

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Wt: 378.5 BDBM26144 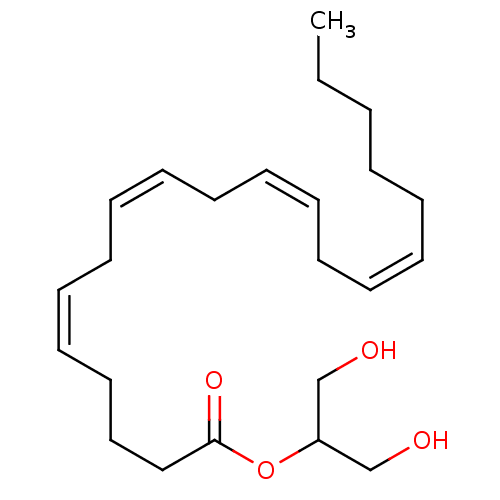 Purchase Purchase | Wt: 452.2 BDBM29075 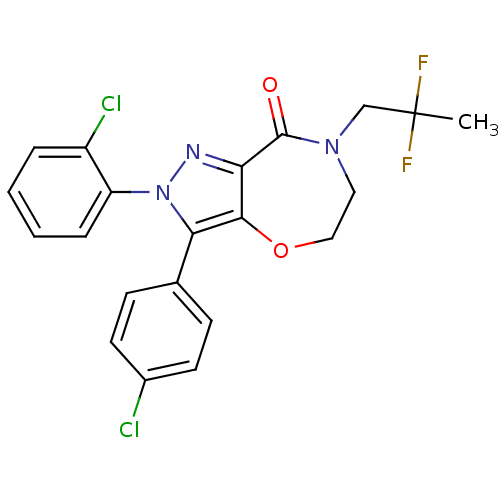 Purchase Purchase | Wt: 325.5 BDBM29080 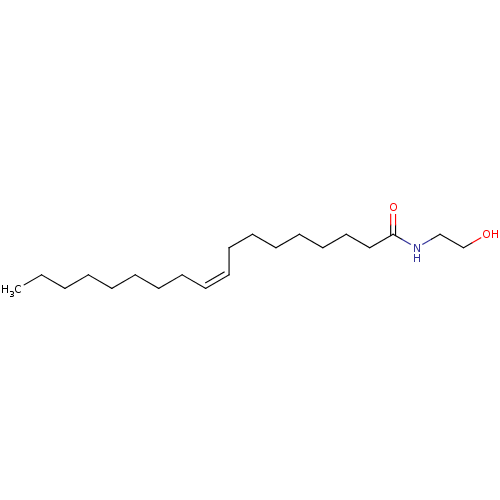 Purchase Purchase | Wt: 299.4 BDBM29083 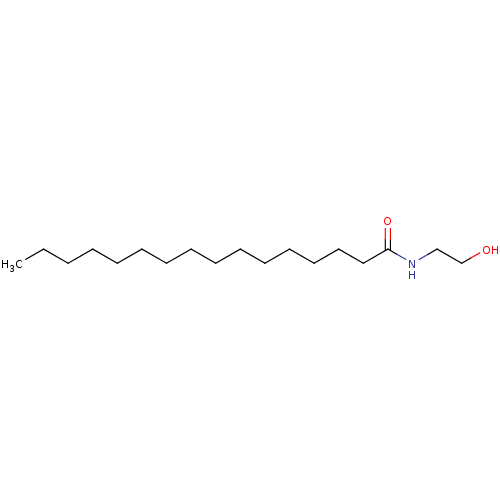 Purchase Purchase | Wt: 258.3 BDBM40144  Purchase Purchase |
| Wt: 512.5 BDBM45519 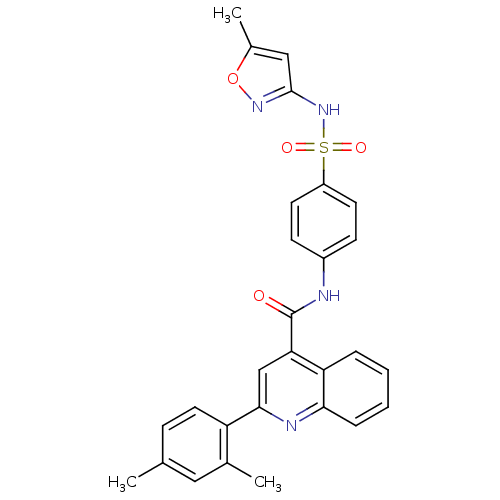 Purchase Purchase | Wt: 527.5 BDBM50459  Purchase Purchase | Wt: 391.4 BDBM51557 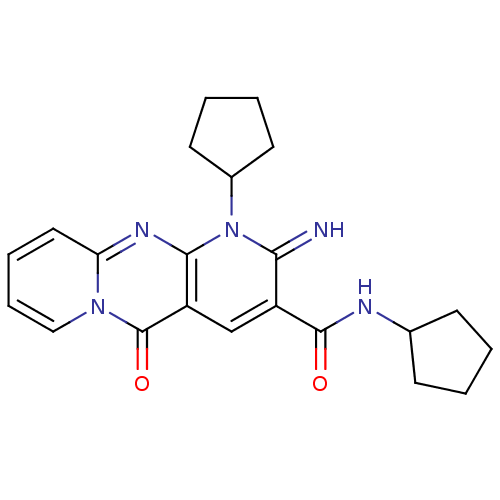 Purchase Purchase | Wt: 314.7 BDBM57190 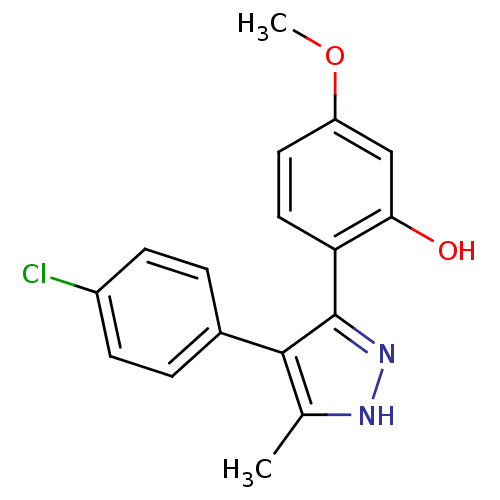 Purchase Purchase | Wt: 491.6 BDBM61031  Purchase Purchase |
| Wt: 474.5 BDBM61037  Purchase Purchase | Wt: 453.5 BDBM61041  Purchase Purchase | Wt: 440.9 BDBM61043 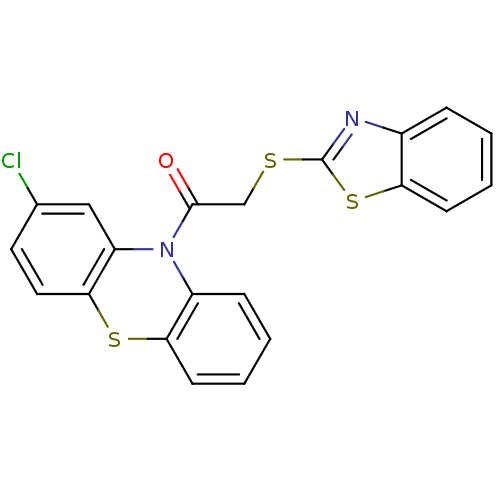 Purchase Purchase | Wt: 363.4 BDBM61054  Purchase Purchase | Wt: 434.5 BDBM61068  Purchase Purchase |
| << First | Previous | Displayed 16 to 30 (of 340 total ) | Next | Last >> |
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29075 (PF-514273) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1... | ACS Med Chem Lett 3: 397-401 (2012) Article DOI: 10.1021/ml3000325 BindingDB Entry DOI: 10.7270/Q22N53BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29075 (PF-514273) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | -12.5 | n/a | n/a | 0.820 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29075 (PF-514273) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta counting | ACS Med Chem Lett 3: 397-401 (2012) Article DOI: 10.1021/ml3000325 BindingDB Entry DOI: 10.7270/Q22N53BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, liver (Mus musculus (Mouse)) | BDBM29080 (CHEMBL280065 | N-oleoylethanolamine | Oleamide MEA...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avanti Polar Lipids | Assay Description The following fluorescent ligand displacement assays at 24 °C were used to further confirm and/or determine if the cytosolic lipidic ligand "chaperon... | Biochemistry 55: 5243-55 (2016) Article DOI: 10.1021/acs.biochem.6b00446 BindingDB Entry DOI: 10.7270/Q2MS3RJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, liver (Mus musculus (Mouse)) | BDBM26144 (1,3-dihydroxypropan-2-yl (5Z,8Z,11Z,14Z)-icosa-5,8...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avanti Polar Lipids | Assay Description The following fluorescent ligand displacement assays at 24 °C were used to further confirm and/or determine if the cytosolic lipidic ligand "chaperon... | Biochemistry 55: 5243-55 (2016) Article DOI: 10.1021/acs.biochem.6b00446 BindingDB Entry DOI: 10.7270/Q2MS3RJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM26144 (1,3-dihydroxypropan-2-yl (5Z,8Z,11Z,14Z)-icosa-5,8...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from rat forebrain membrane which expresses Cannabinoid receptor 2 in the presence of PMSF | J Med Chem 45: 3709-20 (2002) BindingDB Entry DOI: 10.7270/Q2WD3ZXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM26144 (1,3-dihydroxypropan-2-yl (5Z,8Z,11Z,14Z)-icosa-5,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from rat forebrain membrane which expresses Cannabinoid receptor 1 in the presence of PMSF | J Med Chem 45: 3709-20 (2002) BindingDB Entry DOI: 10.7270/Q2WD3ZXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM26144 (1,3-dihydroxypropan-2-yl (5Z,8Z,11Z,14Z)-icosa-5,8...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in human HEK293 cells | J Med Chem 54: 8278-88 (2011) Article DOI: 10.1021/jm200529h BindingDB Entry DOI: 10.7270/Q23J3DC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM26144 (1,3-dihydroxypropan-2-yl (5Z,8Z,11Z,14Z)-icosa-5,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 472 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to rat CB1 receptor | Cell Chem Biol 56: 8224-56 (2013) Article DOI: 10.1021/jm4005626 BindingDB Entry DOI: 10.7270/Q2B859M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM26144 (1,3-dihydroxypropan-2-yl (5Z,8Z,11Z,14Z)-icosa-5,8...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 472 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari Curated by ChEMBL | Assay Description Binding affinity to CB1 receptor (unknown origin) | Bioorg Med Chem 24: 5291-5301 (2016) Article DOI: 10.1016/j.bmc.2016.08.055 BindingDB Entry DOI: 10.7270/Q2HT2SV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM26144 (1,3-dihydroxypropan-2-yl (5Z,8Z,11Z,14Z)-icosa-5,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 472 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Wistar rat brain incubated for 60 mins by radioactive filter binding assay | Eur J Med Chem 112: 66-80 (2016) Article DOI: 10.1016/j.ejmech.2016.02.005 BindingDB Entry DOI: 10.7270/Q24B336C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM26144 (1,3-dihydroxypropan-2-yl (5Z,8Z,11Z,14Z)-icosa-5,8...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in HEK-293-EBNA cell membranes after 90 mins by scintillation counting | J Med Chem 54: 5265-9 (2011) Article DOI: 10.1021/jm2004392 BindingDB Entry DOI: 10.7270/Q25D8S6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM26144 (1,3-dihydroxypropan-2-yl (5Z,8Z,11Z,14Z)-icosa-5,8...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Binding affinity to CB1R (unknown origin) | J Med Chem 60: 4-46 (2017) Article DOI: 10.1021/acs.jmedchem.6b00538 BindingDB Entry DOI: 10.7270/Q2348NZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29080 (CHEMBL280065 | N-oleoylethanolamine | Oleamide MEA...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.10E+3 | -8.26 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29080 (CHEMBL280065 | N-oleoylethanolamine | Oleamide MEA...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Farmaceutiche Curated by ChEMBL | Assay Description Binding affinity to human recombinant CB1 receptor expressed in COS cells | J Med Chem 49: 2333-8 (2006) Article DOI: 10.1021/jm051240y BindingDB Entry DOI: 10.7270/Q2862G15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM26144 (1,3-dihydroxypropan-2-yl (5Z,8Z,11Z,14Z)-icosa-5,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in HEK-293-EBNA cell membranes after 90 mins by scintillation counting | J Med Chem 54: 5265-9 (2011) Article DOI: 10.1021/jm2004392 BindingDB Entry DOI: 10.7270/Q25D8S6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM26144 (1,3-dihydroxypropan-2-yl (5Z,8Z,11Z,14Z)-icosa-5,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor expressed in human HEK293 cells | J Med Chem 54: 8278-88 (2011) Article DOI: 10.1021/jm200529h BindingDB Entry DOI: 10.7270/Q23J3DC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM26144 (1,3-dihydroxypropan-2-yl (5Z,8Z,11Z,14Z)-icosa-5,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor (unknown origin) | Bioorg Med Chem 24: 5291-5301 (2016) Article DOI: 10.1016/j.bmc.2016.08.055 BindingDB Entry DOI: 10.7270/Q2HT2SV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM26144 (1,3-dihydroxypropan-2-yl (5Z,8Z,11Z,14Z)-icosa-5,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor | Cell Chem Biol 56: 8224-56 (2013) Article DOI: 10.1021/jm4005626 BindingDB Entry DOI: 10.7270/Q2B859M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29083 (Impulsin | MimyX | Palmidrol | Palmityoletanolamid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+3 | >-7.35 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29083 (Impulsin | MimyX | Palmidrol | Palmityoletanolamid...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+3 | >-7.35 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM26144 (1,3-dihydroxypropan-2-yl (5Z,8Z,11Z,14Z)-icosa-5,8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University Curated by ChEMBL | Assay Description Inhibition of Fatty-acid amide hydrolase (FAAH) activity in human lymphoma U937 cell using [3H]-AEA as substrate in the 0-25 uM conc | J Med Chem 45: 3709-20 (2002) BindingDB Entry DOI: 10.7270/Q2WD3ZXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM29080 (CHEMBL280065 | N-oleoylethanolamine | Oleamide MEA...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Tested for binding affinity to Cannabinoid receptor 1 | J Med Chem 42: 896-902 (1999) Article DOI: 10.1021/jm980461j BindingDB Entry DOI: 10.7270/Q2XG9Q9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29075 (PF-514273) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29080 (CHEMBL280065 | N-oleoylethanolamine | Oleamide MEA...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Farmaceutiche Curated by ChEMBL | Assay Description Binding affinity to human recombinant CB2 receptor expressed in COS cells | J Med Chem 49: 2333-8 (2006) Article DOI: 10.1021/jm051240y BindingDB Entry DOI: 10.7270/Q2862G15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29075 (PF-514273) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB2 receptor expressed in CHO-K1 cells after 60 mins by beta counting | ACS Med Chem Lett 3: 397-401 (2012) Article DOI: 10.1021/ml3000325 BindingDB Entry DOI: 10.7270/Q22N53BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29080 (CHEMBL280065 | N-oleoylethanolamine | Oleamide MEA...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+4 | >-6.93 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM29080 (CHEMBL280065 | N-oleoylethanolamine | Oleamide MEA...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Binding affinity towards cannabinoid receptor 1 from rat forebrain membranes in the presence of phenylmethanesulfonylfluoride (PMSF) using 0.8 nM [3H... | J Med Chem 41: 5353-61 (1999) Article DOI: 10.1021/jm970257g BindingDB Entry DOI: 10.7270/Q2ZS2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM29080 (CHEMBL280065 | N-oleoylethanolamine | Oleamide MEA...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Binding affinity towards Cannabinoid receptor 2 from mouse spleen membranes using 0.8 nM [3H]-CP-55,940 as radioligand | J Med Chem 41: 5353-61 (1999) Article DOI: 10.1021/jm970257g BindingDB Entry DOI: 10.7270/Q2ZS2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM29080 (CHEMBL280065 | N-oleoylethanolamine | Oleamide MEA...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Binding affinity towards cannabinoid receptor 1 from rat forebrain membranes in the absence of phenylmethanesulfonylfluoride (PMSF) using 0.8 nM [3H]... | J Med Chem 41: 5353-61 (1999) Article DOI: 10.1021/jm970257g BindingDB Entry DOI: 10.7270/Q2ZS2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acid ceramidase (Homo sapiens (Human)) | BDBM29080 (CHEMBL280065 | N-oleoylethanolamine | Oleamide MEA...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of acid ceramidase (unknown origin) | J Med Chem 56: 3518-30 (2013) Article DOI: 10.1021/jm301879g BindingDB Entry DOI: 10.7270/Q27D2WH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM29080 (CHEMBL280065 | N-oleoylethanolamine | Oleamide MEA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.65E+3 | n/a | n/a | n/a | n/a |
Universita del Piemonte Orientale | Assay Description The effect of the substances on Ca2+ influx was determined by using HEK-293 cells stably overexpressing recombinant human TRPV1 cDNA. The cells were ... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM29083 (Impulsin | MimyX | Palmidrol | Palmityoletanolamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Universita del Piemonte Orientale | Assay Description The effect of the substances on Ca2+ influx was determined by using HEK-293 cells stably overexpressing recombinant human TRPV1 cDNA. The cells were ... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM40144 (2-Butylsulfanyl-9H-1,3,4,9-tetraaza-fluorene | 3-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Molecular Libraries Screening Center Curated by PubChem BioAssay | Assay Description NIH Molecular Libraries Screening Centers Network [MLSCN] Emory Chemical Biology Discovery Center in MLSCN Assay provider: John A. Katzenellenbogen, ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2QJ7FQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM45519 (2-(2,4-dimethylphenyl)-N-(4-{[(5-methyl-3-isoxazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PCMD Curated by PubChem BioAssay | Assay Description Molecular Library Screening Center Network (MLSCN) Penn Center for Molecular Discovery (PCMD) Assay Provider: Scott L. Diamond, University of Pennsy... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q21G0JQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50459 (MLS000862518 | N-(4-{[(3,4-dimethyl-5-isoxazolyl)a...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 4.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Molecular Libraries Screening Center Curated by PubChem BioAssay | Assay Description NIH Molecular Libraries Screening Centers Network [MLSCN] Emory Chemical Biology Discovery Center in MLSCN Assay provider: Nikolovska-Coleska, Univer... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q23X8539 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dTDP-4-dehydrorhamnose 3,5-epimerase (Mycobacterium tuberculosis H37Rv) | BDBM51557 (2-azanylidene-N,1-dicyclopentyl-5-oxidanylidene-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 2.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PCMD Curated by PubChem BioAssay | Assay Description Molecular Library Screening Center Network (MLSCN) Penn Center for Molecular Discovery (PCMD) Assay Provider: Michael McNeil, Colorado State Universi... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2TD9VRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 55 (Homo sapiens (Human)) | BDBM50459 (MLS000862518 | N-(4-{[(3,4-dimethyl-5-isoxazolyl)a...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2348HSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Streptokinase A (Streptococcus pyogenes M1 GAS) | BDBM57190 ((6Z)-6-[4-(4-chlorophenyl)-5-methyl-1,2-dihydropyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 9.86E+3 | n/a | n/a | n/a | n/a |
Broad Institute Curated by PubChem BioAssay | Assay Description Keywords: Group A streptococcus, GAS, streptokinase, expression, virulence, inhibition, dose response, EC50 Assay Overview: The goal of this assa... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2736PBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 55 (Homo sapiens (Human)) | BDBM61031 (MLS000540201 | N,N-diallyl-4-({[([1,1'-bipheny...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2GF0RZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 55 (Homo sapiens (Human)) | BDBM61037 (2-(1,3-benzothiazol-2-ylsulfanyl)-1-[2-(trifluorom...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2GF0RZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 55 (Homo sapiens (Human)) | BDBM61041 (4-phenyl-N-[[4-(propan-2-ylsulfamoyl)anilino]-sulf...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 544 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2GF0RZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 55 (Homo sapiens (Human)) | BDBM61043 (10-[(1,3-benzothiazol-2-ylthio)acetyl]-2-chloro-10...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 840 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2GF0RZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 55 (Homo sapiens (Human)) | BDBM61054 (MLS000579482 | N-(2-methoxy-3-dibenzofuranyl)-2-(p...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 671 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2GF0RZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 55 (Homo sapiens (Human)) | BDBM61068 (2-(4-acetamidophenyl)sulfanyl-N-(2-methoxydibenzof...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 806 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2GF0RZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Streptokinase A (Streptococcus pyogenes M1 GAS) | BDBM45519 (2-(2,4-dimethylphenyl)-N-(4-{[(5-methyl-3-isoxazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a |
Broad Institute Curated by PubChem BioAssay | Assay Description Keywords: Group A streptococcus, GAS, streptokinase, expression, virulence, inhibition, dose response, EC50 Assay Overview: The goal of this assa... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2736PBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock factor protein 1 (Mus musculus) | BDBM61068 (2-(4-acetamidophenyl)sulfanyl-N-(2-methoxydibenzof...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | >1.95E+5 | n/a | n/a | n/a | n/a |
Broad Institute Curated by PubChem BioAssay | Assay Description Keywords: Heat Shock Factor-1 (HSF-1), Stress Response, MG132, NIH3T3, Luminescence Assay Overview: Modified NIH3T3, transformed to express firefly... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2MW2FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM61037 (2-(1,3-benzothiazol-2-ylsulfanyl)-1-[2-(trifluorom...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics Source Affiliation: Sanford-Burnham Medical Research Institute Network: NIH Molecular Lib... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2K072QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM61031 (MLS000540201 | N,N-diallyl-4-({[([1,1'-bipheny...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics Source Affiliation: Sanford-Burnham Medical Research Institute Network: NIH Molecular Lib... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2K072QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM61068 (2-(4-acetamidophenyl)sulfanyl-N-(2-methoxydibenzof...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics Source Affiliation: Sanford-Burnham Medical Research Institute Network: NIH Molecular Lib... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2K072QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 132 total ) | Next | Last >> |