against FGFR1 (FGFR1 Kinase Enzyme System: Promega), were evaluated by mixing the FGFR1 prote... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM50026612 (BIBF-1120 | Nintedanib | US10981896, Compound Nint...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay was performed in buffer containing 40 mM Tris pH 7.5, 20 mM MgCl2, 0.1 mg/mL BSA, 1 mM DTT and 2 mM MnCl2. The inhibitory activities of compoun... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XP784N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM50026612 (BIBF-1120 | Nintedanib | US10981896, Compound Nint...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activities of compounds of the invention against VEGFR1 (VEGFR1 Kinase Enzyme System: Promega), were evaluated by mixing the VEGFR1 pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 425 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of His-tagged BTK (unknown origin) after 1.5 hrs by HTRF analysis | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM31096 (CHEMBL290084 | Staurosporine | cid_451705) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | 640 | -8.44 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2NC5ZHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc. Curated by ChEMBL | Assay Description Binding affinity to human ERG | Bioorg Med Chem Lett 28: 2939-2944 (2018) Article DOI: 10.1016/j.bmcl.2018.07.008 BindingDB Entry DOI: 10.7270/Q2QR50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-2 (Homo sapiens (Human)) | BDBM31096 (CHEMBL290084 | Staurosporine | cid_451705) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q25D8Q75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM31096 (CHEMBL290084 | Staurosporine | cid_451705) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | 9.50E+3 | -6.85 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2NC5ZHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM31096 (CHEMBL290084 | Staurosporine | cid_451705) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2GB22FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM31096 (CHEMBL290084 | Staurosporine | cid_451705) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2M32T55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM31096 (CHEMBL290084 | Staurosporine | cid_451705) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2NC5ZHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| eIF-2-alpha kinase GCN2 (Homo sapiens (Human)) | BDBM31096 (CHEMBL290084 | Staurosporine | cid_451705) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q28W3BP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-6 (Homo sapiens (Human)) | BDBM31096 (CHEMBL290084 | Staurosporine | cid_451705) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2WW7G1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM31096 (CHEMBL290084 | Staurosporine | cid_451705) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | 1.90E+4 | -6.44 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2NC5ZHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM31096 (CHEMBL290084 | Staurosporine | cid_451705) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | 3.80E+4 | -6.03 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2NC5ZHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-1 (Homo sapiens (Human)) | BDBM31096 (CHEMBL290084 | Staurosporine | cid_451705) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2959FZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50355501 (INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Binding affinity to recombinant human LXRbeta-LBD expressed in Escherichia coli BL21 (DE3) assessed as inhibitory constant incubated for 30 mins by f... | Eur J Med Chem 178: 458-467 (2019) Article DOI: 10.1016/j.ejmech.2019.06.011 BindingDB Entry DOI: 10.7270/Q2BC42WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM31096 (CHEMBL290084 | Staurosporine | cid_451705) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2TD9VQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM31096 (CHEMBL290084 | Staurosporine | cid_451705) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q21N7ZHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM21 (CHEMBL24828 | N-(4-bromo-2-fluorophenyl)-6-methoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PCBioAssay | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2NC5ZHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 3 of Ribosomal protein S6 kinase alpha-2 (3) (Homo sapiens (Human)) | BDBM31096 (CHEMBL290084 | Staurosporine | cid_451705) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2CC0Z29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-6 (Homo sapiens (Human)) | BDBM21 (CHEMBL24828 | N-(4-bromo-2-fluorophenyl)-6-methoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PCBioAssay | 2.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q27P8WRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM21 (CHEMBL24828 | N-(4-bromo-2-fluorophenyl)-6-methoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PCBioAssay | 2.60E+5 | -4.89 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2NC5ZHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM21 (CHEMBL24828 | N-(4-bromo-2-fluorophenyl)-6-methoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PCBioAssay | 2.90E+5 | -4.82 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2NC5ZHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-1 (Homo sapiens (Human)) | BDBM21 (CHEMBL24828 | N-(4-bromo-2-fluorophenyl)-6-methoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PCBioAssay | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2765CQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4675 total ) | Next | Last >> |