

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Penicillin-binding protein 1B (Pseudomonas aeruginosa) | BDBM50378857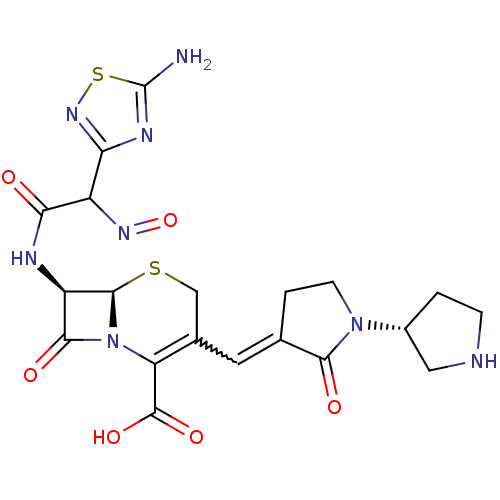 (CEFTOBIPROLE | Zeftera | Zevtera) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 935 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of bocillin FL binding to Pseudomonas aeruginosa PAO1 penicillin-binding protein 1b | Antimicrob Agents Chemother 51: 2621-4 (2007) Article DOI: 10.1128/aac.00029-07 BindingDB Entry DOI: 10.7270/Q2VH5RN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillin-binding protein 1B (Pseudomonas aeruginosa) | BDBM50213266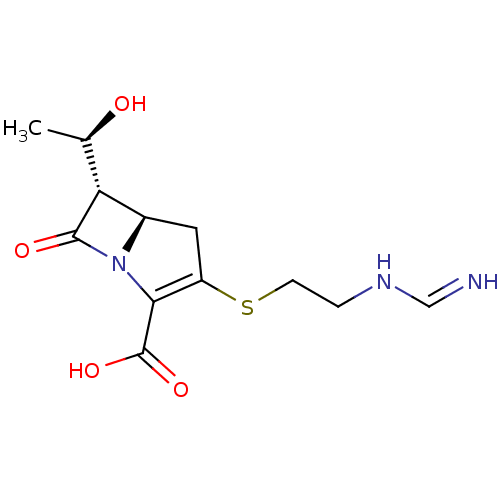 (CHEBI:471744 | Imipenem | MK-0787) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of bocillin FL binding to Pseudomonas aeruginosa PAO1 penicillin-binding protein 1b | Antimicrob Agents Chemother 51: 2621-4 (2007) Article DOI: 10.1128/aac.00029-07 BindingDB Entry DOI: 10.7270/Q2VH5RN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillin-binding protein 1B (Pseudomonas aeruginosa) | BDBM50350470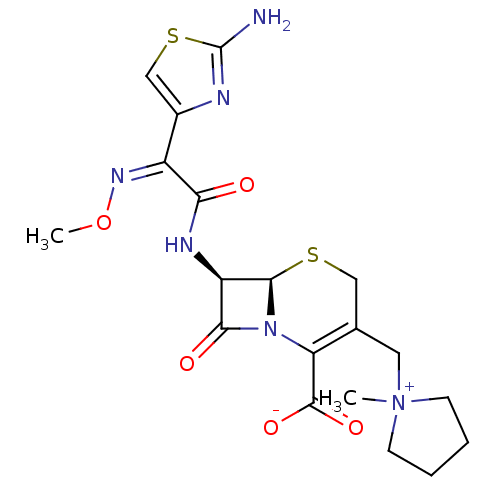 (CEFEPIME | Maxipime) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of bocillin FL binding to Pseudomonas aeruginosa PAO1 penicillin-binding protein 1b | Antimicrob Agents Chemother 51: 2621-4 (2007) Article DOI: 10.1128/aac.00029-07 BindingDB Entry DOI: 10.7270/Q2VH5RN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillin-binding protein 1B (Pseudomonas aeruginosa) | BDBM50240480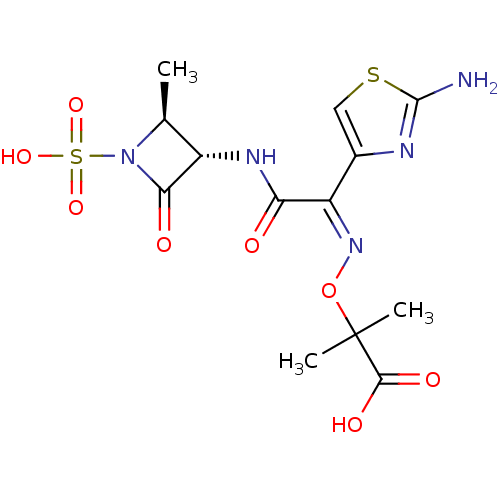 ((2S,3S)-3-({(2Z)-2-(2-ammonio-1,3-thiazol-4-yl)-2-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of bocillin FL binding to Pseudomonas aeruginosa PAO1 penicillin-binding protein 1b | Antimicrob Agents Chemother 51: 2621-4 (2007) Article DOI: 10.1128/aac.00029-07 BindingDB Entry DOI: 10.7270/Q2VH5RN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillin-binding protein 1B (Pseudomonas aeruginosa) | BDBM50420259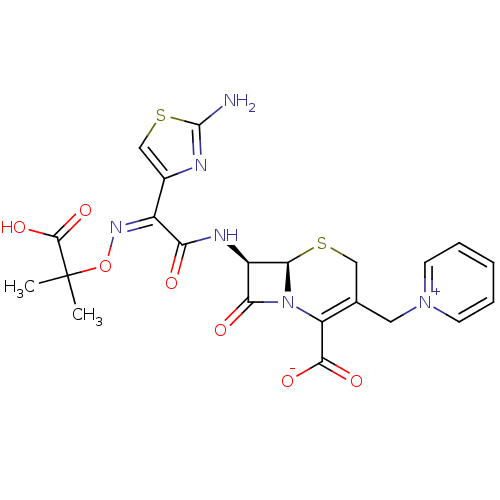 (CEFTAZIDIME) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of bocillin FL binding to Pseudomonas aeruginosa PAO1 penicillin-binding protein 1b | Antimicrob Agents Chemother 51: 2621-4 (2007) Article DOI: 10.1128/aac.00029-07 BindingDB Entry DOI: 10.7270/Q2VH5RN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillin-binding protein 1B (Acinetobacter baumannii) | BDBM50458507 (CHEMBL4203272) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of Acinetobacter baumannii PBP1b transglycosylase activity using NBD-lipid 2 as substrate after 2 hrs by muramidase enzyme coupled HPLC an... | Eur J Med Chem 150: 729-741 (2018) Article DOI: 10.1016/j.ejmech.2018.03.034 BindingDB Entry DOI: 10.7270/Q27M0BJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillin-binding protein 1B (Acinetobacter baumannii) | BDBM50458508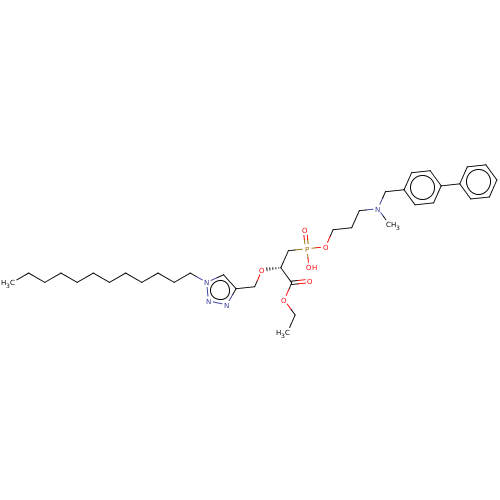 (CHEMBL4211545) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.53E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of Acinetobacter baumannii PBP1b transglycosylase activity using NBD-lipid 2 as substrate after 2 hrs by muramidase enzyme coupled HPLC an... | Eur J Med Chem 150: 729-741 (2018) Article DOI: 10.1016/j.ejmech.2018.03.034 BindingDB Entry DOI: 10.7270/Q27M0BJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillin-binding protein 1B (Acinetobacter baumannii) | BDBM50458509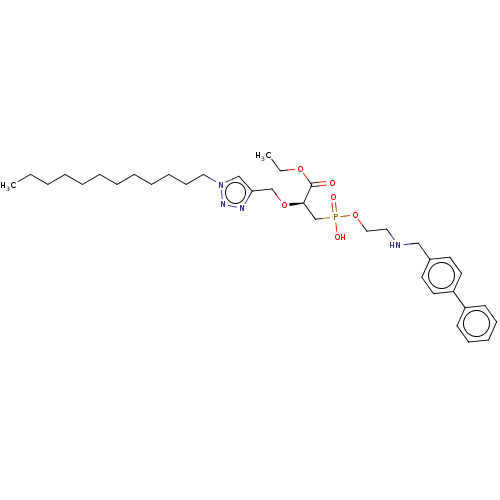 (CHEMBL4208140) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.57E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of Acinetobacter baumannii PBP1b transglycosylase activity using NBD-lipid 2 as substrate after 2 hrs by muramidase enzyme coupled HPLC an... | Eur J Med Chem 150: 729-741 (2018) Article DOI: 10.1016/j.ejmech.2018.03.034 BindingDB Entry DOI: 10.7270/Q27M0BJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillin-binding protein 1B (Acinetobacter baumannii) | BDBM50458510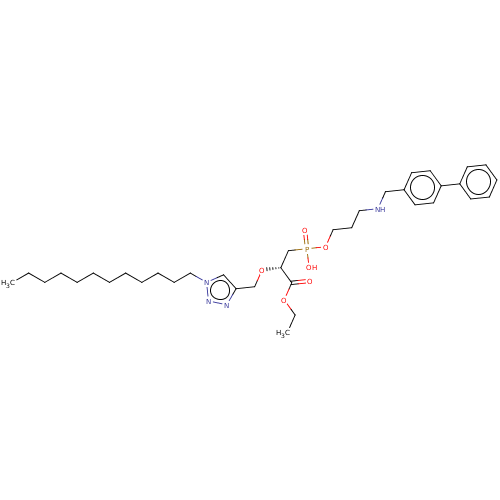 (CHEMBL4211590) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.32E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of Acinetobacter baumannii PBP1b transglycosylase activity using NBD-lipid 2 as substrate after 2 hrs by muramidase enzyme coupled HPLC an... | Eur J Med Chem 150: 729-741 (2018) Article DOI: 10.1016/j.ejmech.2018.03.034 BindingDB Entry DOI: 10.7270/Q27M0BJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillin-binding protein 1B (Escherichia coli (strain K12)) | BDBM50053179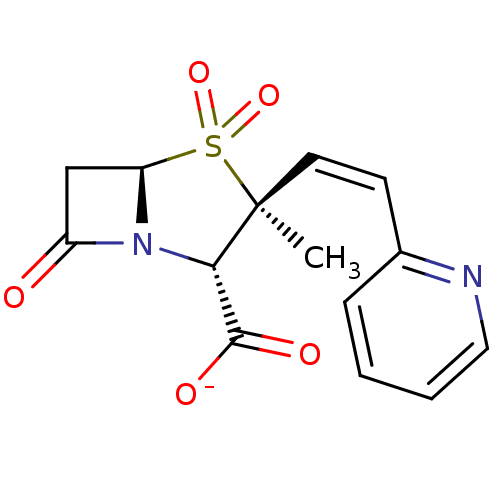 (CHEMBL121924 | Sodium; (2S,3S,5R)-3-methyl-4,4,7-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Penicillin-binding protein 1b from Escherichia coli. | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillin-binding protein 1B (Escherichia coli (strain K12)) | BDBM50053175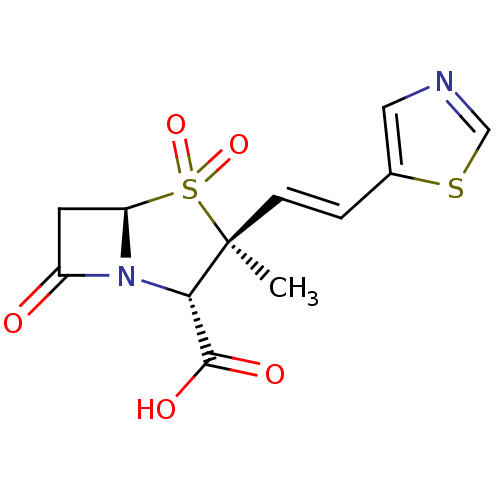 ((2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-((E)-2-thiazol-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Penicillin-binding protein 1b from Escherichia coli. | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillin-binding protein 1B (Escherichia coli (strain K12)) | BDBM50053180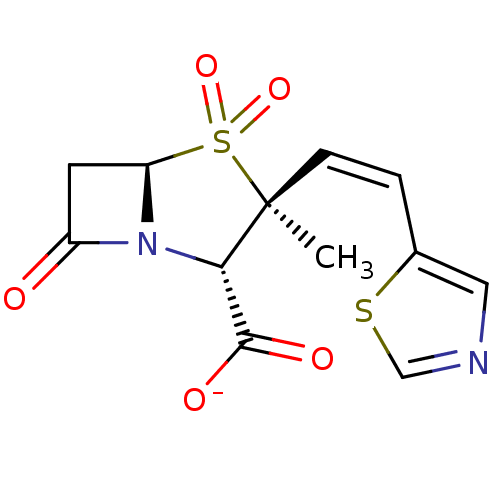 (CHEMBL332565 | Sodium; (2S,3S,5R)-3-methyl-4,4,7-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Penicillin-binding protein 1b from Escherichia coli. | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillin-binding protein 1B (Escherichia coli (strain K12)) | BDBM50053183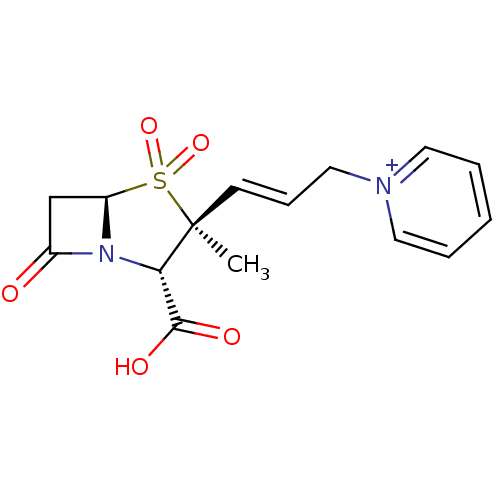 (CHEMBL123481 | Trifluoro-methanesulfonate1-[(E)-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Penicillin-binding protein 1b from Escherichia coli. | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||