

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM227598 (Penem 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | n/a | 30 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Case Western Reserve University | Assay Description Steady-state kinetics were performed on an Agilent 8453 diode array spectrophotometer (Palo Alto, CA) in 50 mM sodium phosphate buffer (pH 7.2) suppl... | J Biol Chem 289: 6152-64 (2014) Article DOI: 10.1074/jbc.M113.533562 BindingDB Entry DOI: 10.7270/Q2CN72SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM227599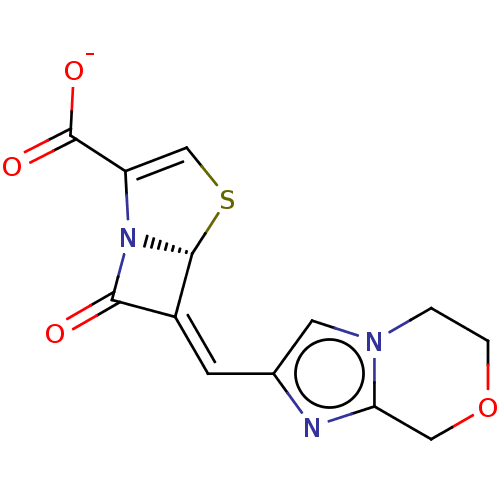 (Penem 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 380 | n/a | 60 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Case Western Reserve University | Assay Description Steady-state kinetics were performed on an Agilent 8453 diode array spectrophotometer (Palo Alto, CA) in 50 mM sodium phosphate buffer (pH 7.2) suppl... | J Biol Chem 289: 6152-64 (2014) Article DOI: 10.1074/jbc.M113.533562 BindingDB Entry DOI: 10.7270/Q2CN72SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-10 (Pseudomonas aeruginosa) | BDBM50293713 (CHEMBL553476 | Tribenzyl 2-(2-phenoxyacetamido)ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Competitive inhibition of Pseudomonas aeruginosa OXA10 beta-lactamase at pH 7 | Bioorg Med Chem Lett 19: 3593-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.149 BindingDB Entry DOI: 10.7270/Q2W9597J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-10 (Pseudomonas aeruginosa) | BDBM50293712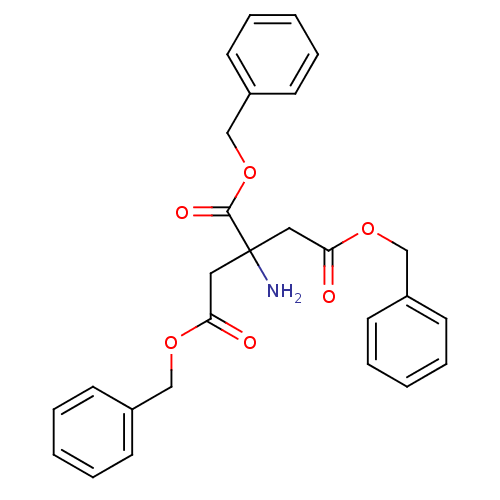 (CHEMBL561821 | Tribenzyl 2-aminopropane-1,2,3-tric...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inactivation of Pseudomonas aeruginosa OXA10 beta-lactamase at pH 7 | Bioorg Med Chem Lett 19: 3593-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.149 BindingDB Entry DOI: 10.7270/Q2W9597J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50212634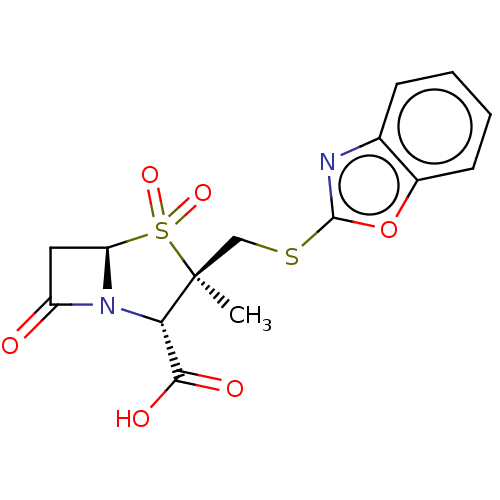 (CHEMBL309009) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 83.7 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli HA209 Class-V OXA-1 type beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50212638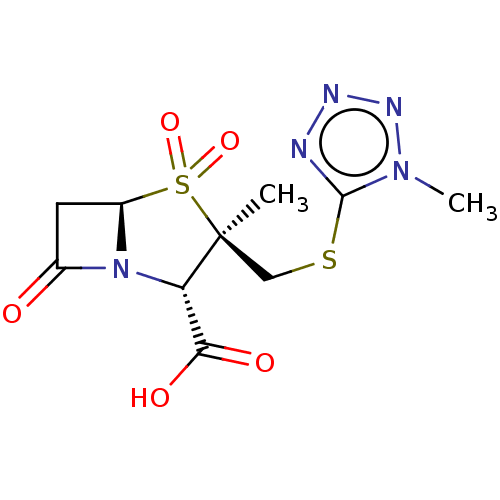 (CHEMBL302512) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 92.1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli HA209 Class-V OXA-1 type beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50076680 (CHEMBL6461 | Ro-48-1220 | Sodium; (2S,3R,5R)-3-((Z...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Beta-lactamase from OXA-1 | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50076680 (CHEMBL6461 | Ro-48-1220 | Sodium; (2S,3R,5R)-3-((Z...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Beta-lactamase from OXA-1 | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50212637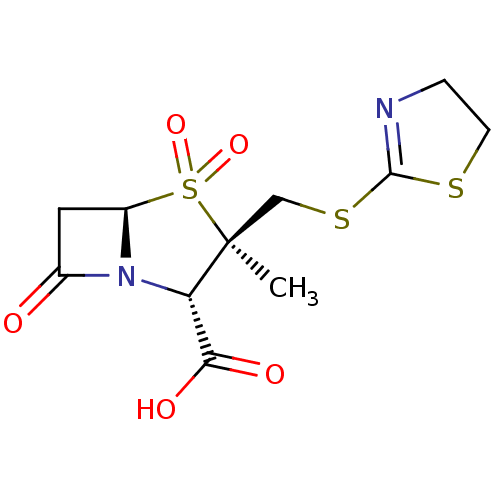 (CHEMBL72046) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli HA209 Class-V OXA-1 type beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50212646 (CHEMBL72972) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli HA209 Class-V OXA-1 type beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50212640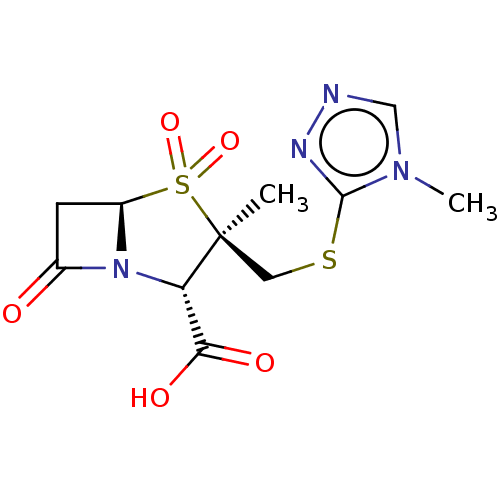 (CHEMBL423309) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 375 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli HA209 Class-V OXA-1 type beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 401 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli HA209 Class-V OXA-1 type beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50053173 ((2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 432 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli HA209 Class-V OXA-1 type beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50212639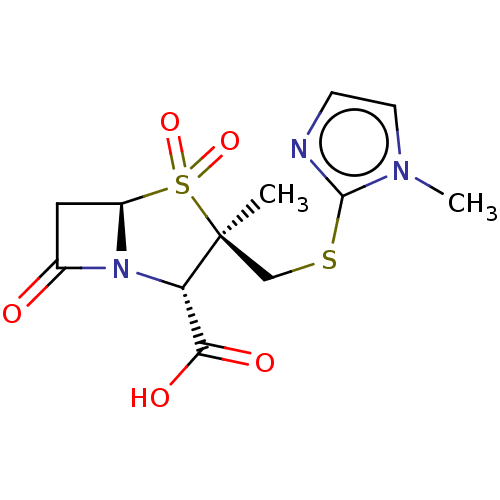 (CHEMBL302241) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 463 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli HA209 Class-V OXA-1 type beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50212641 (Brobactam) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | n/a | n/a | 713 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli HA209 Class-V OXA-1 type beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50021954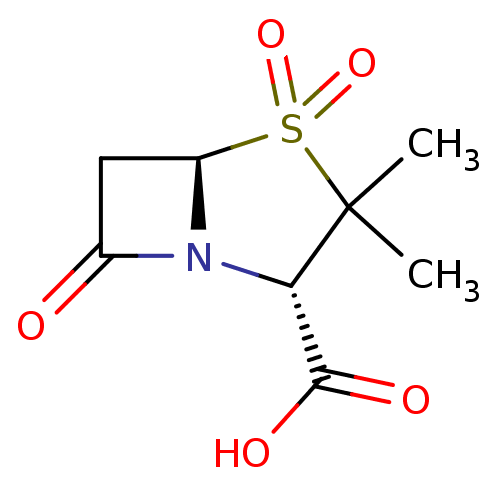 ((2S,5R)-3,3-Dimethyl-4,4,7-trioxo-4lambda*6*-thia-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli HA209 Class-V OXA-1 type beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50212643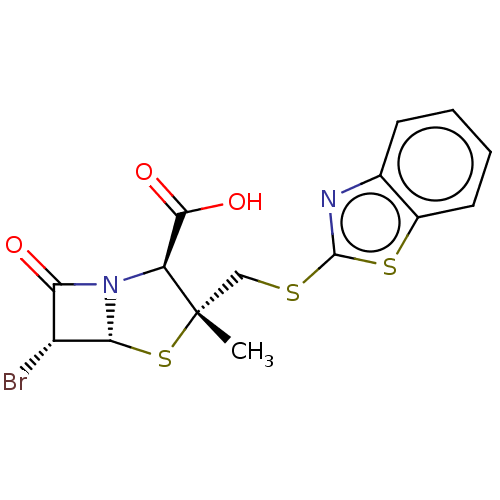 (CHEMBL308516) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli HA209 Class-V OXA-1 type beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50212636 (CHEMBL310221) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli HA209 Class-V OXA-1 type beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50212642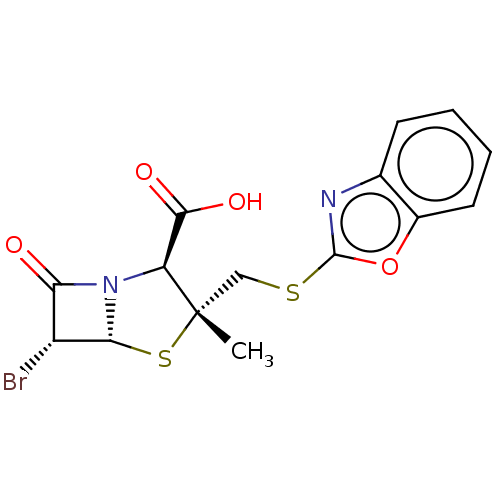 (CHEMBL70269) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 2.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli HA209 Class-V OXA-1 type beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Beta-lactamase type OXA1 (penicillinase) from E. coli OXA1 using ampicillin (40 uM) as a substrate | J Med Chem 30: 1469-74 (1987) BindingDB Entry DOI: 10.7270/Q28G8JQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50053173 ((2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Beta-lactamase from Bacteroides fragilis 36 | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50212645 (CHEMBL305908) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 5.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli HA209 Class-V OXA-1 type beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50212635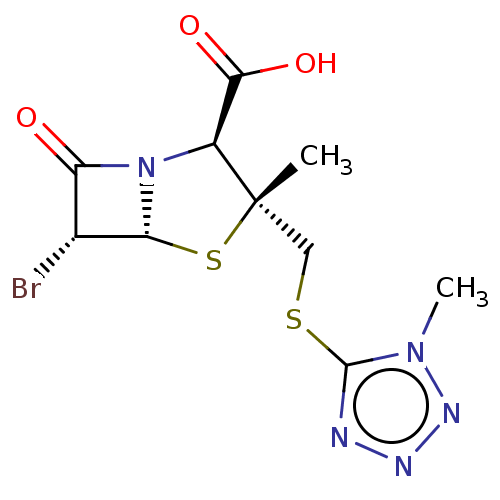 (CHEMBL415266) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 8.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli HA209 Class-V OXA-1 type beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50212644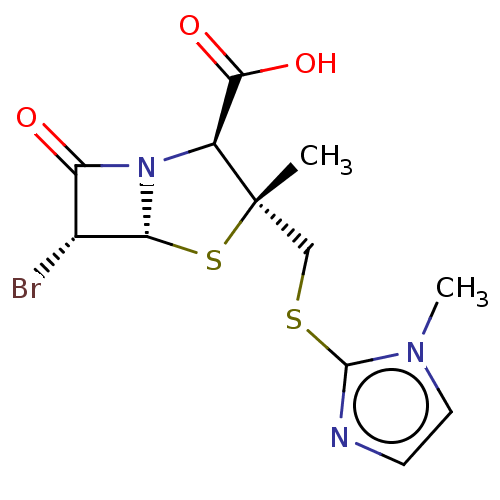 (CHEMBL73450) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli HA209 Class-V OXA-1 type beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50021954 ((2S,5R)-3,3-Dimethyl-4,4,7-trioxo-4lambda*6*-thia-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Beta-lactamase type OXA1 (penicillinase) from E. coli OXA1 using ampicillin (40 uM) as a substrate | J Med Chem 30: 1469-74 (1987) BindingDB Entry DOI: 10.7270/Q28G8JQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-10 (Pseudomonas aeruginosa) | BDBM50247467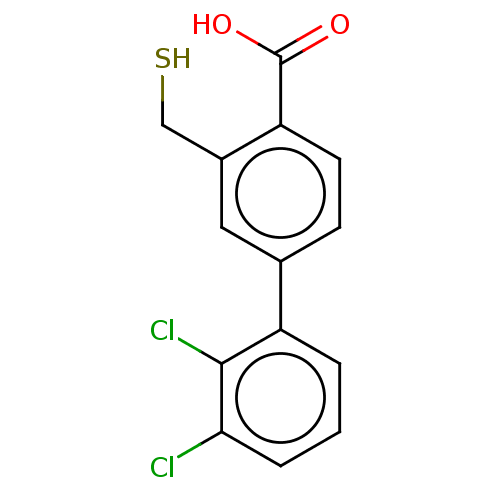 (CHEMBL4083640) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds Curated by ChEMBL | Assay Description Inhibition of recombinant Pseudomonas aeruginosa OXA-10 expressed in Escherichia coli BL21 (DE3) cells using FC5 as substrate preincubated up to 360 ... | J Med Chem 61: 1255-1260 (2018) Article DOI: 10.1021/acs.jmedchem.7b01728 BindingDB Entry DOI: 10.7270/Q2GQ716Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 1.75E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Concentration required for its inhibitory activity against Escherichia coli K12(OXA1) class D beta-lactamase | Citation and Details Article DOI: 10.1016/S0960-894X(01)80506-0 BindingDB Entry DOI: 10.7270/Q21V5HPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50292429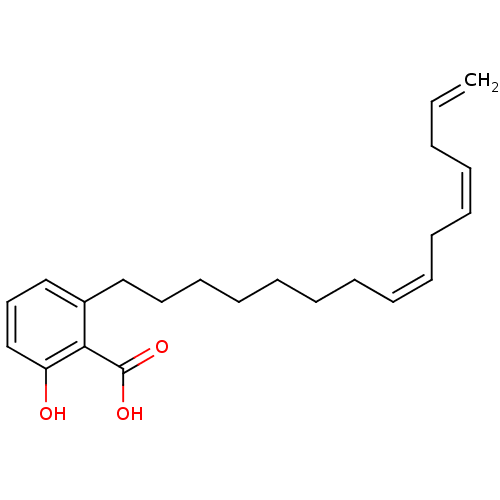 (2-Hydroxy-6-pentadecyl-benzoic acid | 2-Pentadecyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7.29E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Concentration required for its inhibitory activity against Escherichia coli K12(OXA1) class D beta-lactamase | Citation and Details Article DOI: 10.1016/S0960-894X(01)80506-0 BindingDB Entry DOI: 10.7270/Q21V5HPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50469665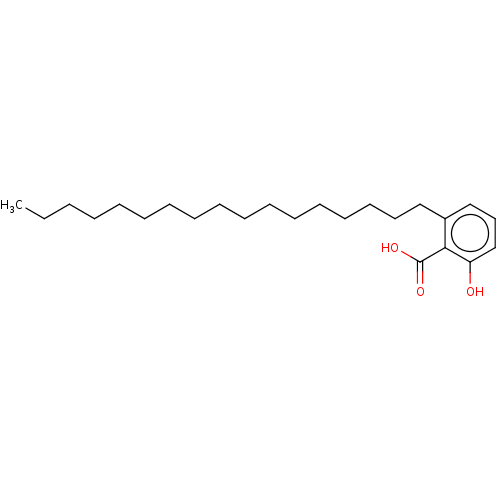 (CHEMBL30914) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article | n/a | n/a | 7.70E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Concentration required for its inhibitory activity against Escherichia coli K12(OXA1) class D beta-lactamase | Citation and Details Article DOI: 10.1016/S0960-894X(01)80506-0 BindingDB Entry DOI: 10.7270/Q21V5HPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50469660 (CHEMBL30915) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article | n/a | n/a | >9.10E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Concentration required for its inhibitory activity against Escherichia coli K12(OXA1) class D beta-lactamase | Citation and Details Article DOI: 10.1016/S0960-894X(01)80506-0 BindingDB Entry DOI: 10.7270/Q21V5HPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50082305 (3-Pentadecyl-phenol | 3-pentadecyl phenol | 3-pent...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | >1.08E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Concentration required for its inhibitory activity against Escherichia coli K12(OXA1) class D beta-lactamase | Citation and Details Article DOI: 10.1016/S0960-894X(01)80506-0 BindingDB Entry DOI: 10.7270/Q21V5HPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50469664 (3-Decyl-Phenol | CHEMBL33202) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article | n/a | n/a | >1.40E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Concentration required for its inhibitory activity against Escherichia coli K12(OXA1) class D beta-lactamase | Citation and Details Article DOI: 10.1016/S0960-894X(01)80506-0 BindingDB Entry DOI: 10.7270/Q21V5HPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50469657 (CHEMBL33995 | SB-202742) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article | n/a | n/a | 2.15E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Concentration required for its inhibitory activity against Escherichia coli K12(OXA1) class D beta-lactamase | Citation and Details Article DOI: 10.1016/S0960-894X(01)80506-0 BindingDB Entry DOI: 10.7270/Q21V5HPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50469667 (6-Decylsalicylic Acid | CHEMBL416038) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article | n/a | n/a | 1.29E+9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Concentration required for its inhibitory activity against Escherichia coli K12(OXA1) class D beta-lactamase | Citation and Details Article DOI: 10.1016/S0960-894X(01)80506-0 BindingDB Entry DOI: 10.7270/Q21V5HPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM50469661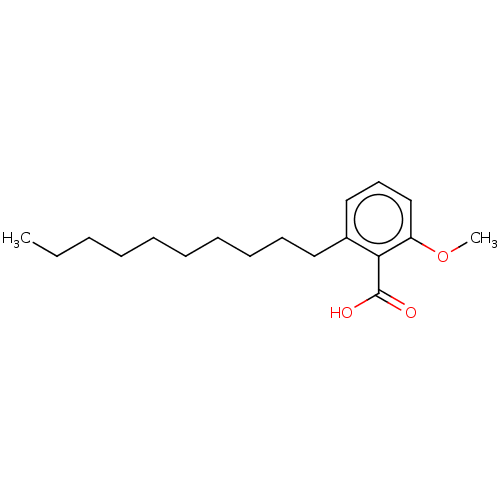 (CHEMBL30870) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article | n/a | n/a | 2.15E+9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Concentration required for its inhibitory activity against Escherichia coli K12(OXA1) class D beta-lactamase | Citation and Details Article DOI: 10.1016/S0960-894X(01)80506-0 BindingDB Entry DOI: 10.7270/Q21V5HPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||