

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin Z (Homo sapiens (Human)) | BDBM50255753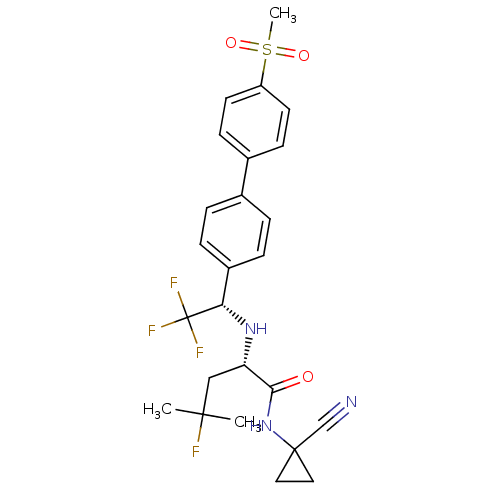 (CHEMBL481611 | MK-0822 | Odanacatib) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of cathepsin Z | Bioorg Med Chem Lett 18: 923-8 (2008) Article DOI: 10.1016/j.bmcl.2007.12.047 BindingDB Entry DOI: 10.7270/Q21J9BM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin Z (Homo sapiens (Human)) | BDBM50303441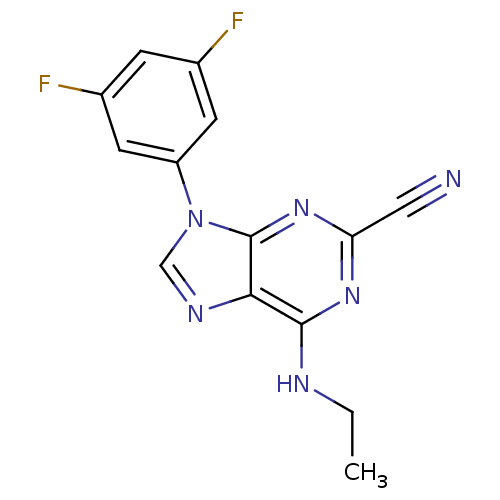 (9-(3,5-Difluorophenyl)-6-(ethylamino)-9H-purine-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Inhibition of human Cathepsin X/Z | J Med Chem 53: 52-60 (2010) Article DOI: 10.1021/jm901069a BindingDB Entry DOI: 10.7270/Q2TX3FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin Z (Homo sapiens (Human)) | BDBM50303420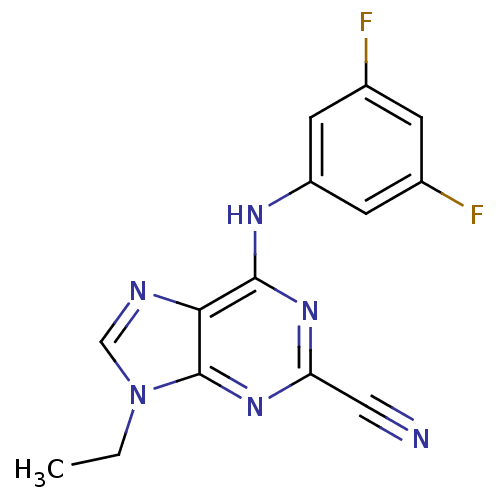 (6-(3,5-Difluorophenylamino)-9-ethyl-9H-purine-2-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 933 | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Inhibition of human Cathepsin X/Z | J Med Chem 53: 52-60 (2010) Article DOI: 10.1021/jm901069a BindingDB Entry DOI: 10.7270/Q2TX3FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin Z (Rattus norvegicus) | BDBM36325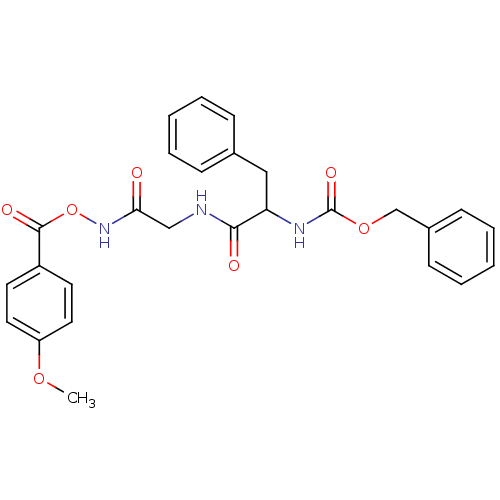 (Cathepsin Inhibitor III) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Stanford University | Assay Description Protease enzyme inhibition assay targeting diverse member of the cysteine protease families:cathepsin B, Z, and H | Nat Chem Biol 1: 33-8 (2005) Article DOI: 10.1038/nchembio707 BindingDB Entry DOI: 10.7270/Q22V2DGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin Z (Homo sapiens (Human)) | BDBM50303417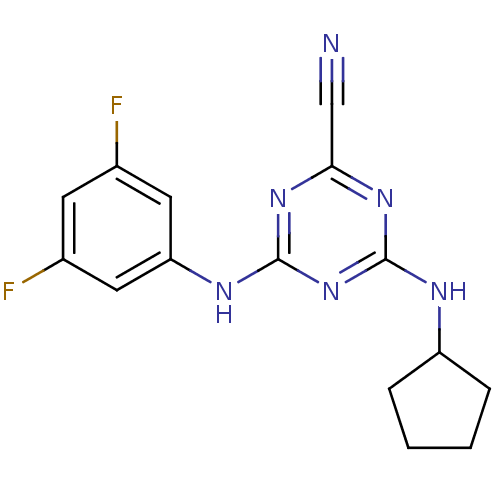 (4-(Cyclopentylamino)-6-(3,5-difluorophenylamino)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Inhibition of human Cathepsin X/Z | J Med Chem 53: 52-60 (2010) Article DOI: 10.1021/jm901069a BindingDB Entry DOI: 10.7270/Q2TX3FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin Z (Rattus norvegicus) | BDBM36331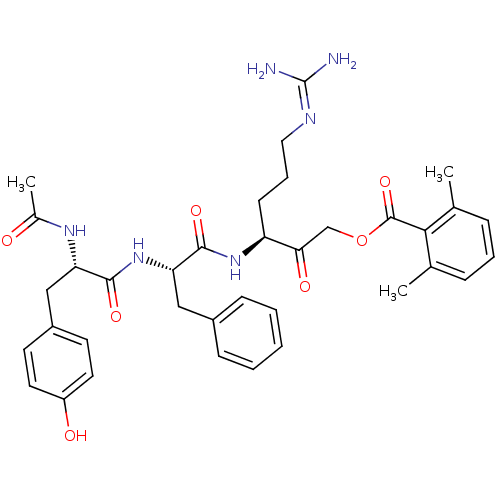 (Ac-YFR-AMOK 10b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Stanford University | Assay Description Protease enzyme inhibition assay targeting diverse member of the cysteine protease families:cathepsin B, Z, and H | Nat Chem Biol 1: 33-8 (2005) Article DOI: 10.1038/nchembio707 BindingDB Entry DOI: 10.7270/Q22V2DGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin Z (Homo sapiens (Human)) | BDBM50303410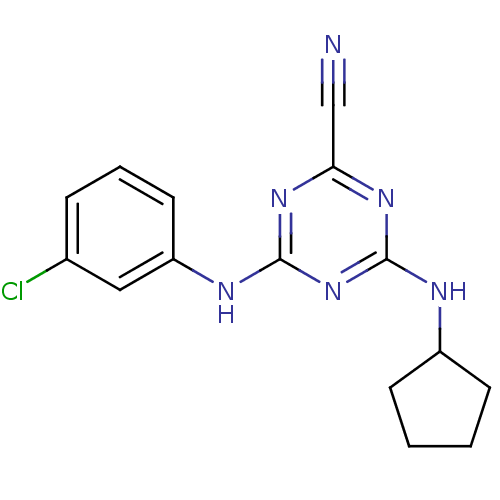 (4-(3-chlorophenylamino)-6-(cyclopentylamino)-1,3,5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Inhibition of human Cathepsin X/Z | J Med Chem 53: 52-60 (2010) Article DOI: 10.1021/jm901069a BindingDB Entry DOI: 10.7270/Q2TX3FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin Z (Homo sapiens (Human)) | BDBM50551801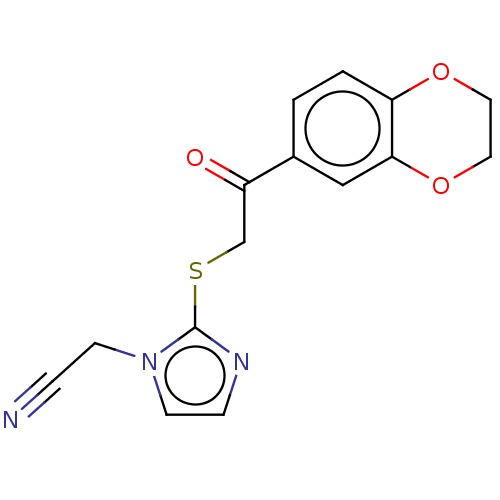 (CHEMBL4754916) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human cathepsin X expressed in Pichia pastoris assessed as residual activity using Abz-FEK(Dnp)-OH as substrate incubated for 30 to 45 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112218 BindingDB Entry DOI: 10.7270/Q2HQ43JF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin Z (Homo sapiens (Human)) | BDBM50551800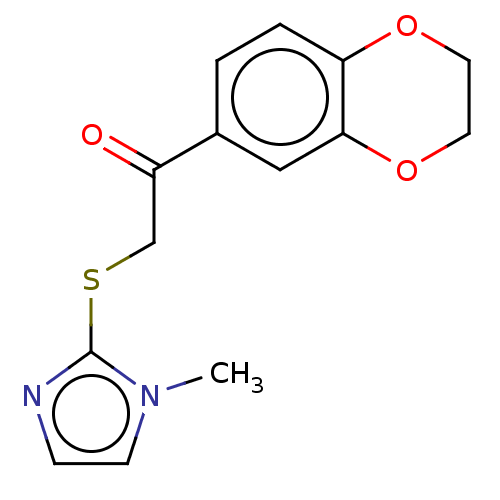 (CHEMBL4743093) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human cathepsin X expressed in Pichia pastoris assessed as residual activity using Abz-FEK(Dnp)-OH as substrate incubated for 30 to 45 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112218 BindingDB Entry DOI: 10.7270/Q2HQ43JF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin Z (Homo sapiens (Human)) | BDBM50302107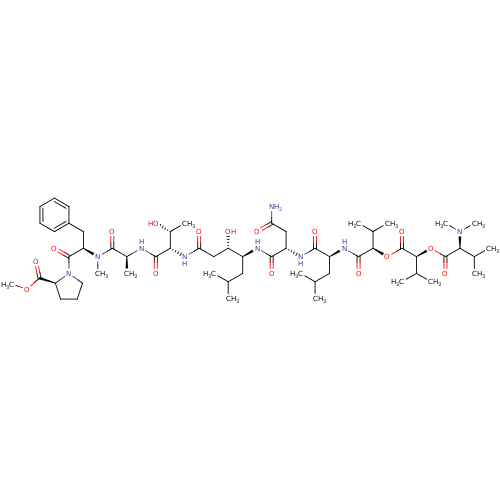 (CHEMBL567893 | Grassystatin A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of cathepsin Z after 10 to 15 mins by fluorescence assay | J Med Chem 52: 5732-47 (2009) Article DOI: 10.1021/jm9009394 BindingDB Entry DOI: 10.7270/Q2BG2PXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin Z (Homo sapiens (Human)) | BDBM397464 (US10676470, Compound 43 | US10730826, Compound 73 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin Z (Homo sapiens (Human)) | BDBM397464 (US10676470, Compound 43 | US10730826, Compound 73 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin Z (Homo sapiens (Human)) | BDBM453332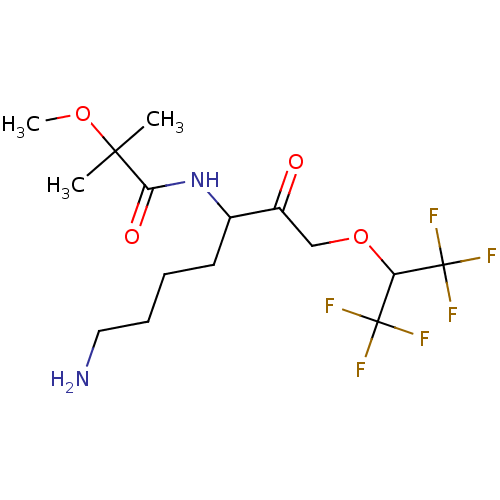 (BDBM553835 | US10730826, Compound 69a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin Z (Homo sapiens (Human)) | BDBM453332 (BDBM553835 | US10730826, Compound 69a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin Z (Homo sapiens (Human)) | BDBM453275 (BDBM453327 | US10730826, Compound 1a-non-racemic |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin Z (Homo sapiens (Human)) | BDBM453275 (BDBM453327 | US10730826, Compound 1a-non-racemic |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin Z (Homo sapiens (Human)) | BDBM50551796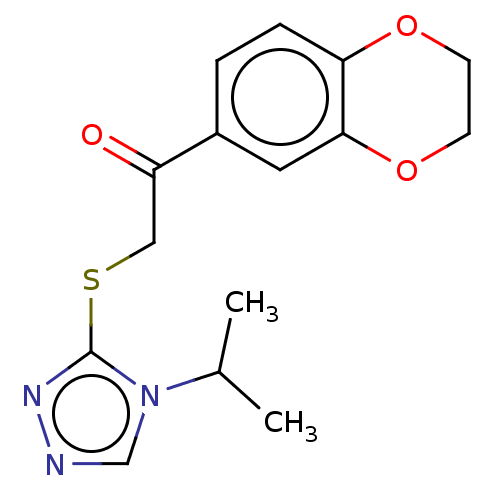 (CHEMBL4798763) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human cathepsin X expressed in Pichia pastoris assessed as residual activity using Abz-FEK(Dnp)-OH as substrate incubated for 30 to 45 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112218 BindingDB Entry DOI: 10.7270/Q2HQ43JF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin Z (Homo sapiens (Human)) | BDBM50551797 (CHEMBL1331625) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human cathepsin X expressed in Pichia pastoris assessed as residual activity using Abz-FEK(Dnp)-OH as substrate incubated for 30 to 45 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112218 BindingDB Entry DOI: 10.7270/Q2HQ43JF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin Z (Homo sapiens (Human)) | BDBM50551798 (CHEMBL4752735) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human cathepsin X expressed in Pichia pastoris assessed as residual activity using Abz-FEK(Dnp)-OH as substrate incubated for 30 to 45 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112218 BindingDB Entry DOI: 10.7270/Q2HQ43JF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin Z (Homo sapiens (Human)) | BDBM50551799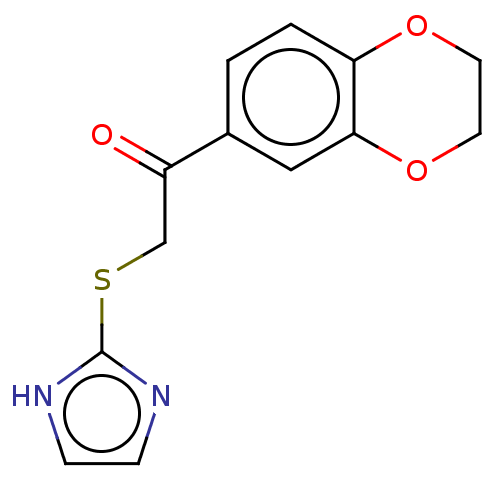 (CHEMBL4744124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human cathepsin X expressed in Pichia pastoris assessed as residual activity using Abz-FEK(Dnp)-OH as substrate incubated for 30 to 45 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112218 BindingDB Entry DOI: 10.7270/Q2HQ43JF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin Z (Homo sapiens (Human)) | BDBM50303436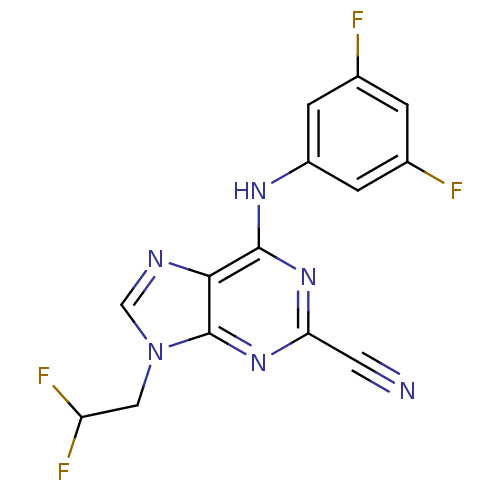 (9-(2,2-difluoroethyl)-6-(3,5-difluorophenylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Inhibition of human Cathepsin X/Z | J Med Chem 53: 52-60 (2010) Article DOI: 10.1021/jm901069a BindingDB Entry DOI: 10.7270/Q2TX3FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin Z (Homo sapiens (Human)) | BDBM50195235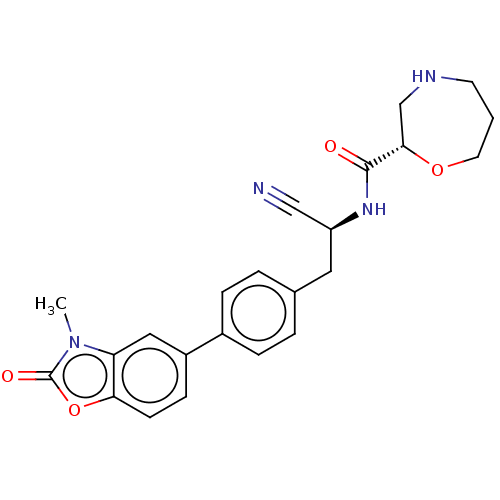 (CHEMBL3900409 | US10287258, Example 2 | US10669245...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CHARLES RIVER DISCOVERY RESEARCH SERVICES UK LIMITED Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin Z | J Med Chem 59: 9457-9472 (2016) Article DOI: 10.1021/acs.jmedchem.6b01127 BindingDB Entry DOI: 10.7270/Q2Z321K4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin Z (Rattus norvegicus) | BDBM36330 (AC-YFG-AMOK 10a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Stanford University | Assay Description Protease enzyme inhibition assay targeting diverse member of the cysteine protease families:cathepsin B, Z, and H | Nat Chem Biol 1: 33-8 (2005) Article DOI: 10.1038/nchembio707 BindingDB Entry DOI: 10.7270/Q22V2DGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||