

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50541929 (CHEMBL1738785) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Ostrinia furnacalis) | BDBM50514507 (CHEMBL1583158) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of Ostrinia furnacalis chitinase h catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by flu... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50554340 (CHEMBL4788866) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged human recombinant CHIT1 catalytic domain (1 to 386 residues) expressed in HEK293F cells assessed as r... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endochitinase B1 (Aspergillus fumigatus) | BDBM50173286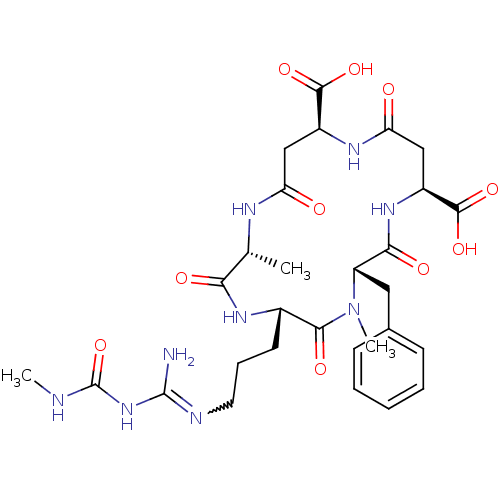 (5-[3-[amino-(methylcarbamoylamino)methylidene]amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhihitory activity against Chitinase B1 (AfChiB1) using fuorometric assay with 4-methylumbelliferyl-b-D-N,N0-diacetylchitobiose as substrate | Bioorg Med Chem Lett 15: 4717-21 (2005) Article DOI: 10.1016/j.bmcl.2005.07.068 BindingDB Entry DOI: 10.7270/Q2MS3S9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM10854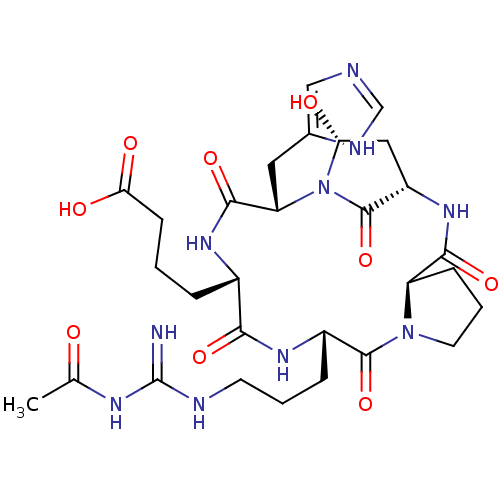 (4-[(1S,4R,10S,13S,16S,18R)-10-{3-[(acetamidomethan...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem | Article PubMed | 20 | -45.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
University of Dundee | Assay Description The IC50s of inhibitor against the human chitinase were determined using the fluorogenic substrate 4MU-NAG3. The fluorescence of the liberated 4MU wa... | Chem Biol 12: 65-76 (2005) Article DOI: 10.1016/j.chembiol.2004.10.013 BindingDB Entry DOI: 10.7270/Q23F4MV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Mus musculus) | BDBM50554340 (CHEMBL4788866) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full-length C-terminal his-tagged mouse CHIT1 expressed in CHO-K1 cells assessed as reduction in chitinolytic activity usin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Mus musculus) | BDBM50514508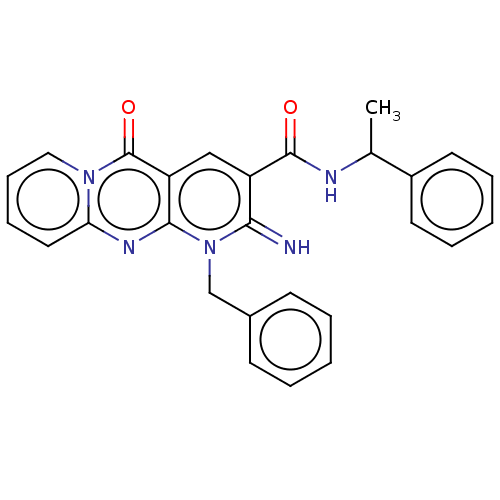 (CHEMBL4549449) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of mouse CHIT1 catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fluorescence based micr... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50514508 (CHEMBL4549449) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of human CHIT1 catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fluorescence based micr... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50514507 (CHEMBL1583158) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of Serratia marcescens chitinase b catalytic domain overexpressed in Escherichia coli BL21(DE3) cells using 4MU-(GlcNAc)2 as substrate aft... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Onchocerca volvulus) | BDBM50022832 (Rafoxanide) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Onchocerca volvulus L3 larvae chitinase using 20 uM 4-methylumbelliferyl-N,N',N''-beta-chitotrioside as substrate after 10 ... | J Med Chem 57: 5792-9 (2014) Article DOI: 10.1021/jm5006435 BindingDB Entry DOI: 10.7270/Q23B61Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50587859 (CHEMBL5180998) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2021.116143 BindingDB Entry DOI: 10.7270/Q28G8QN5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50587858 (9-O-(Benzoyl)Berberrubine Chloride | CHEMBL1223325) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2021.116143 BindingDB Entry DOI: 10.7270/Q28G8QN5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50587857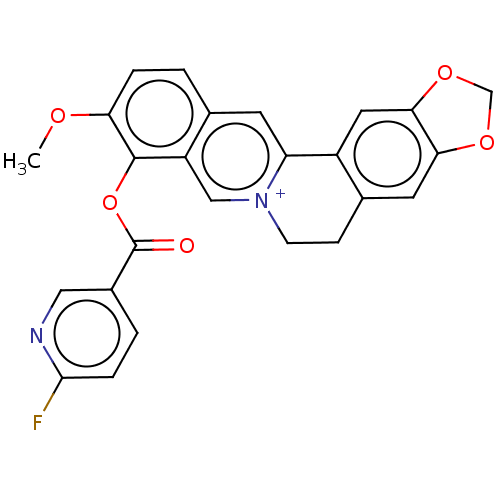 (CHEMBL5199149) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2021.116143 BindingDB Entry DOI: 10.7270/Q28G8QN5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Hypocrea rufa) | BDBM50254171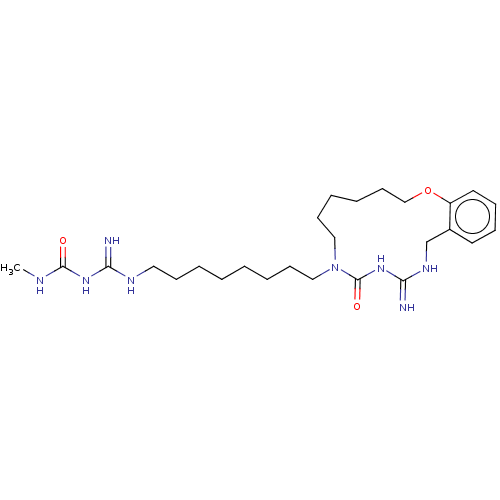 (CHEMBL4069120) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Biotechnology, Chemistry and Pharmacy, University of Siena, I-53100 Siena, Italy. Curated by ChEMBL | Assay Description Inhibition of Trichoderma viride chitinase incubated for 10 mins to 3 hrs using 4-nitrophenyl N-acetyl-beta-D-glucosaminide substrate by spectrophoto... | Bioorg Med Chem Lett 27: 3332-3336 (2017) Article DOI: 10.1016/j.bmcl.2017.06.016 BindingDB Entry DOI: 10.7270/Q2HT2RSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50243745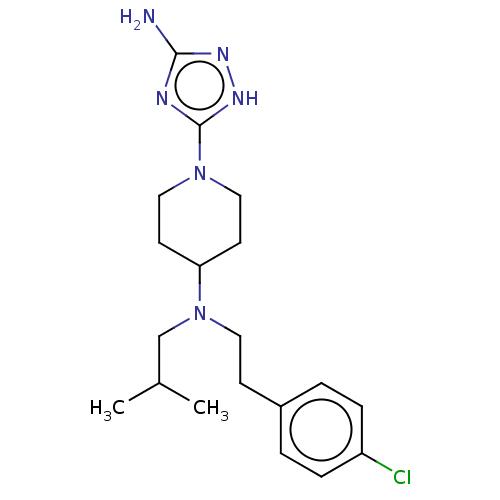 (CHEMBL4076989) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 312 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of human recombinant full length C-terminal His-tagged chitotriosidase expressed in CHO-K1 cells using 4-methylumbelliferyl-beta-D-N,N',N\... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50587857 (CHEMBL5199149) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2021.116143 BindingDB Entry DOI: 10.7270/Q28G8QN5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Ostrinia furnacalis) | BDBM50514508 (CHEMBL4549449) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of Ostrinia furnacalis chitinase h catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by flu... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50514508 (CHEMBL4549449) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of Serratia marcescens chitinase b catalytic domain overexpressed in Escherichia coli BL21(DE3) cells using 4MU-(GlcNAc)2 as substrate aft... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Onchocerca volvulus) | BDBM50063753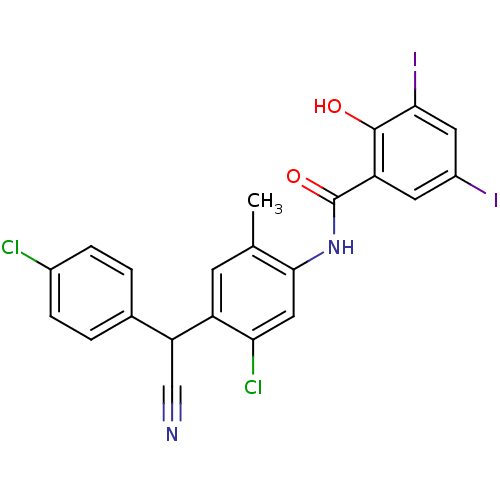 (CHEMBL12131 | Closantel | N-(5-chloro-4-((4-chloro...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 468 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Skaggs Institute for Chemical Biology Curated by ChEMBL | Assay Description Inhibition of Onchocerca volvulus L3 larvae chitinase using 4-methylumbelliferyl-N,N',N''-beta-chitotrioside as a profluorescent substrate after 10 m... | J Med Chem 54: 3963-72 (2011) Article DOI: 10.1021/jm200364n BindingDB Entry DOI: 10.7270/Q21V5FB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Onchocerca volvulus) | BDBM50063753 (CHEMBL12131 | Closantel | N-(5-chloro-4-((4-chloro...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Onchocerca volvulus L3 larvae chitinase using 20 uM 4-methylumbelliferyl-N,N',N''-beta-chitotrioside as substrate after 10 ... | J Med Chem 57: 5792-9 (2014) Article DOI: 10.1021/jm5006435 BindingDB Entry DOI: 10.7270/Q23B61Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Onchocerca volvulus) | BDBM50498224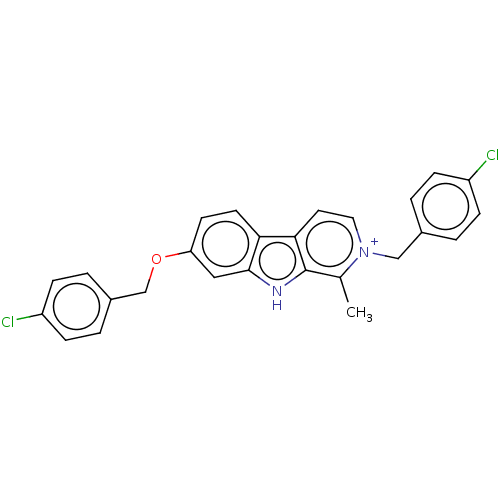 (CHEMBL3577757) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of L3 larval stage of Onchocerca volvulus chitinase using 4-methylumbelliferyl-N-N'-N''-beta-chitotrioside as substrate assess... | ACS Med Chem Lett 6: 339-43 (2015) Article DOI: 10.1021/ml500516r BindingDB Entry DOI: 10.7270/Q2C53PTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50514507 (CHEMBL1583158) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of human CHIT1 catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fluorescence based micr... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Mus musculus) | BDBM50514507 (CHEMBL1583158) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of mouse CHIT1 catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fluorescence based micr... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Mus musculus) | BDBM50243745 (CHEMBL4076989) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of mouse recombinant full length C-terminal His-tagged chitotriosidase expressed in CHO-K1 cells using 4-methylumbelliferyl-beta-D-N,N',N\... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Onchocerca volvulus) | BDBM100152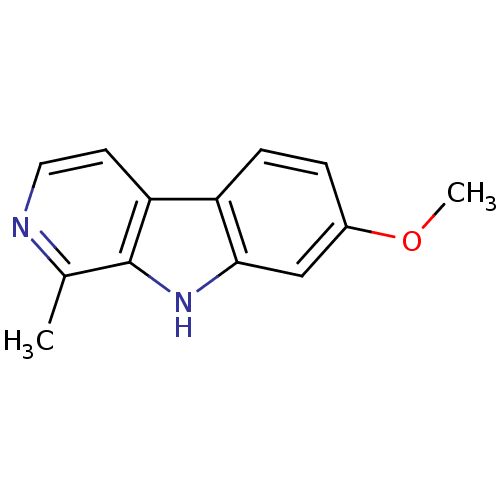 (7-methoxy-1-methyl-9H-beta-carboline;hydrochloride...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 3.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of L3 larval stage of Onchocerca volvulus chitinase using 4-methylumbelliferyl-N-N'-N''-beta-chitotrioside as substrate assess... | ACS Med Chem Lett 6: 339-43 (2015) Article DOI: 10.1021/ml500516r BindingDB Entry DOI: 10.7270/Q2C53PTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | CHEMBL5265903 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | CHEMBL5280316 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | CHEMBL5272737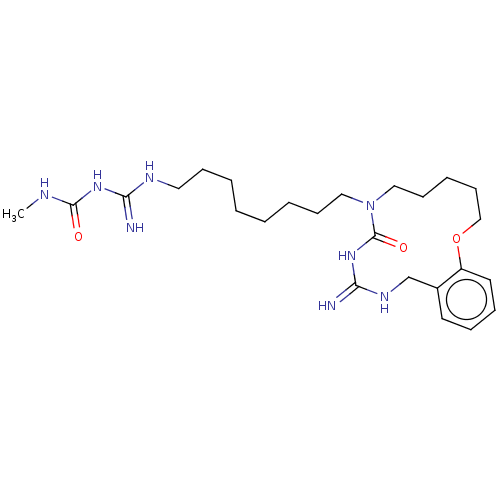 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | CHEMBL5288210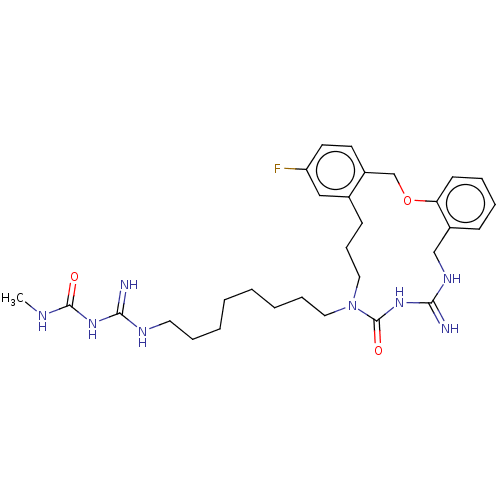 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | CHEMBL5269695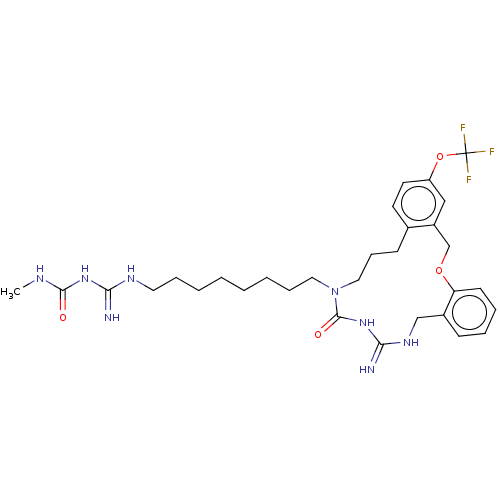 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | CHEMBL5287414 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | CHEMBL5289877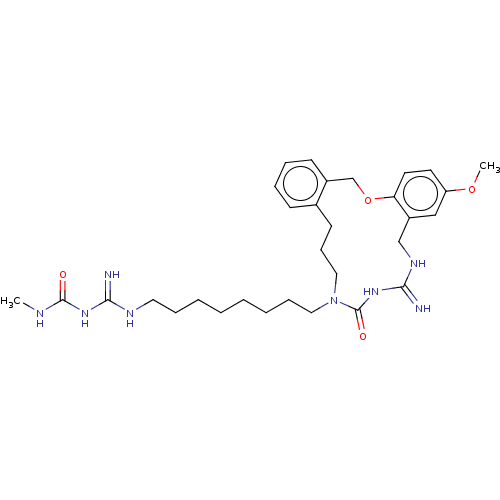 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | CHEMBL5279325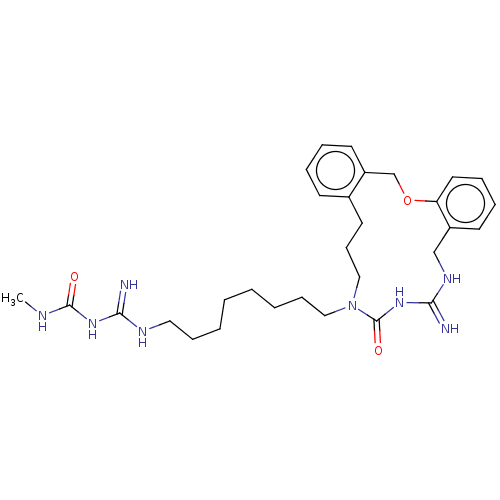 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50254171 (CHEMBL4069120) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | CHEMBL5270383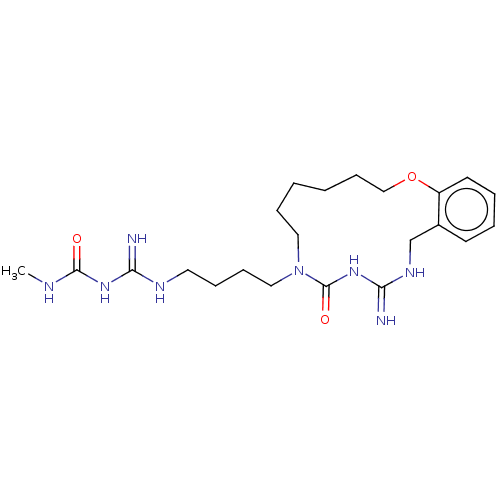 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | CHEMBL5268683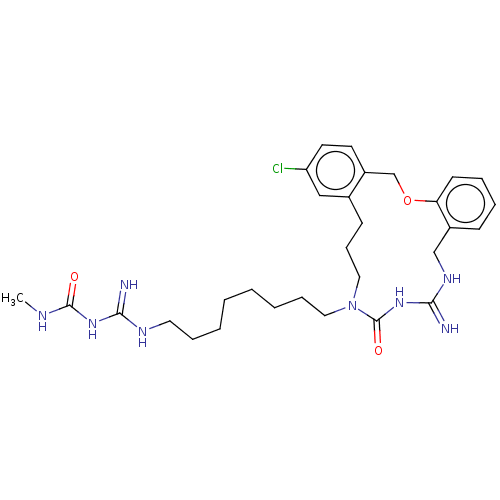 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | CHEMBL5280623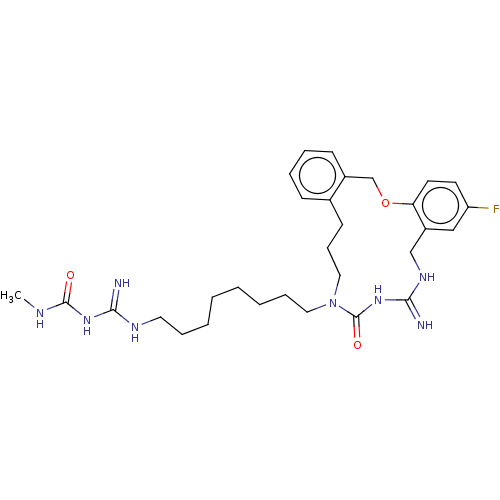 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | CHEMBL5270387 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Hypocrea rufa) | BDBM50254176 (CHEMBL3040884) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Biotechnology, Chemistry and Pharmacy, University of Siena, I-53100 Siena, Italy. Curated by ChEMBL | Assay Description Inhibition of Trichoderma viride chitinase incubated for 10 mins to 3 hrs using 4-nitrophenyl N-acetyl-beta-D-glucosaminide substrate by spectrophoto... | Bioorg Med Chem Lett 27: 3332-3336 (2017) Article DOI: 10.1016/j.bmcl.2017.06.016 BindingDB Entry DOI: 10.7270/Q2HT2RSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | CHEMBL5272989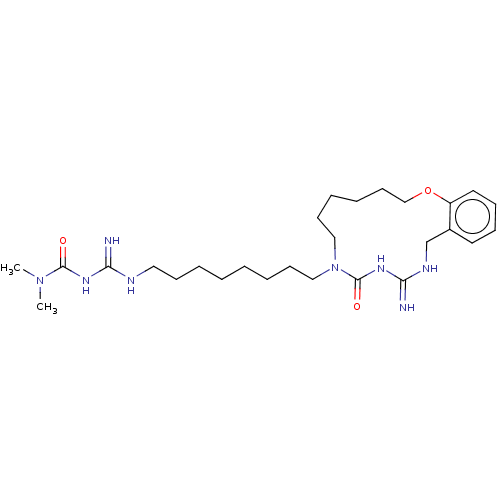 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | 1.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Hypocrea rufa) | BDBM50254177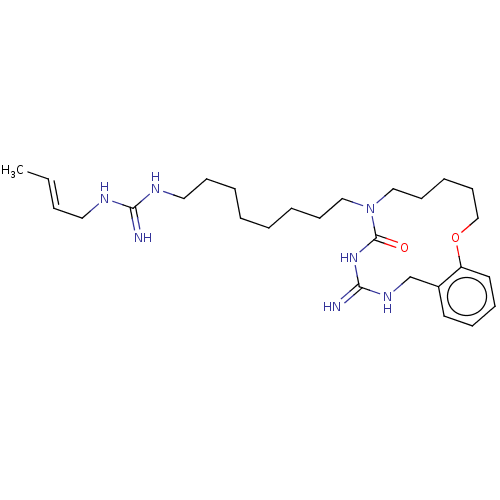 (CHEMBL3040820) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Biotechnology, Chemistry and Pharmacy, University of Siena, I-53100 Siena, Italy. Curated by ChEMBL | Assay Description Inhibition of Trichoderma viride chitinase incubated for 10 mins to 3 hrs using 4-nitrophenyl N-acetyl-beta-D-glucosaminide substrate by spectrophoto... | Bioorg Med Chem Lett 27: 3332-3336 (2017) Article DOI: 10.1016/j.bmcl.2017.06.016 BindingDB Entry DOI: 10.7270/Q2HT2RSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Hypocrea rufa) | BDBM50254178 (CHEMBL3558827) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Biotechnology, Chemistry and Pharmacy, University of Siena, I-53100 Siena, Italy. Curated by ChEMBL | Assay Description Inhibition of Trichoderma viride chitinase incubated for 10 mins to 3 hrs using 4-nitrophenyl N-acetyl-beta-D-glucosaminide substrate by spectrophoto... | Bioorg Med Chem Lett 27: 3332-3336 (2017) Article DOI: 10.1016/j.bmcl.2017.06.016 BindingDB Entry DOI: 10.7270/Q2HT2RSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | CHEMBL5290508 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | 3.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM10853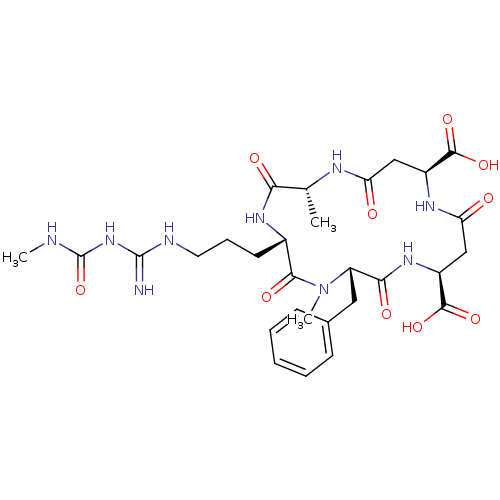 ((2R,5S,8S,11S,15S)-8-benzyl-2,7-dimethyl-5-[3-({[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 3.30E+4 | -26.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
University of Dundee | Assay Description The IC50s of inhibitor against the human chitinase were determined using the fluorogenic substrate 4MU-NAG3. The fluorescence of the liberated 4MU wa... | Chem Biol 12: 65-76 (2005) Article DOI: 10.1016/j.chembiol.2004.10.013 BindingDB Entry DOI: 10.7270/Q23F4MV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Hypocrea rufa) | BDBM50254180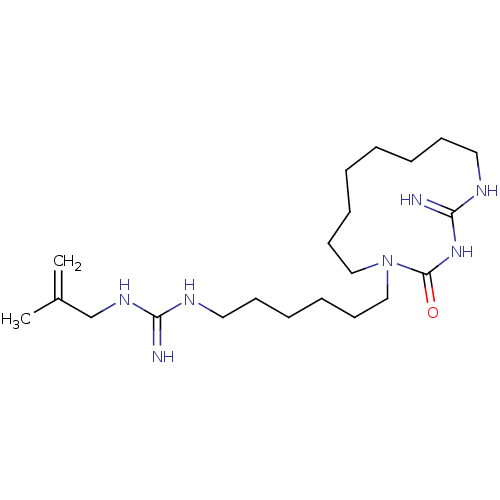 (CHEMBL3558806) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Biotechnology, Chemistry and Pharmacy, University of Siena, I-53100 Siena, Italy. Curated by ChEMBL | Assay Description Inhibition of Trichoderma viride chitinase incubated for 10 mins to 3 hrs using 4-nitrophenyl N-acetyl-beta-D-glucosaminide substrate by spectrophoto... | Bioorg Med Chem Lett 27: 3332-3336 (2017) Article DOI: 10.1016/j.bmcl.2017.06.016 BindingDB Entry DOI: 10.7270/Q2HT2RSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Hypocrea rufa) | BDBM50254181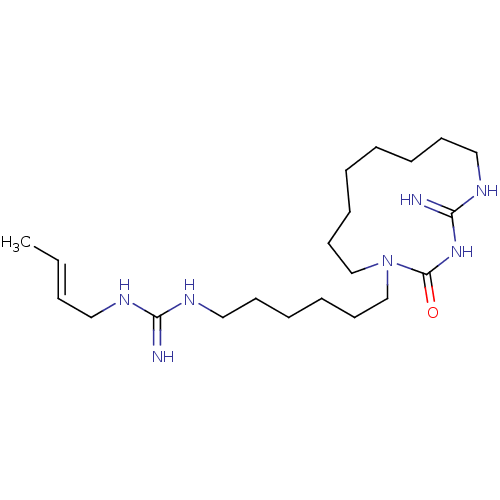 (CHEMBL3558803) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Biotechnology, Chemistry and Pharmacy, University of Siena, I-53100 Siena, Italy. Curated by ChEMBL | Assay Description Inhibition of Trichoderma viride chitinase incubated for 10 mins to 3 hrs using 4-nitrophenyl N-acetyl-beta-D-glucosaminide substrate by spectrophoto... | Bioorg Med Chem Lett 27: 3332-3336 (2017) Article DOI: 10.1016/j.bmcl.2017.06.016 BindingDB Entry DOI: 10.7270/Q2HT2RSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endochitinase B1 (Aspergillus fumigatus) | BDBM10850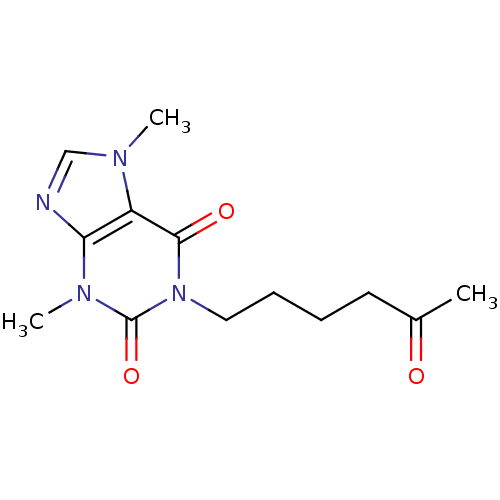 (1-(5-Oxohexyl)theobromine (pentoxifylline) | 3,7-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3.70E+4 | -26.3 | 1.26E+5 | n/a | n/a | n/a | n/a | 5.5 | 37 |
University of Dundee | Assay Description The IC50s of inhibitor against the human chitinase were determined using the fluorogenic substrate 4MU-NAG3. The fluorescence of the liberated 4MU wa... | Chem Biol 12: 973-80 (2005) Article DOI: 10.1016/j.chembiol.2005.07.009 BindingDB Entry DOI: 10.7270/Q2765CJG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Chitinase (Hypocrea rufa) | BDBM50254182 (CHEMBL3040830) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Biotechnology, Chemistry and Pharmacy, University of Siena, I-53100 Siena, Italy. Curated by ChEMBL | Assay Description Inhibition of Trichoderma viride chitinase incubated for 10 mins to 3 hrs using 4-nitrophenyl N-acetyl-beta-D-glucosaminide substrate by spectrophoto... | Bioorg Med Chem Lett 27: 3332-3336 (2017) Article DOI: 10.1016/j.bmcl.2017.06.016 BindingDB Entry DOI: 10.7270/Q2HT2RSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Hypocrea rufa) | BDBM50254179 (CHEMBL3558802) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Biotechnology, Chemistry and Pharmacy, University of Siena, I-53100 Siena, Italy. Curated by ChEMBL | Assay Description Inhibition of Trichoderma viride chitinase incubated for 10 mins to 3 hrs using 4-nitrophenyl N-acetyl-beta-D-glucosaminide substrate by spectrophoto... | Bioorg Med Chem Lett 27: 3332-3336 (2017) Article DOI: 10.1016/j.bmcl.2017.06.016 BindingDB Entry DOI: 10.7270/Q2HT2RSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | CHEMBL5268303 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 970 total ) | Next | Last >> |