 Found 28 hits Enz. Inhib. hit(s) with Target = 'Antithrombin-III'
Found 28 hits Enz. Inhib. hit(s) with Target = 'Antithrombin-III' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Antithrombin-III
(Homo sapiens (Human)) | BDBM50037996
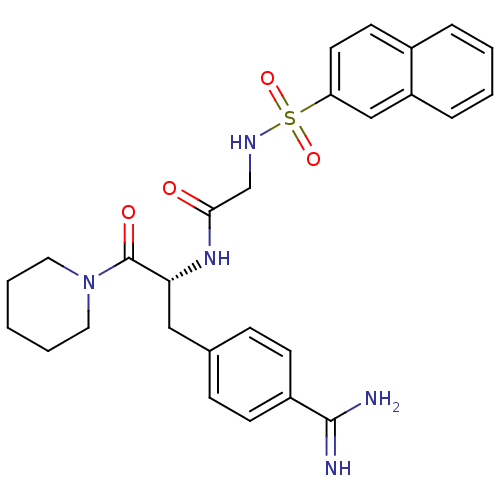
(1-[N-(naphthalen-2-ylsulfonyl)glycyl-4-carbamimido...)Show SMILES NC(=N)c1ccc(C[C@@H](NC(=O)CNS(=O)(=O)c2ccc3ccccc3c2)C(=O)N2CCCCC2)cc1 Show InChI InChI=1S/C27H31N5O4S/c28-26(29)21-10-8-19(9-11-21)16-24(27(34)32-14-4-1-5-15-32)31-25(33)18-30-37(35,36)23-13-12-20-6-2-3-7-22(20)17-23/h2-3,6-13,17,24,30H,1,4-5,14-16,18H2,(H3,28,29)(H,31,33)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry
Curated by ChEMBL
| Assay Description
Antithrombin III (ATIII)-mediated anti-Xa activity was determined |
Bioorg Med Chem Lett 9: 2013-8 (1999)
BindingDB Entry DOI: 10.7270/Q26H4HXG |
More data for this
Ligand-Target Pair | |
Antithrombin-III/Prothrombin
(Homo sapiens (Human)) | BDBM264613

(US9718760, C157)Show SMILES CCCCCCCCCCN(S(=O)(=O)c1ccc(O)c(O)c1)S(=O)(=O)c1ccc(O)c(O)c1 Show InChI InChI=1S/C22H31NO8S2/c1-2-3-4-5-6-7-8-9-14-23(32(28,29)17-10-12-19(24)21(26)15-17)33(30,31)18-11-13-20(25)22(27)16-18/h10-13,15-16,24-27H,2-9,14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.44E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY
US Patent
| Assay Description
PAI-1 inhibitor compounds were dissolved in DMSO to a final concentration of (10-50 mM), depending upon solubility. Compounds were then diluted in ph... |
US Patent US9718760 (2017)
BindingDB Entry DOI: 10.7270/Q2K939HK |
More data for this
Ligand-Target Pair | |
Antithrombin-III/Prothrombin
(Homo sapiens (Human)) | BDBM264597

(US9718760, C152)Show SMILES CCCCCCCCN(S(=O)(=O)c1ccc(O)c(O)c1)S(=O)(=O)c1cc(F)c(O)c(F)c1 Show InChI InChI=1S/C20H25F2NO7S2/c1-2-3-4-5-6-7-10-23(31(27,28)14-8-9-18(24)19(25)13-14)32(29,30)15-11-16(21)20(26)17(22)12-15/h8-9,11-13,24-26H,2-7,10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.70E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY
US Patent
| Assay Description
PAI-1 inhibitor compounds were dissolved in DMSO to a final concentration of (10-50 mM), depending upon solubility. Compounds were then diluted in ph... |
US Patent US9718760 (2017)
BindingDB Entry DOI: 10.7270/Q2K939HK |
More data for this
Ligand-Target Pair | |
Antithrombin-III/Coagulation factor X
(Homo sapiens (Human)) | BDBM50393527
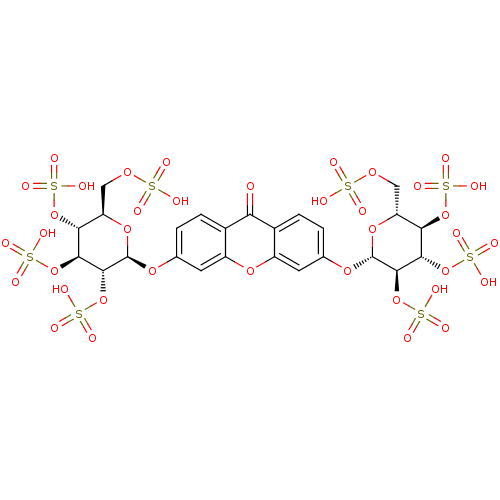
(CHEMBL2158198)Show SMILES OS(=O)(=O)OC[C@H]1O[C@@H](Oc2ccc3c(c2)oc2cc(O[C@@H]4O[C@H](COS(O)(=O)=O)[C@@H](OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@H]4OS(O)(=O)=O)ccc2c3=O)[C@H](OS(O)(=O)=O)[C@@H](OS(O)(=O)=O)[C@@H]1OS(O)(=O)=O |r| Show InChI InChI=1S/C25H28O38S8/c26-17-11-3-1-9(53-24-22(62-70(45,46)47)20(60-68(39,40)41)18(58-66(33,34)35)15(56-24)7-51-64(27,28)29)5-13(11)55-14-6-10(2-4-12(14)17)54-25-23(63-71(48,49)50)21(61-69(42,43)44)19(59-67(36,37)38)16(57-25)8-52-65(30,31)32/h1-6,15-16,18-25H,7-8H2,(H,27,28,29)(H,30,31,32)(H,33,34,35)(H,36,37,38)(H,39,40,41)(H,42,43,44)(H,45,46,47)(H,48,49,50)/t15-,16-,18-,19-,20+,21+,22-,23-,24-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto (CEQUIMED-UP)
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a/antithrombin 3 assessed as CBS 31.39 substrate hydrolysis by spectrophotometric analysis |
J Med Chem 54: 5373-84 (2011)
Article DOI: 10.1021/jm2006589
BindingDB Entry DOI: 10.7270/Q27P90HT |
More data for this
Ligand-Target Pair | |
Antithrombin-III
(Homo sapiens (Human)) | BDBM50310306
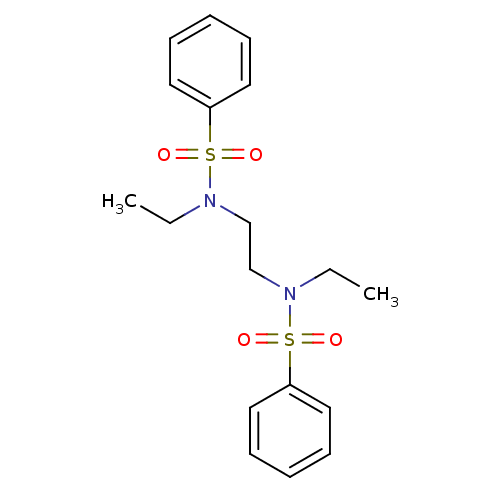
(CHEMBL611972 | N,N'-(ethane-1,2-diyl)bis(N-ethylbe...)Show SMILES CCN(CCN(CC)S(=O)(=O)c1ccccc1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C18H24N2O4S2/c1-3-19(25(21,22)17-11-7-5-8-12-17)15-16-20(4-2)26(23,24)18-13-9-6-10-14-18/h5-14H,3-4,15-16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Michigan University
Curated by ChEMBL
| Assay Description
Inhibition of antithrombin-3 assessed as residual alpha-thrombin activity |
Bioorg Med Chem Lett 20: 966-70 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.051
BindingDB Entry DOI: 10.7270/Q24M94P3 |
More data for this
Ligand-Target Pair | |
Antithrombin-III
(Homo sapiens (Human)) | BDBM50310305
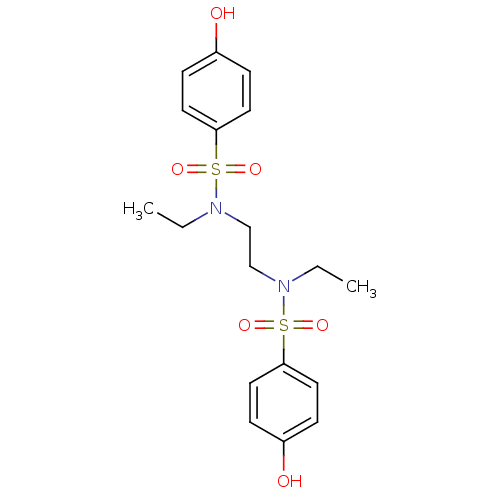
(CHEMBL596859 | N,N'-(ethane-1,2-diyl)bis(N-ethyl-4...)Show SMILES CCN(CCN(CC)S(=O)(=O)c1ccc(O)cc1)S(=O)(=O)c1ccc(O)cc1 Show InChI InChI=1S/C18H24N2O6S2/c1-3-19(27(23,24)17-9-5-15(21)6-10-17)13-14-20(4-2)28(25,26)18-11-7-16(22)8-12-18/h5-12,21-22H,3-4,13-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Michigan University
Curated by ChEMBL
| Assay Description
Inhibition of antithrombin-3 assessed as residual alpha-thrombin activity |
Bioorg Med Chem Lett 20: 966-70 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.051
BindingDB Entry DOI: 10.7270/Q24M94P3 |
More data for this
Ligand-Target Pair | |
Antithrombin-III
(Homo sapiens (Human)) | BDBM50310299
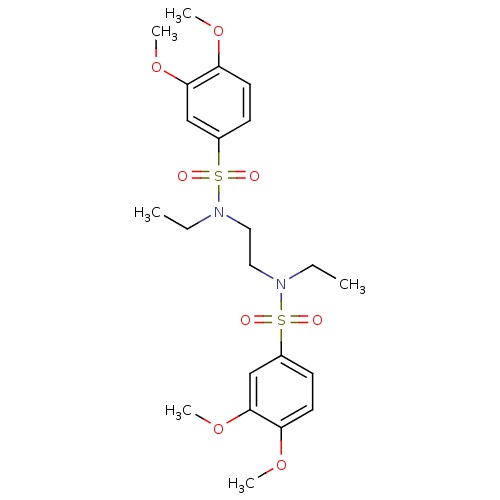
(CHEMBL599342 | N,N'-(ethane-1,2-diyl)bis(N-ethyl-3...)Show SMILES CCN(CCN(CC)S(=O)(=O)c1ccc(OC)c(OC)c1)S(=O)(=O)c1ccc(OC)c(OC)c1 Show InChI InChI=1S/C22H32N2O8S2/c1-7-23(33(25,26)17-9-11-19(29-3)21(15-17)31-5)13-14-24(8-2)34(27,28)18-10-12-20(30-4)22(16-18)32-6/h9-12,15-16H,7-8,13-14H2,1-6H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Michigan University
Curated by ChEMBL
| Assay Description
Inhibition of antithrombin-3 assessed as residual alpha-thrombin activity |
Bioorg Med Chem Lett 20: 966-70 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.051
BindingDB Entry DOI: 10.7270/Q24M94P3 |
More data for this
Ligand-Target Pair | |
Antithrombin-III
(Homo sapiens (Human)) | BDBM50310312
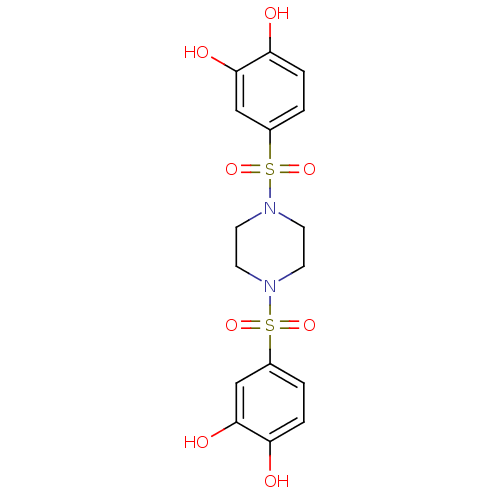
(CHEMBL597509 | N,N'-bis(3,4-dihydroxybenzenesulfon...)Show SMILES Oc1ccc(cc1O)S(=O)(=O)N1CCN(CC1)S(=O)(=O)c1ccc(O)c(O)c1 Show InChI InChI=1S/C16H18N2O8S2/c19-13-3-1-11(9-15(13)21)27(23,24)17-5-7-18(8-6-17)28(25,26)12-2-4-14(20)16(22)10-12/h1-4,9-10,19-22H,5-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Michigan University
Curated by ChEMBL
| Assay Description
Inhibition of antithrombin-3 assessed as residual alpha-thrombin activity |
Bioorg Med Chem Lett 20: 966-70 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.051
BindingDB Entry DOI: 10.7270/Q24M94P3 |
More data for this
Ligand-Target Pair | |
Antithrombin-III
(Homo sapiens (Human)) | BDBM50310309
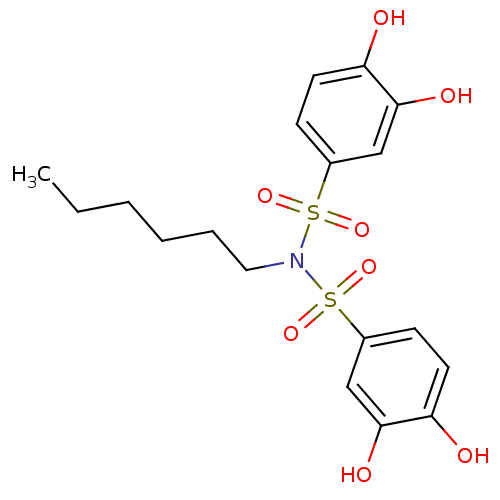
(CHEMBL596879 | N-(3,4-dihydroxyphenylsulfonyl)-N-h...)Show SMILES CCCCCCN(S(=O)(=O)c1ccc(O)c(O)c1)S(=O)(=O)c1ccc(O)c(O)c1 Show InChI InChI=1S/C18H23NO8S2/c1-2-3-4-5-10-19(28(24,25)13-6-8-15(20)17(22)11-13)29(26,27)14-7-9-16(21)18(23)12-14/h6-9,11-12,20-23H,2-5,10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Michigan University
Curated by ChEMBL
| Assay Description
Inhibition of antithrombin-3 assessed as residual alpha-thrombin activity |
Bioorg Med Chem Lett 20: 966-70 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.051
BindingDB Entry DOI: 10.7270/Q24M94P3 |
More data for this
Ligand-Target Pair | |
Antithrombin-III
(Homo sapiens (Human)) | BDBM50310313
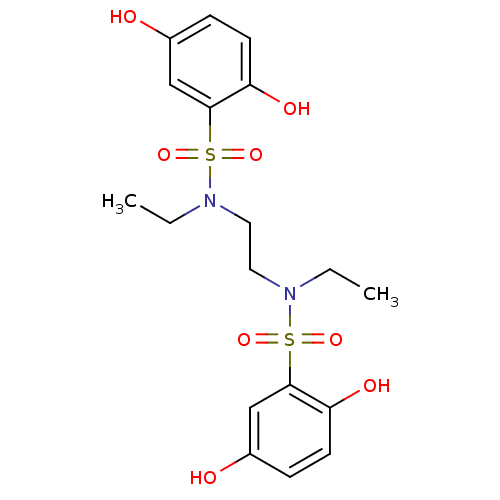
(CHEMBL596860 | N,N'-(ethane-1,2-diyl)bis(N-ethyl-2...)Show SMILES CCN(CCN(CC)S(=O)(=O)c1cc(O)ccc1O)S(=O)(=O)c1cc(O)ccc1O Show InChI InChI=1S/C18H24N2O8S2/c1-3-19(29(25,26)17-11-13(21)5-7-15(17)23)9-10-20(4-2)30(27,28)18-12-14(22)6-8-16(18)24/h5-8,11-12,21-24H,3-4,9-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Michigan University
Curated by ChEMBL
| Assay Description
Inhibition of antithrombin-3 assessed as residual alpha-thrombin activity |
Bioorg Med Chem Lett 20: 966-70 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.051
BindingDB Entry DOI: 10.7270/Q24M94P3 |
More data for this
Ligand-Target Pair | |
Antithrombin-III
(Homo sapiens (Human)) | BDBM50310310
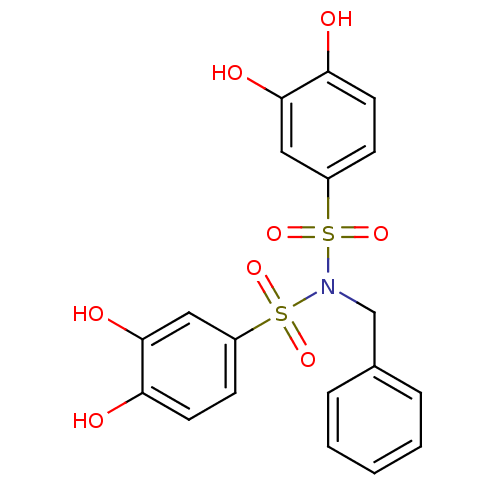
(CHEMBL596880 | N-benzyl-N-(3,4-dihydroxyphenylsulf...)Show SMILES Oc1ccc(cc1O)S(=O)(=O)N(Cc1ccccc1)S(=O)(=O)c1ccc(O)c(O)c1 Show InChI InChI=1S/C19H17NO8S2/c21-16-8-6-14(10-18(16)23)29(25,26)20(12-13-4-2-1-3-5-13)30(27,28)15-7-9-17(22)19(24)11-15/h1-11,21-24H,12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Michigan University
Curated by ChEMBL
| Assay Description
Inhibition of antithrombin-3 assessed as residual alpha-thrombin activity |
Bioorg Med Chem Lett 20: 966-70 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.051
BindingDB Entry DOI: 10.7270/Q24M94P3 |
More data for this
Ligand-Target Pair | |
Antithrombin-III
(Homo sapiens (Human)) | BDBM50310311

(CHEMBL601265 | N-cyclohexyl-N-(3,4-dihydroxyphenyl...)Show SMILES Oc1ccc(cc1O)S(=O)(=O)N(C1CCCCC1)S(=O)(=O)c1ccc(O)c(O)c1 Show InChI InChI=1S/C18H21NO8S2/c20-15-8-6-13(10-17(15)22)28(24,25)19(12-4-2-1-3-5-12)29(26,27)14-7-9-16(21)18(23)11-14/h6-12,20-23H,1-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Michigan University
Curated by ChEMBL
| Assay Description
Inhibition of antithrombin-3 assessed as residual alpha-thrombin activity |
Bioorg Med Chem Lett 20: 966-70 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.051
BindingDB Entry DOI: 10.7270/Q24M94P3 |
More data for this
Ligand-Target Pair | |
Antithrombin-III/Prothrombin
(Homo sapiens (Human)) | BDBM264614

(US9718760, C158)Show SMILES CCCCCCCCCCCCN(S(=O)(=O)c1ccc(O)c(O)c1)S(=O)(=O)c1ccc(O)c(O)c1 Show InChI InChI=1S/C24H35NO8S2/c1-2-3-4-5-6-7-8-9-10-11-16-25(34(30,31)19-12-14-21(26)23(28)17-19)35(32,33)20-13-15-22(27)24(29)18-20/h12-15,17-18,26-29H,2-11,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.47E+5 | n/a | n/a | n/a | n/a | n/a | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY
US Patent
| Assay Description
PAI-1 inhibitor compounds were dissolved in DMSO to a final concentration of (10-50 mM), depending upon solubility. Compounds were then diluted in ph... |
US Patent US9718760 (2017)
BindingDB Entry DOI: 10.7270/Q2K939HK |
More data for this
Ligand-Target Pair | |
Antithrombin-III
(Homo sapiens (Human)) | BDBM50310308

(CHEMBL597478 | N-(3,4-dihydroxyphenylsulfonyl)-3,4...)Show SMILES CCCN(S(=O)(=O)c1ccc(O)c(O)c1)S(=O)(=O)c1ccc(O)c(O)c1 Show InChI InChI=1S/C15H17NO8S2/c1-2-7-16(25(21,22)10-3-5-12(17)14(19)8-10)26(23,24)11-4-6-13(18)15(20)9-11/h3-6,8-9,17-20H,2,7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Michigan University
Curated by ChEMBL
| Assay Description
Inhibition of antithrombin-3 assessed as residual alpha-thrombin activity |
Bioorg Med Chem Lett 20: 966-70 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.051
BindingDB Entry DOI: 10.7270/Q24M94P3 |
More data for this
Ligand-Target Pair | |
Antithrombin-III
(Homo sapiens (Human)) | BDBM50310304
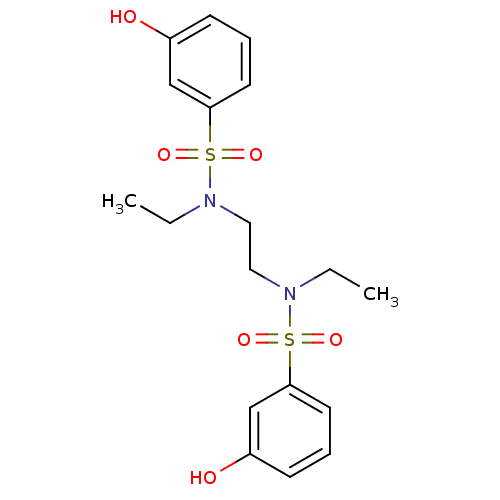
(CHEMBL597510 | N,N'-(ethane-1,2-diyl)bis(N-ethyl-3...)Show SMILES CCN(CCN(CC)S(=O)(=O)c1cccc(O)c1)S(=O)(=O)c1cccc(O)c1 Show InChI InChI=1S/C18H24N2O6S2/c1-3-19(27(23,24)17-9-5-7-15(21)13-17)11-12-20(4-2)28(25,26)18-10-6-8-16(22)14-18/h5-10,13-14,21-22H,3-4,11-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Michigan University
Curated by ChEMBL
| Assay Description
Inhibition of antithrombin-3 assessed as residual alpha-thrombin activity |
Bioorg Med Chem Lett 20: 966-70 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.051
BindingDB Entry DOI: 10.7270/Q24M94P3 |
More data for this
Ligand-Target Pair | |
Antithrombin-III
(Homo sapiens (Human)) | BDBM50310301
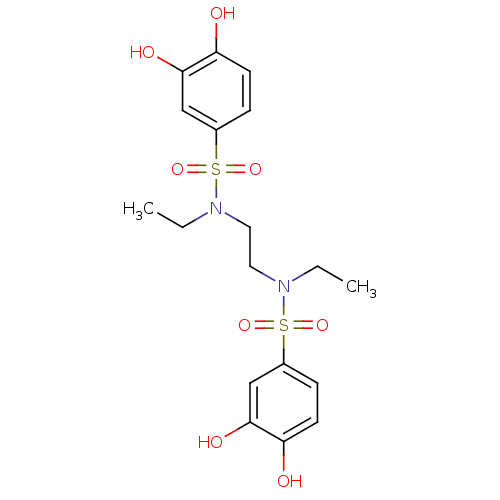
(CHEMBL599742 | N,N'-(ethane-1,2-diyl)bis(N-ethyl-3...)Show SMILES CCN(CCN(CC)S(=O)(=O)c1ccc(O)c(O)c1)S(=O)(=O)c1ccc(O)c(O)c1 Show InChI InChI=1S/C18H24N2O8S2/c1-3-19(29(25,26)13-5-7-15(21)17(23)11-13)9-10-20(4-2)30(27,28)14-6-8-16(22)18(24)12-14/h5-8,11-12,21-24H,3-4,9-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Michigan University
Curated by ChEMBL
| Assay Description
Inhibition of antithrombin-3 assessed as residual alpha-thrombin activity |
Bioorg Med Chem Lett 20: 966-70 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.051
BindingDB Entry DOI: 10.7270/Q24M94P3 |
More data for this
Ligand-Target Pair | |
Antithrombin-III/Prothrombin
(Homo sapiens (Human)) | BDBM264648

(US9718760, C161)Show InChI InChI=1S/C15H23NO4/c1-2-3-4-5-6-7-8-16-15(20)11-9-12(17)14(19)13(18)10-11/h9-10,17-19H,2-8H2,1H3,(H,16,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.67E+6 | n/a | n/a | n/a | n/a | n/a | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY
US Patent
| Assay Description
PAI-1 inhibitor compounds were dissolved in DMSO to a final concentration of (10-50 mM), depending upon solubility. Compounds were then diluted in ph... |
US Patent US9718760 (2017)
BindingDB Entry DOI: 10.7270/Q2K939HK |
More data for this
Ligand-Target Pair | |
Antithrombin-III/Prothrombin
(Homo sapiens (Human)) | BDBM264627

(US9718760, C160)Show InChI InChI=1S/C13H13NO4S/c15-12-7-6-11(8-13(12)16)19(17,18)14-9-10-4-2-1-3-5-10/h1-8,14-16H,9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.33E+6 | n/a | n/a | n/a | n/a | n/a | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY
US Patent
| Assay Description
PAI-1 inhibitor compounds were dissolved in DMSO to a final concentration of (10-50 mM), depending upon solubility. Compounds were then diluted in ph... |
US Patent US9718760 (2017)
BindingDB Entry DOI: 10.7270/Q2K939HK |
More data for this
Ligand-Target Pair | |
Antithrombin-III/Prothrombin
(Homo sapiens (Human)) | BDBM264598

(US9718760, C155)Show SMILES CCCCCCCCN(S(=O)(=O)c1cc(F)c(O)c(F)c1)S(=O)(=O)c1cc(F)c(O)c(F)c1 Show InChI InChI=1S/C20H23F4NO6S2/c1-2-3-4-5-6-7-8-25(32(28,29)13-9-15(21)19(26)16(22)10-13)33(30,31)14-11-17(23)20(27)18(24)12-14/h9-12,26-27H,2-8H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.33E+6 | n/a | n/a | n/a | n/a | n/a | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY
US Patent
| Assay Description
PAI-1 inhibitor compounds were dissolved in DMSO to a final concentration of (10-50 mM), depending upon solubility. Compounds were then diluted in ph... |
US Patent US9718760 (2017)
BindingDB Entry DOI: 10.7270/Q2K939HK |
More data for this
Ligand-Target Pair | |
Antithrombin-III
(Homo sapiens (Human)) | BDBM50310302

(CHEMBL611992 | N,N'-(butane-1,4-diyl)bis(3,4-dihyd...)Show SMILES Oc1ccc(cc1O)S(=O)(=O)NCCCCNS(=O)(=O)c1ccc(O)c(O)c1 Show InChI InChI=1S/C16H20N2O8S2/c19-13-5-3-11(9-15(13)21)27(23,24)17-7-1-2-8-18-28(25,26)12-4-6-14(20)16(22)10-12/h3-6,9-10,17-22H,1-2,7-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Michigan University
Curated by ChEMBL
| Assay Description
Inhibition of antithrombin-3 assessed as residual alpha-thrombin activity |
Bioorg Med Chem Lett 20: 966-70 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.051
BindingDB Entry DOI: 10.7270/Q24M94P3 |
More data for this
Ligand-Target Pair | |
Antithrombin-III
(Homo sapiens (Human)) | BDBM50310303
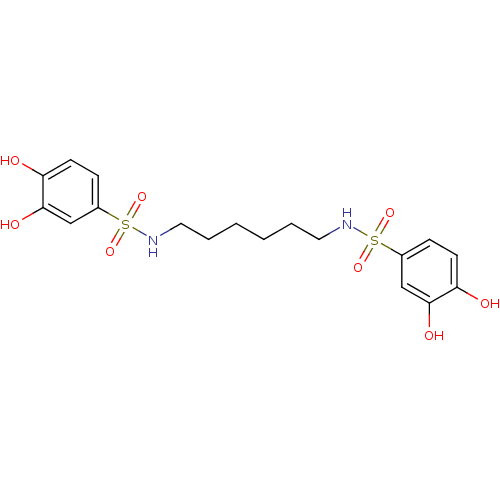
(CHEMBL599769 | N,N'-(hexane-1,6-diyl)bis(3,4-dihyd...)Show SMILES Oc1ccc(cc1O)S(=O)(=O)NCCCCCCNS(=O)(=O)c1ccc(O)c(O)c1 Show InChI InChI=1S/C18H24N2O8S2/c21-15-7-5-13(11-17(15)23)29(25,26)19-9-3-1-2-4-10-20-30(27,28)14-6-8-16(22)18(24)12-14/h5-8,11-12,19-24H,1-4,9-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Michigan University
Curated by ChEMBL
| Assay Description
Inhibition of antithrombin-3 assessed as residual alpha-thrombin activity |
Bioorg Med Chem Lett 20: 966-70 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.051
BindingDB Entry DOI: 10.7270/Q24M94P3 |
More data for this
Ligand-Target Pair | |
Antithrombin-III
(Homo sapiens (Human)) | BDBM50310307
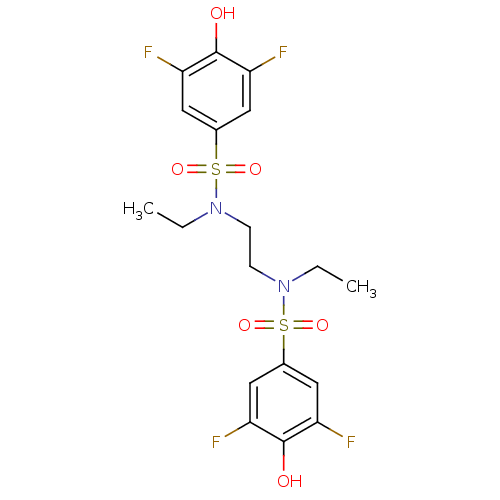
(CHEMBL596861 | N,N'-(ethane-1,2-diyl)bis(N-ethyl-3...)Show SMILES CCN(CCN(CC)S(=O)(=O)c1cc(F)c(O)c(F)c1)S(=O)(=O)c1cc(F)c(O)c(F)c1 Show InChI InChI=1S/C18H20F4N2O6S2/c1-3-23(31(27,28)11-7-13(19)17(25)14(20)8-11)5-6-24(4-2)32(29,30)12-9-15(21)18(26)16(22)10-12/h7-10,25-26H,3-6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >3.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Michigan University
Curated by ChEMBL
| Assay Description
Inhibition of antithrombin-3 assessed as residual alpha-thrombin activity |
Bioorg Med Chem Lett 20: 966-70 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.051
BindingDB Entry DOI: 10.7270/Q24M94P3 |
More data for this
Ligand-Target Pair | |
Antithrombin-III
(Homo sapiens (Human)) | BDBM50310300
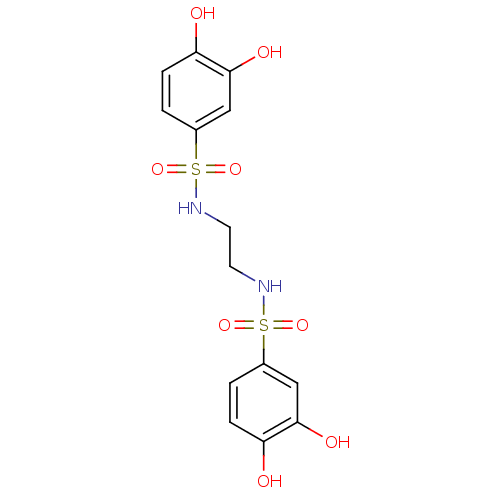
(CHEMBL606451 | N,N'-(ethane-1,2-diyl)bis(3,4-dihyd...)Show SMILES Oc1ccc(cc1O)S(=O)(=O)NCCNS(=O)(=O)c1ccc(O)c(O)c1 Show InChI InChI=1S/C14H16N2O8S2/c17-11-3-1-9(7-13(11)19)25(21,22)15-5-6-16-26(23,24)10-2-4-12(18)14(20)8-10/h1-4,7-8,15-20H,5-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Michigan University
Curated by ChEMBL
| Assay Description
Inhibition of antithrombin-3 assessed as residual alpha-thrombin activity |
Bioorg Med Chem Lett 20: 966-70 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.051
BindingDB Entry DOI: 10.7270/Q24M94P3 |
More data for this
Ligand-Target Pair | |
Antithrombin-III
(Homo sapiens (Human)) | BDBM50421407

(CHEMBL2303814)Show SMILES CO[C@H]1O[C@@H](COS(O)(=O)=O)[C@@H](O[C@@H]2O[C@H]([C@H](O[C@H]3O[C@@H](COS(O)(=O)=O)[C@@H](O[C@@H]4O[C@H]([C@@H](C[C@H]5O[C@@H](COS(O)(=O)=O)[C@@H](OC)[C@H](OC)[C@@H]5OC)[C@H](OC)[C@@H]4OC)C(O)=O)[C@H](OS(O)(=O)=O)[C@@H]3OS(O)(=O)=O)[C@H](OC)[C@H]2OC)C(O)=O)[C@H](OS(O)(=O)=O)[C@@H]1OS(O)(=O)=O |r| Show InChI InChI=1S/C39H66O48S7/c1-65-18-13(9-14-20(66-2)24(68-4)21(67-3)15(76-14)10-73-88(44,45)46)19(34(40)41)79-37(30(18)70-6)80-23-17(12-75-90(50,51)52)78-39(33(87-94(62,63)64)28(23)85-92(56,57)58)82-26-25(69-5)31(71-7)38(83-29(26)35(42)43)81-22-16(11-74-89(47,48)49)77-36(72-8)32(86-93(59,60)61)27(22)84-91(53,54)55/h13-33,36-39H,9-12H2,1-8H3,(H,40,41)(H,42,43)(H,44,45,46)(H,47,48,49)(H,50,51,52)(H,53,54,55)(H,56,57,58)(H,59,60,61)(H,62,63,64)/t13-,14+,15-,16-,17-,18-,19+,20+,21+,22+,23+,24+,25-,26+,27-,28-,29+,30-,31+,32-,33-,36-,37-,38+,39+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against antithrombin III (AT III) was determined by fluorescence spectroscopy |
Bioorg Med Chem Lett 7: 1507-1510 (1997)
Article DOI: 10.1016/S0960-894X(97)00252-7
BindingDB Entry DOI: 10.7270/Q2JD4X9F |
More data for this
Ligand-Target Pair | |
Antithrombin-III
(Homo sapiens (Human)) | BDBM50378854
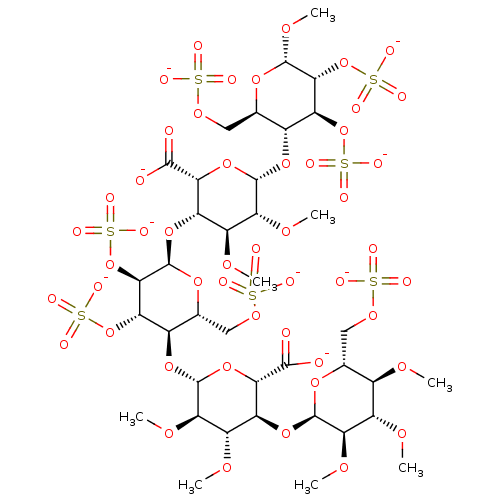
(IDRAPARINUX SODIUM)Show SMILES CO[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H]([C@@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](O[C@@H]4O[C@@H]([C@@H](O[C@H]5O[C@H](COS([O-])(=O)=O)[C@@H](OC)[C@H](OC)[C@H]5OC)[C@H](OC)[C@H]4OC)C([O-])=O)[C@H](OS([O-])(=O)=O)[C@H]3OS([O-])(=O)=O)[C@H](OC)[C@H]2OC)C([O-])=O)[C@H](OS([O-])(=O)=O)[C@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C38H64O49S7/c1-64-15-12(9-72-88(43,44)45)76-35(27(68-5)18(15)65-2)80-21-19(66-3)28(69-6)37(82-25(21)32(39)40)79-17-14(11-74-90(49,50)51)77-38(31(87-94(61,62)63)24(17)85-92(55,56)57)81-22-20(67-4)29(70-7)36(83-26(22)33(41)42)78-16-13(10-73-89(46,47)48)75-34(71-8)30(86-93(58,59)60)23(16)84-91(52,53)54/h12-31,34-38H,9-11H2,1-8H3,(H,39,40)(H,41,42)(H,43,44,45)(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)/p-9/t12-,13-,14-,15-,16-,17-,18+,19+,20+,21+,22+,23+,24+,25+,26-,27-,28-,29-,30-,31-,34+,35-,36+,37-,38-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to antithrombin 3 |
J Med Chem 53: 6243-74 (2010)
Article DOI: 10.1021/jm100146h
BindingDB Entry DOI: 10.7270/Q2CR5VBB |
More data for this
Ligand-Target Pair | |
Antithrombin-III
(Homo sapiens (Human)) | BDBM50369254
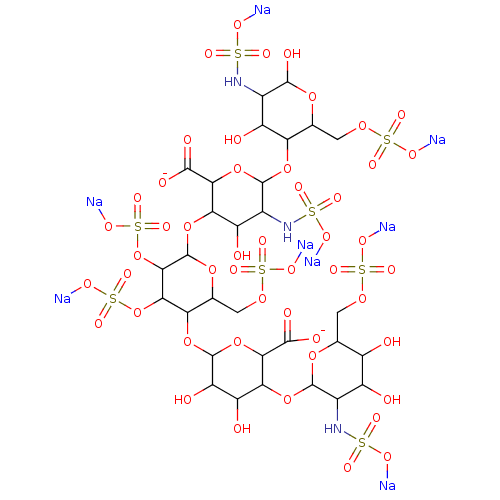
(CHEMBL609990)Show SMILES OC1OC(COS(=O)(=O)O[Na])C(OC2OC(C(OC3OC(COS(=O)(=O)O[Na])C(OC4OC(C(OC5OC(COS(=O)(=O)O[Na])C(O)C(O)C5NS(=O)(=O)O[Na])C(O)C4O)C([O-])=O)C(OS(=O)(=O)O[Na])C3OS(=O)(=O)O[Na])C(O)C2NS(=O)(=O)O[Na])C([O-])=O)C(O)C1NS(=O)(=O)O[Na] Show InChI InChI=1S/C30H51N3O49S8.8Na/c34-10-4(1-69-86(54,55)56)73-27(8(11(10)35)32-84(48,49)50)77-19-14(38)15(39)29(80-22(19)25(42)43)76-17-6(3-71-88(60,61)62)74-30(23(82-90(66,67)68)20(17)81-89(63,64)65)78-18-13(37)9(33-85(51,52)53)28(79-21(18)24(40)41)75-16-5(2-70-87(57,58)59)72-26(44)7(12(16)36)31-83(45,46)47;;;;;;;;/h4-23,26-39,44H,1-3H2,(H,40,41)(H,42,43)(H,45,46,47)(H,48,49,50)(H,51,52,53)(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68);;;;;;;;/q;8*+1/p-10 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by ChEMBL
| Assay Description
Dissociation constant for Antithrombin-III |
J Med Chem 40: 1600-7 (1997)
Article DOI: 10.1021/jm960726z
BindingDB Entry DOI: 10.7270/Q2TH8NC2 |
More data for this
Ligand-Target Pair | |
Antithrombin-III
(Homo sapiens (Human)) | BDBM50369253

(CHEMBL1909451)Show SMILES CO[C@@H]1O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H]([C@H](O[C@@H]3O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]4O[C@@H]([C@@H](O[C@@H]5O[C@@H](COS([O-])(=O)=O)[C@H](OC)[C@@H](OC)[C@@H]5NS([O-])(=O)=O)[C@@H](OC)[C@@H]4OC)C([O-])=O)[C@@H](OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H](OC)[C@@H]2OS([O-])(=O)=O)C([O-])=O)[C@H](OC)[C@H]1NS([O-])(=O)=O |r,@:7,66,81,@@:23,39,51,72,93| Show InChI InChI=1S/C37H64N2O50S8/c1-68-16-11(8-75-92(50,51)52)79-34(15(19(16)69-2)39-91(47,48)49)83-23-21(71-4)28(73-6)35(85-26(23)31(40)41)82-18-13(10-77-94(56,57)58)80-36(30(89-97(65,66)67)25(18)87-95(59,60)61)84-24-22(72-5)29(88-96(62,63)64)37(86-27(24)32(42)43)81-17-12(9-76-93(53,54)55)78-33(74-7)14(20(17)70-3)38-90(44,45)46/h11-30,33-39H,8-10H2,1-7H3,(H,40,41)(H,42,43)(H,44,45,46)(H,47,48,49)(H,50,51,52)(H,53,54,55)(H,56,57,58)(H,59,60,61)(H,62,63,64)(H,65,66,67)/p-10/t11-,12+,13+,14+,15-,16-,17+,18+,19-,20+,21+,22+,23-,24+,25+,26-,27+,28-,29-,30-,33+,34-,35-,36-,37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by ChEMBL
| Assay Description
Dissociation constant for Antithrombin-III |
J Med Chem 40: 1600-7 (1997)
Article DOI: 10.1021/jm960726z
BindingDB Entry DOI: 10.7270/Q2TH8NC2 |
More data for this
Ligand-Target Pair | |
Antithrombin-III
(Homo sapiens (Human)) | BDBM50421408
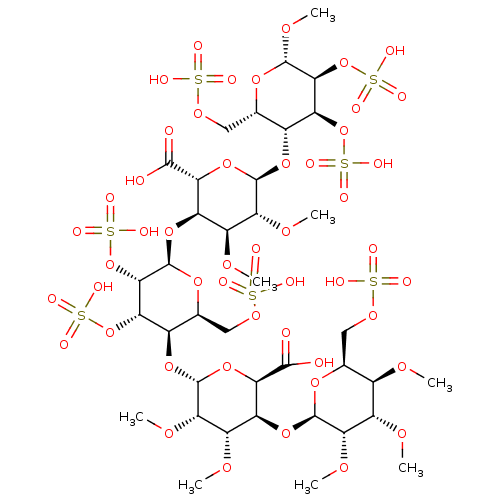
(CHEMBL2303815)Show SMILES CO[C@H]1O[C@@H](COS(O)(=O)=O)[C@@H](O[C@@H]2O[C@H]([C@H](O[C@H]3O[C@@H](COS(O)(=O)=O)[C@@H](O[C@@H]4O[C@H]([C@@H](O[C@H]5O[C@@H](COS(O)(=O)=O)[C@@H](OC)[C@H](OC)[C@@H]5OC)[C@H](OC)[C@@H]4OC)C(O)=O)[C@H](OS(O)(=O)=O)[C@@H]3OS(O)(=O)=O)[C@H](OC)[C@H]2OC)C(O)=O)[C@H](OS(O)(=O)=O)[C@@H]1OS(O)(=O)=O |r| Show InChI InChI=1S/C38H64O49S7/c1-64-15-12(9-72-88(43,44)45)76-35(27(68-5)18(15)65-2)80-21-19(66-3)28(69-6)37(82-25(21)32(39)40)79-17-14(11-74-90(49,50)51)77-38(31(87-94(61,62)63)24(17)85-92(55,56)57)81-22-20(67-4)29(70-7)36(83-26(22)33(41)42)78-16-13(10-73-89(46,47)48)75-34(71-8)30(86-93(58,59)60)23(16)84-91(52,53)54/h12-31,34-38H,9-11H2,1-8H3,(H,39,40)(H,41,42)(H,43,44,45)(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)/t12-,13-,14-,15+,16+,17+,18-,19-,20-,21-,22+,23-,24-,25+,26+,27-,28-,29+,30-,31-,34-,35+,36+,37+,38+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against antithrombin III (AT III) was determined by fluorescence spectroscopy |
Bioorg Med Chem Lett 7: 1507-1510 (1997)
Article DOI: 10.1016/S0960-894X(97)00252-7
BindingDB Entry DOI: 10.7270/Q2JD4X9F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data