 Found 25 hits for monomerid = 50113118,50113126,50113130,50113131,50113138,50113144,50121347,50228108,50228105,50228106,50228100,50228089,50228090,50228091,50228092
Found 25 hits for monomerid = 50113118,50113126,50113130,50113131,50113138,50113144,50121347,50228108,50228105,50228106,50228100,50228089,50228090,50228091,50228092 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113130
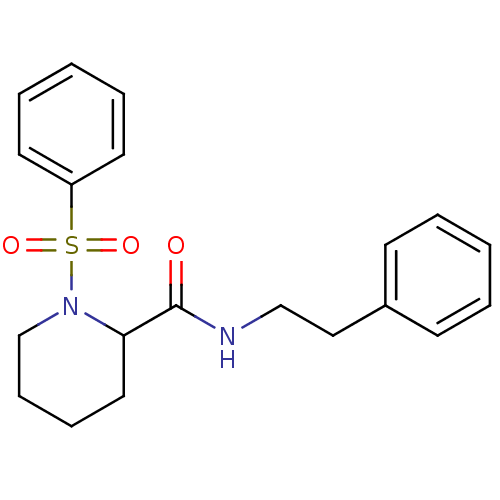
(1-Benzenesulfonyl-piperidine-2-carboxylic acid phe...)Show InChI InChI=1S/C20H24N2O3S/c23-20(21-15-14-17-9-3-1-4-10-17)19-13-7-8-16-22(19)26(24,25)18-11-5-2-6-12-18/h1-6,9-12,19H,7-8,13-16H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113126
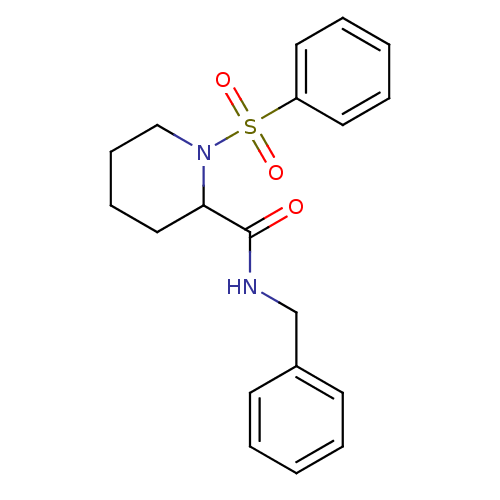
(1-Benzenesulfonyl-piperidine-2-carboxylic acid ben...)Show InChI InChI=1S/C19H22N2O3S/c22-19(20-15-16-9-3-1-4-10-16)18-13-7-8-14-21(18)25(23,24)17-11-5-2-6-12-17/h1-6,9-12,18H,7-8,13-15H2,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113144

(1-Benzenesulfonyl-piperidine-2-carboxylic acid but...)Show InChI InChI=1S/C16H24N2O3S/c1-2-3-12-17-16(19)15-11-7-8-13-18(15)22(20,21)14-9-5-4-6-10-14/h4-6,9-10,15H,2-3,7-8,11-13H2,1H3,(H,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113138

(1-(Toluene-4-sulfonyl)-pyrrolidine-2-carboxylic ac...)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCCC1C(=O)NCCCc1ccccc1 Show InChI InChI=1S/C21H26N2O3S/c1-17-11-13-19(14-12-17)27(25,26)23-16-6-10-20(23)21(24)22-15-5-9-18-7-3-2-4-8-18/h2-4,7-8,11-14,20H,5-6,9-10,15-16H2,1H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113131
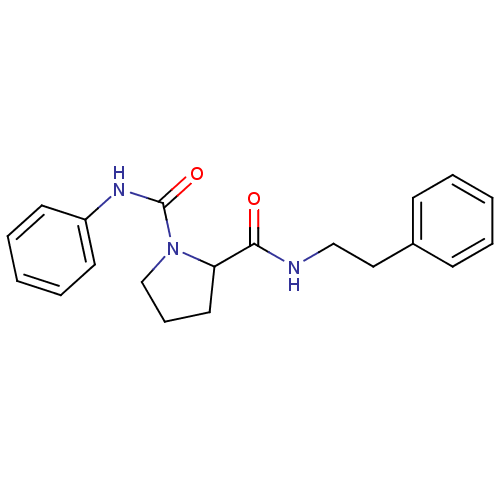
(CHEMBL33413 | Pyrrolidine-1,2-dicarboxylic acid 2-...)Show InChI InChI=1S/C20H23N3O2/c24-19(21-14-13-16-8-3-1-4-9-16)18-12-7-15-23(18)20(25)22-17-10-5-2-6-11-17/h1-6,8-11,18H,7,12-15H2,(H,21,24)(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113118

(1-Benzenesulfonyl-pyrrolidine-2-carboxylic acid ph...)Show InChI InChI=1S/C19H22N2O3S/c22-19(20-14-13-16-8-3-1-4-9-16)18-12-7-15-21(18)25(23,24)17-10-5-2-6-11-17/h1-6,8-11,18H,7,12-15H2,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50121347
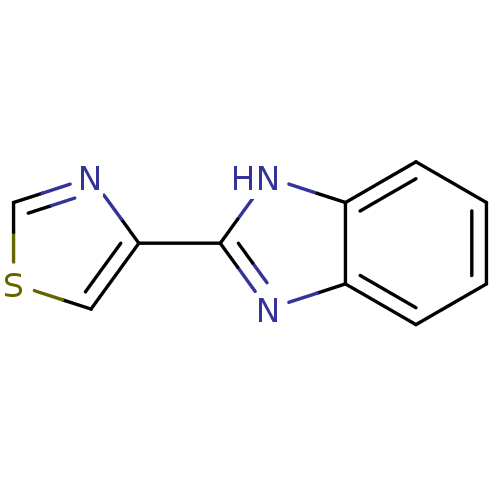
(CHEMBL625 | THIABENDAZOLE)Show InChI InChI=1S/C10H7N3S/c1-2-4-8-7(3-1)12-10(13-8)9-5-14-6-11-9/h1-6H,(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50228106

(4-(2-(4-isopropylphenylthio)acetyl)morpholine-3-ca...)Show SMILES CC(C)c1ccc(SCC(=O)N2CCOCC2C(O)=O)cc1 |w:16.17| Show InChI InChI=1S/C16H21NO4S/c1-11(2)12-3-5-13(6-4-12)22-10-15(18)17-7-8-21-9-14(17)16(19)20/h3-6,11,14H,7-10H2,1-2H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.26E+5 | n/a | n/a | n/a | n/a | n/a |
and University of California at San Diego
Curated by ChEMBL
| Assay Description
Binding affinity to human FKBP12 expressed in Escherichia coli BL21(DE3) by isothermal titration calorimetry |
J Med Chem 50: 6607-17 (2007)
Article DOI: 10.1021/jm0707424
BindingDB Entry DOI: 10.7270/Q2TT4QPH |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50228105

(2-(2-methyl-4-(pyrrolidin-1-ylsulfonyl)phenoxy)-1-...)Show InChI InChI=1S/C17H24N2O5S/c1-14-12-15(25(21,22)19-6-2-3-7-19)4-5-16(14)24-13-17(20)18-8-10-23-11-9-18/h4-5,12H,2-3,6-11,13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.76E+5 | n/a | n/a | n/a | n/a | n/a |
and University of California at San Diego
Curated by ChEMBL
| Assay Description
Binding affinity to human FKBP12 expressed in Escherichia coli BL21(DE3) by isothermal titration calorimetry |
J Med Chem 50: 6607-17 (2007)
Article DOI: 10.1021/jm0707424
BindingDB Entry DOI: 10.7270/Q2TT4QPH |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50228108
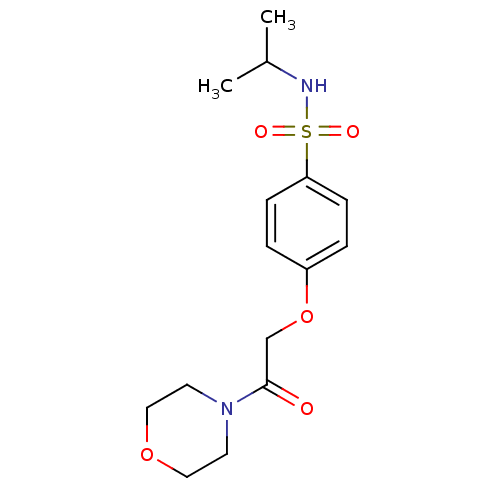
(CHEMBL235927 | N-isopropyl-4-(2-morpholino-2-oxoet...)Show InChI InChI=1S/C15H22N2O5S/c1-12(2)16-23(19,20)14-5-3-13(4-6-14)22-11-15(18)17-7-9-21-10-8-17/h3-6,12,16H,7-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
and University of California at San Diego
Curated by ChEMBL
| Assay Description
Binding affinity to human FKBP12 expressed in Escherichia coli BL21(DE3) by isothermal titration calorimetry |
J Med Chem 50: 6607-17 (2007)
Article DOI: 10.1021/jm0707424
BindingDB Entry DOI: 10.7270/Q2TT4QPH |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50121347

(CHEMBL625 | THIABENDAZOLE)Show InChI InChI=1S/C10H7N3S/c1-2-4-8-7(3-1)12-10(13-8)9-5-14-6-11-9/h1-6H,(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a |
and University of California at San Diego
Curated by ChEMBL
| Assay Description
Binding affinity to human FKBP12 expressed in Escherichia coli BL21(DE3) by isothermal titration calorimetry |
J Med Chem 50: 6607-17 (2007)
Article DOI: 10.1021/jm0707424
BindingDB Entry DOI: 10.7270/Q2TT4QPH |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50121347

(CHEMBL625 | THIABENDAZOLE)Show InChI InChI=1S/C10H7N3S/c1-2-4-8-7(3-1)12-10(13-8)9-5-14-6-11-9/h1-6H,(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 in presence of Co2+ |
J Med Chem 56: 3996-4016 (2013)
Article DOI: 10.1021/jm400227z
BindingDB Entry DOI: 10.7270/Q2SJ1N1Z |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50121347

(CHEMBL625 | THIABENDAZOLE)Show InChI InChI=1S/C10H7N3S/c1-2-4-8-7(3-1)12-10(13-8)9-5-14-6-11-9/h1-6H,(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 in presence of Zn2+ |
J Med Chem 56: 3996-4016 (2013)
Article DOI: 10.1021/jm400227z
BindingDB Entry DOI: 10.7270/Q2SJ1N1Z |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50121347

(CHEMBL625 | THIABENDAZOLE)Show InChI InChI=1S/C10H7N3S/c1-2-4-8-7(3-1)12-10(13-8)9-5-14-6-11-9/h1-6H,(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 in presence of Mn2+ |
J Med Chem 56: 3996-4016 (2013)
Article DOI: 10.1021/jm400227z
BindingDB Entry DOI: 10.7270/Q2SJ1N1Z |
More data for this
Ligand-Target Pair | |
Multidrug and toxin extrusion protein 1
(Homo sapiens (Human)) | BDBM50121347

(CHEMBL625 | THIABENDAZOLE)Show InChI InChI=1S/C10H7N3S/c1-2-4-8-7(3-1)12-10(13-8)9-5-14-6-11-9/h1-6H,(H,12,13) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human MATE1-mediated ASP+ uptake expressed in HEK293 cells after 1.5 mins by fluorescence assay |
J Med Chem 56: 781-95 (2013)
Article DOI: 10.1021/jm301302s
BindingDB Entry DOI: 10.7270/Q2F76DWZ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50121347

(CHEMBL625 | THIABENDAZOLE)Show InChI InChI=1S/C10H7N3S/c1-2-4-8-7(3-1)12-10(13-8)9-5-14-6-11-9/h1-6H,(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Heidelberg
Curated by ChEMBL
| Assay Description
Inhibition of human MetAP1 |
Bioorg Med Chem Lett 20: 4038-44 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.093
BindingDB Entry DOI: 10.7270/Q2RF5W0K |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50121347

(CHEMBL625 | THIABENDAZOLE)Show InChI InChI=1S/C10H7N3S/c1-2-4-8-7(3-1)12-10(13-8)9-5-14-6-11-9/h1-6H,(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Heidelberg
Curated by ChEMBL
| Assay Description
Inhibition of human MetAP2 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 20: 4038-44 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.093
BindingDB Entry DOI: 10.7270/Q2RF5W0K |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Homo sapiens (Human)) | BDBM50121347

(CHEMBL625 | THIABENDAZOLE)Show InChI InChI=1S/C10H7N3S/c1-2-4-8-7(3-1)12-10(13-8)9-5-14-6-11-9/h1-6H,(H,12,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human BSEP expressed in baculovirus transfected fall armyworm Sf21 cell membranes vesicles assessed as reduction in ATP-dependent [3H]-... |
Drug Metab Dispos 40: 2332-41 (2012)
Article DOI: 10.1124/dmd.112.047068
BindingDB Entry DOI: 10.7270/Q2ZP488M |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50228091

(2-(4'-methyl-biphenyl-4-yloxy)-1-morpholin-4-yl-et...)Show InChI InChI=1S/C19H21NO3/c1-15-2-4-16(5-3-15)17-6-8-18(9-7-17)23-14-19(21)20-10-12-22-13-11-20/h2-9H,10-14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 7.55E+5 | n/a | n/a | n/a | n/a | n/a |
and University of California at San Diego
Curated by ChEMBL
| Assay Description
Binding affinity to human FKBP12 expressed in Escherichia coli BL21(DE3) by isothermal titration calorimetry |
J Med Chem 50: 6607-17 (2007)
Article DOI: 10.1021/jm0707424
BindingDB Entry DOI: 10.7270/Q2TT4QPH |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50228092

(1-(3-(trifluoromethyl)benzyl)-2-(thiazol-4-yl)-1H-...)Show InChI InChI=1S/C18H12F3N3S/c19-18(20,21)13-5-3-4-12(8-13)9-24-16-7-2-1-6-14(16)23-17(24)15-10-25-11-22-15/h1-8,10-11H,9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >3.00E+6 | n/a | n/a | n/a | n/a | n/a |
and University of California at San Diego
Curated by ChEMBL
| Assay Description
Binding affinity to human FKBP12 expressed in Escherichia coli BL21(DE3) by isothermal titration calorimetry |
J Med Chem 50: 6607-17 (2007)
Article DOI: 10.1021/jm0707424
BindingDB Entry DOI: 10.7270/Q2TT4QPH |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50228090

(1-morpholino-2-(p-tolylthio)ethanone | CHEMBL23638...)Show InChI InChI=1S/C13H17NO2S/c1-11-2-4-12(5-3-11)17-10-13(15)14-6-8-16-9-7-14/h2-5H,6-10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a |
and University of California at San Diego
Curated by ChEMBL
| Assay Description
Binding affinity to human FKBP12 expressed in Escherichia coli BL21(DE3) by isothermal titration calorimetry |
J Med Chem 50: 6607-17 (2007)
Article DOI: 10.1021/jm0707424
BindingDB Entry DOI: 10.7270/Q2TT4QPH |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50228089
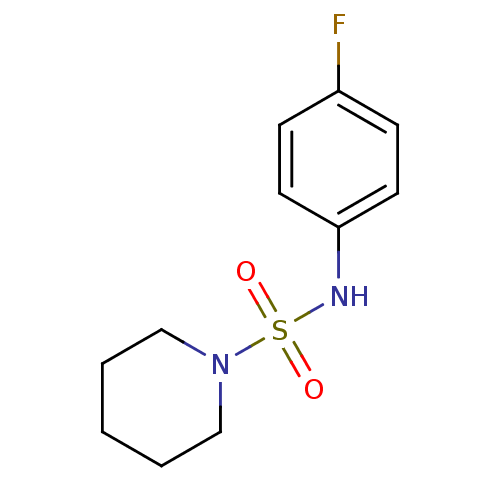
(CHEMBL393271 | N-(4-fluorophenyl)piperidine-1-sulf...)Show InChI InChI=1S/C11H15FN2O2S/c12-10-4-6-11(7-5-10)13-17(15,16)14-8-2-1-3-9-14/h4-7,13H,1-3,8-9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 3.19E+5 | n/a | n/a | n/a | n/a | n/a |
and University of California at San Diego
Curated by ChEMBL
| Assay Description
Binding affinity to human FKBP12 expressed in Escherichia coli BL21(DE3) by isothermal titration calorimetry |
J Med Chem 50: 6607-17 (2007)
Article DOI: 10.1021/jm0707424
BindingDB Entry DOI: 10.7270/Q2TT4QPH |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase
(Escherichia coli (strain K12)) | BDBM50121347

(CHEMBL625 | THIABENDAZOLE)Show InChI InChI=1S/C10H7N3S/c1-2-4-8-7(3-1)12-10(13-8)9-5-14-6-11-9/h1-6H,(H,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 472 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of Co2+ loaded MetAP expressed in Escherichia coli |
J Med Chem 49: 511-22 (2006)
Article DOI: 10.1021/jm050476z
BindingDB Entry DOI: 10.7270/Q2N58KXD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50121347

(CHEMBL625 | THIABENDAZOLE)Show InChI InChI=1S/C10H7N3S/c1-2-4-8-7(3-1)12-10(13-8)9-5-14-6-11-9/h1-6H,(H,12,13) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Messina
Curated by ChEMBL
| Assay Description
HIV-1 reverse transcriptase (RT) activity using Poly (rC)/oligo (dG) as template/primer and [3H]dGTP |
J Med Chem 45: 5410-3 (2002)
BindingDB Entry DOI: 10.7270/Q2TT4Q9K |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50228100

(1-(2,6-dimethylmorpholino)-2-(phenylthio)ethanone ...)Show InChI InChI=1S/C14H19NO2S/c1-11-8-15(9-12(2)17-11)14(16)10-18-13-6-4-3-5-7-13/h3-7,11-12H,8-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 7.26E+5 | n/a | n/a | n/a | n/a | n/a |
and University of California at San Diego
Curated by ChEMBL
| Assay Description
Binding affinity to human FKBP12 expressed in Escherichia coli BL21(DE3) by isothermal titration calorimetry |
J Med Chem 50: 6607-17 (2007)
Article DOI: 10.1021/jm0707424
BindingDB Entry DOI: 10.7270/Q2TT4QPH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
 Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase