 Found 54 hits for monomerid = 162723,162738,50036880,50036932,50026450,50042847,50027586,50027649
Found 54 hits for monomerid = 162723,162738,50036880,50036932,50026450,50042847,50027586,50027649 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM162723

(US9056843, 14)Show InChI InChI=1S/C13H12F3N3O2/c1-3-19(2)11(20)9-6-4-8(5-7-9)10-17-12(21-18-10)13(14,15)16/h4-7H,3H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
A similar assay procedure as described in Test 2 was used for HDAC1. Human recombinant full length HDAC1 expressed in a baculovirus expression system... |
US Patent US9056843 (2015)
BindingDB Entry DOI: 10.7270/Q24F1PG8 |
More data for this
Ligand-Target Pair | |
Biofilm regulatory protein A
(Streptococcus mutans serotype c (strain ATCC 70061...) | BDBM50036932
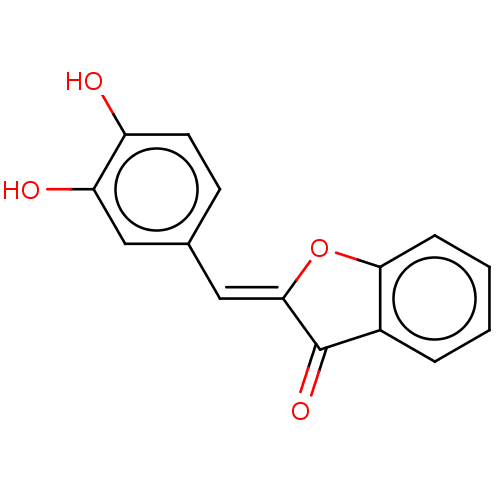
(CHEMBL1478023 | US11866416, Compound SN-II-118)Show InChI InChI=1S/C15H10O4/c16-11-6-5-9(7-12(11)17)8-14-15(18)10-3-1-2-4-13(10)19-14/h1-8,16-17H/b14-8- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 2.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM162723

(US9056843, 14)Show InChI InChI=1S/C13H12F3N3O2/c1-3-19(2)11(20)9-6-4-8(5-7-9)10-17-12(21-18-10)13(14,15)16/h4-7H,3H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... |
US Patent US9056843 (2015)
BindingDB Entry DOI: 10.7270/Q24F1PG8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM162738

(US9056843, 29)Show InChI InChI=1S/C15H14F3N3O2/c16-15(17,18)14-20-12(21-23-14)9-5-7-10(8-6-9)13(22)19-11-3-1-2-4-11/h5-8,11H,1-4H2,(H,19,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... |
US Patent US9056843 (2015)
BindingDB Entry DOI: 10.7270/Q24F1PG8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM162723

(US9056843, 14)Show InChI InChI=1S/C13H12F3N3O2/c1-3-19(2)11(20)9-6-4-8(5-7-9)10-17-12(21-18-10)13(14,15)16/h4-7H,3H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
A similar assay procedure as described in Test 2 was used for HDAC6. Human recombinant full length HDAC6 expressed in a baculovirus expression system... |
US Patent US9056843 (2015)
BindingDB Entry DOI: 10.7270/Q24F1PG8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM162738

(US9056843, 29)Show InChI InChI=1S/C15H14F3N3O2/c16-15(17,18)14-20-12(21-23-14)9-5-7-10(8-6-9)13(22)19-11-3-1-2-4-11/h5-8,11H,1-4H2,(H,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
A similar assay procedure as described in Test 2 was used for HDAC6. Human recombinant full length HDAC6 expressed in a baculovirus expression system... |
US Patent US9056843 (2015)
BindingDB Entry DOI: 10.7270/Q24F1PG8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50027586
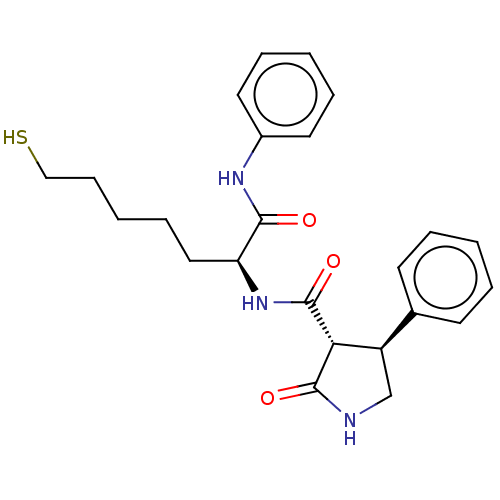
(CHEMBL3356926)Show SMILES SCCCCC[C@H](NC(=O)[C@@H]1[C@H](CNC1=O)c1ccccc1)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C24H29N3O3S/c28-22(26-18-12-6-2-7-13-18)20(14-8-3-9-15-31)27-24(30)21-19(16-25-23(21)29)17-10-4-1-5-11-17/h1-2,4-7,10-13,19-21,31H,3,8-9,14-16H2,(H,25,29)(H,26,28)(H,27,30)/t19-,20+,21-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite SpA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) using fluorogenic tetrapeptide RHKK(Ac) substrate by fluorescence assay |
J Med Chem 57: 8358-77 (2014)
Article DOI: 10.1021/jm5008209
BindingDB Entry DOI: 10.7270/Q2JW8GGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50027649
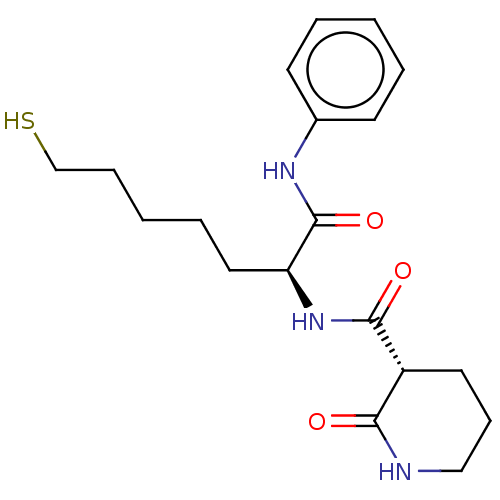
(CHEMBL3356527)Show SMILES SCCCCC[C@H](NC(=O)[C@@H]1CCCNC1=O)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C19H27N3O3S/c23-17-15(10-7-12-20-17)18(24)22-16(11-5-2-6-13-26)19(25)21-14-8-3-1-4-9-14/h1,3-4,8-9,15-16,26H,2,5-7,10-13H2,(H,20,23)(H,21,25)(H,22,24)/t15-,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite SpA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using fluorogenic tetrapeptide RHKK(Ac) substrate by fluorescence assay |
J Med Chem 57: 8358-77 (2014)
Article DOI: 10.1021/jm5008209
BindingDB Entry DOI: 10.7270/Q2JW8GGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50027586

(CHEMBL3356926)Show SMILES SCCCCC[C@H](NC(=O)[C@@H]1[C@H](CNC1=O)c1ccccc1)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C24H29N3O3S/c28-22(26-18-12-6-2-7-13-18)20(14-8-3-9-15-31)27-24(30)21-19(16-25-23(21)29)17-10-4-1-5-11-17/h1-2,4-7,10-13,19-21,31H,3,8-9,14-16H2,(H,25,29)(H,26,28)(H,27,30)/t19-,20+,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite SpA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using fluorogenic tetrapeptide RHKK(Ac) substrate by fluorescence assay |
J Med Chem 57: 8358-77 (2014)
Article DOI: 10.1021/jm5008209
BindingDB Entry DOI: 10.7270/Q2JW8GGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50027649

(CHEMBL3356527)Show SMILES SCCCCC[C@H](NC(=O)[C@@H]1CCCNC1=O)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C19H27N3O3S/c23-17-15(10-7-12-20-17)18(24)22-16(11-5-2-6-13-26)19(25)21-14-8-3-1-4-9-14/h1,3-4,8-9,15-16,26H,2,5-7,10-13H2,(H,20,23)(H,21,25)(H,22,24)/t15-,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite SpA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) using fluorogenic tetrapeptide RHK(Ac)K(Ac) substrate by fluorescence assay |
J Med Chem 57: 8358-77 (2014)
Article DOI: 10.1021/jm5008209
BindingDB Entry DOI: 10.7270/Q2JW8GGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50027586

(CHEMBL3356926)Show SMILES SCCCCC[C@H](NC(=O)[C@@H]1[C@H](CNC1=O)c1ccccc1)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C24H29N3O3S/c28-22(26-18-12-6-2-7-13-18)20(14-8-3-9-15-31)27-24(30)21-19(16-25-23(21)29)17-10-4-1-5-11-17/h1-2,4-7,10-13,19-21,31H,3,8-9,14-16H2,(H,25,29)(H,26,28)(H,27,30)/t19-,20+,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite SpA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) using fluorogenic tetrapeptide RHK(Ac)K(Ac) substrate by fluorescence assay |
J Med Chem 57: 8358-77 (2014)
Article DOI: 10.1021/jm5008209
BindingDB Entry DOI: 10.7270/Q2JW8GGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50027586

(CHEMBL3356926)Show SMILES SCCCCC[C@H](NC(=O)[C@@H]1[C@H](CNC1=O)c1ccccc1)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C24H29N3O3S/c28-22(26-18-12-6-2-7-13-18)20(14-8-3-9-15-31)27-24(30)21-19(16-25-23(21)29)17-10-4-1-5-11-17/h1-2,4-7,10-13,19-21,31H,3,8-9,14-16H2,(H,25,29)(H,26,28)(H,27,30)/t19-,20+,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite SpA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) using fluorogenic tetrapeptide RHKK(Ac) substrate by fluorescence assay |
J Med Chem 57: 8358-77 (2014)
Article DOI: 10.1021/jm5008209
BindingDB Entry DOI: 10.7270/Q2JW8GGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50027649

(CHEMBL3356527)Show SMILES SCCCCC[C@H](NC(=O)[C@@H]1CCCNC1=O)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C19H27N3O3S/c23-17-15(10-7-12-20-17)18(24)22-16(11-5-2-6-13-26)19(25)21-14-8-3-1-4-9-14/h1,3-4,8-9,15-16,26H,2,5-7,10-13H2,(H,20,23)(H,21,25)(H,22,24)/t15-,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite SpA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) using fluorogenic tetrapeptide RHKK(Ac) substrate by fluorescence assay |
J Med Chem 57: 8358-77 (2014)
Article DOI: 10.1021/jm5008209
BindingDB Entry DOI: 10.7270/Q2JW8GGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50027586

(CHEMBL3356926)Show SMILES SCCCCC[C@H](NC(=O)[C@@H]1[C@H](CNC1=O)c1ccccc1)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C24H29N3O3S/c28-22(26-18-12-6-2-7-13-18)20(14-8-3-9-15-31)27-24(30)21-19(16-25-23(21)29)17-10-4-1-5-11-17/h1-2,4-7,10-13,19-21,31H,3,8-9,14-16H2,(H,25,29)(H,26,28)(H,27,30)/t19-,20+,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite SpA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) using fluorogenic tetrapeptide RHKK(Ac) substrate by fluorescence assay |
J Med Chem 57: 8358-77 (2014)
Article DOI: 10.1021/jm5008209
BindingDB Entry DOI: 10.7270/Q2JW8GGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50027649

(CHEMBL3356527)Show SMILES SCCCCC[C@H](NC(=O)[C@@H]1CCCNC1=O)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C19H27N3O3S/c23-17-15(10-7-12-20-17)18(24)22-16(11-5-2-6-13-26)19(25)21-14-8-3-1-4-9-14/h1,3-4,8-9,15-16,26H,2,5-7,10-13H2,(H,20,23)(H,21,25)(H,22,24)/t15-,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite SpA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) using fluorogenic tetrapeptide RHKK(Ac) substrate by fluorescence assay |
J Med Chem 57: 8358-77 (2014)
Article DOI: 10.1021/jm5008209
BindingDB Entry DOI: 10.7270/Q2JW8GGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50027649

(CHEMBL3356527)Show SMILES SCCCCC[C@H](NC(=O)[C@@H]1CCCNC1=O)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C19H27N3O3S/c23-17-15(10-7-12-20-17)18(24)22-16(11-5-2-6-13-26)19(25)21-14-8-3-1-4-9-14/h1,3-4,8-9,15-16,26H,2,5-7,10-13H2,(H,20,23)(H,21,25)(H,22,24)/t15-,16+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite SpA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) using fluorogenic tetrapeptide RHKK(Ac) substrate by fluorescence assay |
J Med Chem 57: 8358-77 (2014)
Article DOI: 10.1021/jm5008209
BindingDB Entry DOI: 10.7270/Q2JW8GGN |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50027649

(CHEMBL3356527)Show SMILES SCCCCC[C@H](NC(=O)[C@@H]1CCCNC1=O)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C19H27N3O3S/c23-17-15(10-7-12-20-17)18(24)22-16(11-5-2-6-13-26)19(25)21-14-8-3-1-4-9-14/h1,3-4,8-9,15-16,26H,2,5-7,10-13H2,(H,20,23)(H,21,25)(H,22,24)/t15-,16+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite SpA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) using fluorogenic tetrapeptide RHKK(Ac) substrate by fluorescence assay |
J Med Chem 57: 8358-77 (2014)
Article DOI: 10.1021/jm5008209
BindingDB Entry DOI: 10.7270/Q2JW8GGN |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50027586

(CHEMBL3356926)Show SMILES SCCCCC[C@H](NC(=O)[C@@H]1[C@H](CNC1=O)c1ccccc1)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C24H29N3O3S/c28-22(26-18-12-6-2-7-13-18)20(14-8-3-9-15-31)27-24(30)21-19(16-25-23(21)29)17-10-4-1-5-11-17/h1-2,4-7,10-13,19-21,31H,3,8-9,14-16H2,(H,25,29)(H,26,28)(H,27,30)/t19-,20+,21-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite SpA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) using fluorogenic tetrapeptide RHKK(Ac) substrate by fluorescence assay |
J Med Chem 57: 8358-77 (2014)
Article DOI: 10.1021/jm5008209
BindingDB Entry DOI: 10.7270/Q2JW8GGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50027649

(CHEMBL3356527)Show SMILES SCCCCC[C@H](NC(=O)[C@@H]1CCCNC1=O)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C19H27N3O3S/c23-17-15(10-7-12-20-17)18(24)22-16(11-5-2-6-13-26)19(25)21-14-8-3-1-4-9-14/h1,3-4,8-9,15-16,26H,2,5-7,10-13H2,(H,20,23)(H,21,25)(H,22,24)/t15-,16+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite SpA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC11 (unknown origin) using fluorogenic tetrapeptide RHKK(Ac) substrate by fluorescence assay |
J Med Chem 57: 8358-77 (2014)
Article DOI: 10.1021/jm5008209
BindingDB Entry DOI: 10.7270/Q2JW8GGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50027586

(CHEMBL3356926)Show SMILES SCCCCC[C@H](NC(=O)[C@@H]1[C@H](CNC1=O)c1ccccc1)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C24H29N3O3S/c28-22(26-18-12-6-2-7-13-18)20(14-8-3-9-15-31)27-24(30)21-19(16-25-23(21)29)17-10-4-1-5-11-17/h1-2,4-7,10-13,19-21,31H,3,8-9,14-16H2,(H,25,29)(H,26,28)(H,27,30)/t19-,20+,21-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite SpA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC11 (unknown origin) using fluorogenic tetrapeptide RHKK(Ac) substrate by fluorescence assay |
J Med Chem 57: 8358-77 (2014)
Article DOI: 10.1021/jm5008209
BindingDB Entry DOI: 10.7270/Q2JW8GGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50026450

(CHEMBL3335283)Show InChI InChI=1S/C21H23N3O2/c25-21(23-26)12-5-4-11-20-13-14-24(22-20)16-17-7-6-10-19(15-17)18-8-2-1-3-9-18/h1-3,6-10,13-15,26H,4-5,11-12,16H2,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 after 60 mins by fluorimetric assay |
Eur J Med Chem 86: 639-52 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.024
BindingDB Entry DOI: 10.7270/Q2NV9KTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50026450

(CHEMBL3335283)Show InChI InChI=1S/C21H23N3O2/c25-21(23-26)12-5-4-11-20-13-14-24(22-20)16-17-7-6-10-19(15-17)18-8-2-1-3-9-18/h1-3,6-10,13-15,26H,4-5,11-12,16H2,(H,23,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC9 after 60 mins by fluorimetric assay |
Eur J Med Chem 86: 639-52 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.024
BindingDB Entry DOI: 10.7270/Q2NV9KTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50026450

(CHEMBL3335283)Show InChI InChI=1S/C21H23N3O2/c25-21(23-26)12-5-4-11-20-13-14-24(22-20)16-17-7-6-10-19(15-17)18-8-2-1-3-9-18/h1-3,6-10,13-15,26H,4-5,11-12,16H2,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 after 60 mins by fluorimetric assay |
Eur J Med Chem 86: 639-52 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.024
BindingDB Entry DOI: 10.7270/Q2NV9KTB |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50026450

(CHEMBL3335283)Show InChI InChI=1S/C21H23N3O2/c25-21(23-26)12-5-4-11-20-13-14-24(22-20)16-17-7-6-10-19(15-17)18-8-2-1-3-9-18/h1-3,6-10,13-15,26H,4-5,11-12,16H2,(H,23,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 after 60 mins by fluorimetric assay |
Eur J Med Chem 86: 639-52 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.024
BindingDB Entry DOI: 10.7270/Q2NV9KTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50026450

(CHEMBL3335283)Show InChI InChI=1S/C21H23N3O2/c25-21(23-26)12-5-4-11-20-13-14-24(22-20)16-17-7-6-10-19(15-17)18-8-2-1-3-9-18/h1-3,6-10,13-15,26H,4-5,11-12,16H2,(H,23,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC11 after 60 mins by fluorimetric assay |
Eur J Med Chem 86: 639-52 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.024
BindingDB Entry DOI: 10.7270/Q2NV9KTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50026450

(CHEMBL3335283)Show InChI InChI=1S/C21H23N3O2/c25-21(23-26)12-5-4-11-20-13-14-24(22-20)16-17-7-6-10-19(15-17)18-8-2-1-3-9-18/h1-3,6-10,13-15,26H,4-5,11-12,16H2,(H,23,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC4 after 60 mins by fluorimetric assay |
Eur J Med Chem 86: 639-52 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.024
BindingDB Entry DOI: 10.7270/Q2NV9KTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50026450

(CHEMBL3335283)Show InChI InChI=1S/C21H23N3O2/c25-21(23-26)12-5-4-11-20-13-14-24(22-20)16-17-7-6-10-19(15-17)18-8-2-1-3-9-18/h1-3,6-10,13-15,26H,4-5,11-12,16H2,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 after 60 mins by fluorimetric assay |
Eur J Med Chem 86: 639-52 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.024
BindingDB Entry DOI: 10.7270/Q2NV9KTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50026450

(CHEMBL3335283)Show InChI InChI=1S/C21H23N3O2/c25-21(23-26)12-5-4-11-20-13-14-24(22-20)16-17-7-6-10-19(15-17)18-8-2-1-3-9-18/h1-3,6-10,13-15,26H,4-5,11-12,16H2,(H,23,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 after 60 mins by fluorimetric assay |
Eur J Med Chem 86: 639-52 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.024
BindingDB Entry DOI: 10.7270/Q2NV9KTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50026450

(CHEMBL3335283)Show InChI InChI=1S/C21H23N3O2/c25-21(23-26)12-5-4-11-20-13-14-24(22-20)16-17-7-6-10-19(15-17)18-8-2-1-3-9-18/h1-3,6-10,13-15,26H,4-5,11-12,16H2,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 397 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 after 60 mins by fluorimetric assay |
Eur J Med Chem 86: 639-52 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.024
BindingDB Entry DOI: 10.7270/Q2NV9KTB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50042847
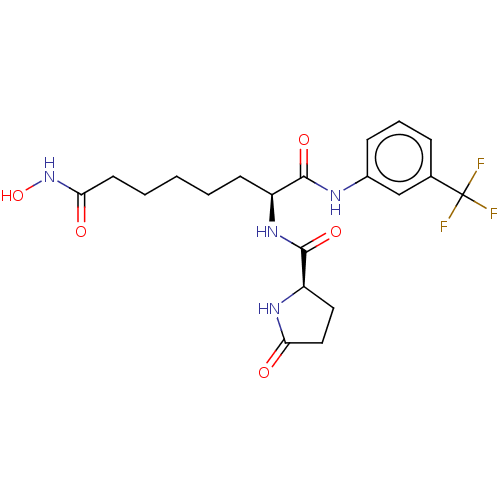
(CHEMBL3098602)Show SMILES ONC(=O)CCCCC[C@H](NC(=O)[C@H]1CCC(=O)N1)C(=O)Nc1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H25F3N4O5/c21-20(22,23)12-5-4-6-13(11-12)24-18(30)14(7-2-1-3-8-17(29)27-32)26-19(31)15-9-10-16(28)25-15/h4-6,11,14-15,32H,1-3,7-10H2,(H,24,30)(H,25,28)(H,26,31)(H,27,29)/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite S.p.A.
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC2 using RHKK(Ac) as substrate by fluorimetric analysis |
Bioorg Med Chem Lett 25: 459-61 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.051
BindingDB Entry DOI: 10.7270/Q2445P3R |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50042847

(CHEMBL3098602)Show SMILES ONC(=O)CCCCC[C@H](NC(=O)[C@H]1CCC(=O)N1)C(=O)Nc1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H25F3N4O5/c21-20(22,23)12-5-4-6-13(11-12)24-18(30)14(7-2-1-3-8-17(29)27-32)26-19(31)15-9-10-16(28)25-15/h4-6,11,14-15,32H,1-3,7-10H2,(H,24,30)(H,25,28)(H,26,31)(H,27,29)/t14-,15+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite S.p.A.
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC3 using RHKK(Ac) as substrate by fluorimetric analysis |
Bioorg Med Chem Lett 25: 459-61 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.051
BindingDB Entry DOI: 10.7270/Q2445P3R |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50042847

(CHEMBL3098602)Show SMILES ONC(=O)CCCCC[C@H](NC(=O)[C@H]1CCC(=O)N1)C(=O)Nc1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H25F3N4O5/c21-20(22,23)12-5-4-6-13(11-12)24-18(30)14(7-2-1-3-8-17(29)27-32)26-19(31)15-9-10-16(28)25-15/h4-6,11,14-15,32H,1-3,7-10H2,(H,24,30)(H,25,28)(H,26,31)(H,27,29)/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite S.p.A.
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC6 using RHKK(Ac) as substrate by fluorimetric analysis |
Bioorg Med Chem Lett 25: 459-61 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.051
BindingDB Entry DOI: 10.7270/Q2445P3R |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50042847

(CHEMBL3098602)Show SMILES ONC(=O)CCCCC[C@H](NC(=O)[C@H]1CCC(=O)N1)C(=O)Nc1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H25F3N4O5/c21-20(22,23)12-5-4-6-13(11-12)24-18(30)14(7-2-1-3-8-17(29)27-32)26-19(31)15-9-10-16(28)25-15/h4-6,11,14-15,32H,1-3,7-10H2,(H,24,30)(H,25,28)(H,26,31)(H,27,29)/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite S.p.A.
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC8 using RHK(Ac)K(Ac) as substrate by fluorimetric analysis |
Bioorg Med Chem Lett 25: 459-61 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.051
BindingDB Entry DOI: 10.7270/Q2445P3R |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50042847

(CHEMBL3098602)Show SMILES ONC(=O)CCCCC[C@H](NC(=O)[C@H]1CCC(=O)N1)C(=O)Nc1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H25F3N4O5/c21-20(22,23)12-5-4-6-13(11-12)24-18(30)14(7-2-1-3-8-17(29)27-32)26-19(31)15-9-10-16(28)25-15/h4-6,11,14-15,32H,1-3,7-10H2,(H,24,30)(H,25,28)(H,26,31)(H,27,29)/t14-,15+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite S.p.A.
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC10 using RHKK(Ac) as substrate by fluorimetric analysis |
Bioorg Med Chem Lett 25: 459-61 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.051
BindingDB Entry DOI: 10.7270/Q2445P3R |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50042847

(CHEMBL3098602)Show SMILES ONC(=O)CCCCC[C@H](NC(=O)[C@H]1CCC(=O)N1)C(=O)Nc1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H25F3N4O5/c21-20(22,23)12-5-4-6-13(11-12)24-18(30)14(7-2-1-3-8-17(29)27-32)26-19(31)15-9-10-16(28)25-15/h4-6,11,14-15,32H,1-3,7-10H2,(H,24,30)(H,25,28)(H,26,31)(H,27,29)/t14-,15+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite S.p.A.
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC11 using RHKK(Ac) as substrate by fluorimetric analysis |
Bioorg Med Chem Lett 25: 459-61 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.051
BindingDB Entry DOI: 10.7270/Q2445P3R |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50042847

(CHEMBL3098602)Show SMILES ONC(=O)CCCCC[C@H](NC(=O)[C@H]1CCC(=O)N1)C(=O)Nc1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H25F3N4O5/c21-20(22,23)12-5-4-6-13(11-12)24-18(30)14(7-2-1-3-8-17(29)27-32)26-19(31)15-9-10-16(28)25-15/h4-6,11,14-15,32H,1-3,7-10H2,(H,24,30)(H,25,28)(H,26,31)(H,27,29)/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite S.p.A.
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 using RHKK(Ac) as substrate by fluorimetric analysis |
Bioorg Med Chem Lett 25: 459-61 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.051
BindingDB Entry DOI: 10.7270/Q2445P3R |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50036880
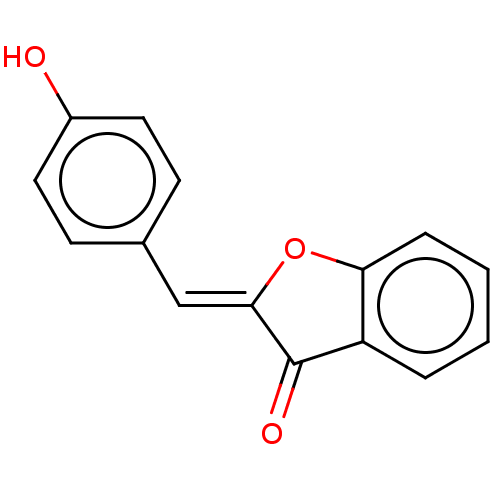
(CHEMBL199974)Show InChI InChI=1S/C15H10O3/c16-11-7-5-10(6-8-11)9-14-15(17)12-3-1-2-4-13(12)18-14/h1-9,16H/b14-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) after 15 mins by fluorescence assay |
Bioorg Med Chem Lett 24: 5497-501 (2015)
Article DOI: 10.1016/j.bmcl.2014.10.019
BindingDB Entry DOI: 10.7270/Q2X3502T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50036932

(CHEMBL1478023 | US11866416, Compound SN-II-118)Show InChI InChI=1S/C15H10O4/c16-11-6-5-9(7-12(11)17)8-14-15(18)10-3-1-2-4-13(10)19-14/h1-8,16-17H/b14-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) after 15 mins by fluorescence assay |
Bioorg Med Chem Lett 24: 5497-501 (2015)
Article DOI: 10.1016/j.bmcl.2014.10.019
BindingDB Entry DOI: 10.7270/Q2X3502T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50036880

(CHEMBL199974)Show InChI InChI=1S/C15H10O3/c16-11-7-5-10(6-8-11)9-14-15(17)12-3-1-2-4-13(12)18-14/h1-9,16H/b14-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) after 15 mins by fluorescence assay |
Bioorg Med Chem Lett 24: 5497-501 (2015)
Article DOI: 10.1016/j.bmcl.2014.10.019
BindingDB Entry DOI: 10.7270/Q2X3502T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50036932

(CHEMBL1478023 | US11866416, Compound SN-II-118)Show InChI InChI=1S/C15H10O4/c16-11-6-5-9(7-12(11)17)8-14-15(18)10-3-1-2-4-13(10)19-14/h1-8,16-17H/b14-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) after 15 mins by fluorescence assay |
Bioorg Med Chem Lett 24: 5497-501 (2015)
Article DOI: 10.1016/j.bmcl.2014.10.019
BindingDB Entry DOI: 10.7270/Q2X3502T |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50036880

(CHEMBL199974)Show InChI InChI=1S/C15H10O3/c16-11-7-5-10(6-8-11)9-14-15(17)12-3-1-2-4-13(12)18-14/h1-9,16H/b14-9- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell extract after 15 mins by fluorescence assay |
Bioorg Med Chem Lett 24: 5497-501 (2015)
Article DOI: 10.1016/j.bmcl.2014.10.019
BindingDB Entry DOI: 10.7270/Q2X3502T |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50036932

(CHEMBL1478023 | US11866416, Compound SN-II-118)Show InChI InChI=1S/C15H10O4/c16-11-6-5-9(7-12(11)17)8-14-15(18)10-3-1-2-4-13(10)19-14/h1-8,16-17H/b14-8- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell extract after 15 mins by fluorescence assay |
Bioorg Med Chem Lett 24: 5497-501 (2015)
Article DOI: 10.1016/j.bmcl.2014.10.019
BindingDB Entry DOI: 10.7270/Q2X3502T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50036880

(CHEMBL199974)Show InChI InChI=1S/C15H10O3/c16-11-7-5-10(6-8-11)9-14-15(17)12-3-1-2-4-13(12)18-14/h1-9,16H/b14-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.29E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) after 15 mins by fluorescence assay |
Bioorg Med Chem Lett 24: 5497-501 (2015)
Article DOI: 10.1016/j.bmcl.2014.10.019
BindingDB Entry DOI: 10.7270/Q2X3502T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50036932

(CHEMBL1478023 | US11866416, Compound SN-II-118)Show InChI InChI=1S/C15H10O4/c16-11-6-5-9(7-12(11)17)8-14-15(18)10-3-1-2-4-13(10)19-14/h1-8,16-17H/b14-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) after 15 mins by fluorescence assay |
Bioorg Med Chem Lett 24: 5497-501 (2015)
Article DOI: 10.1016/j.bmcl.2014.10.019
BindingDB Entry DOI: 10.7270/Q2X3502T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 17B
(Homo sapiens (Human)) | BDBM50036880

(CHEMBL199974)Show InChI InChI=1S/C15H10O3/c16-11-7-5-10(6-8-11)9-14-15(17)12-3-1-2-4-13(12)18-14/h1-9,16H/b14-9- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged DRAK2 (unknown origin) autophosphorylation after 2 hrs by ADP-glo assay |
Eur J Med Chem 130: 195-208 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.048
BindingDB Entry DOI: 10.7270/Q2XW4N3P |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 17B
(Homo sapiens (Human)) | BDBM50036932

(CHEMBL1478023 | US11866416, Compound SN-II-118)Show InChI InChI=1S/C15H10O4/c16-11-6-5-9(7-12(11)17)8-14-15(18)10-3-1-2-4-13(10)19-14/h1-8,16-17H/b14-8- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory constant for mouse kidney cytidine deaminase |
Eur J Med Chem 130: 195-208 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.048
BindingDB Entry DOI: 10.7270/Q2XW4N3P |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50042847

(CHEMBL3098602)Show SMILES ONC(=O)CCCCC[C@H](NC(=O)[C@H]1CCC(=O)N1)C(=O)Nc1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H25F3N4O5/c21-20(22,23)12-5-4-6-13(11-12)24-18(30)14(7-2-1-3-8-17(29)27-32)26-19(31)15-9-10-16(28)25-15/h4-6,11,14-15,32H,1-3,7-10H2,(H,24,30)(H,25,28)(H,26,31)(H,27,29)/t14-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC3/NCOR2 using RHKK(Ac) as substrate by fluorimetric analysis |
Bioorg Med Chem Lett 24: 61-4 (2014)
Article DOI: 10.1016/j.bmcl.2013.11.072
BindingDB Entry DOI: 10.7270/Q21839GT |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50042847

(CHEMBL3098602)Show SMILES ONC(=O)CCCCC[C@H](NC(=O)[C@H]1CCC(=O)N1)C(=O)Nc1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H25F3N4O5/c21-20(22,23)12-5-4-6-13(11-12)24-18(30)14(7-2-1-3-8-17(29)27-32)26-19(31)15-9-10-16(28)25-15/h4-6,11,14-15,32H,1-3,7-10H2,(H,24,30)(H,25,28)(H,26,31)(H,27,29)/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC8 using RHK(Ac)K(Ac) as substrate by fluorimetric analysis |
Bioorg Med Chem Lett 24: 61-4 (2014)
Article DOI: 10.1016/j.bmcl.2013.11.072
BindingDB Entry DOI: 10.7270/Q21839GT |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50042847

(CHEMBL3098602)Show SMILES ONC(=O)CCCCC[C@H](NC(=O)[C@H]1CCC(=O)N1)C(=O)Nc1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H25F3N4O5/c21-20(22,23)12-5-4-6-13(11-12)24-18(30)14(7-2-1-3-8-17(29)27-32)26-19(31)15-9-10-16(28)25-15/h4-6,11,14-15,32H,1-3,7-10H2,(H,24,30)(H,25,28)(H,26,31)(H,27,29)/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC2 using RHKK(Ac) as substrate by fluorimetric analysis |
Bioorg Med Chem Lett 24: 61-4 (2014)
Article DOI: 10.1016/j.bmcl.2013.11.072
BindingDB Entry DOI: 10.7270/Q21839GT |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50042847

(CHEMBL3098602)Show SMILES ONC(=O)CCCCC[C@H](NC(=O)[C@H]1CCC(=O)N1)C(=O)Nc1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H25F3N4O5/c21-20(22,23)12-5-4-6-13(11-12)24-18(30)14(7-2-1-3-8-17(29)27-32)26-19(31)15-9-10-16(28)25-15/h4-6,11,14-15,32H,1-3,7-10H2,(H,24,30)(H,25,28)(H,26,31)(H,27,29)/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 using RHKK(Ac) as substrate by fluorimetric analysis |
Bioorg Med Chem Lett 24: 61-4 (2014)
Article DOI: 10.1016/j.bmcl.2013.11.072
BindingDB Entry DOI: 10.7270/Q21839GT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
 Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase


