

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50022815 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Binding affinity to human recombinant cyclophilin D by surface plasmon resonance analysis | ACS Med Chem Lett 7: 294-9 (2016) Article DOI: 10.1021/acsmedchemlett.5b00451 BindingDB Entry DOI: 10.7270/Q24T6M8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50022815 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of fluorescein labeled cyclosporin binding to Cyp40 by flourescence polarization competition assay | ACS Med Chem Lett 1: 536-539 (2010) Article DOI: 10.1021/ml1001272 BindingDB Entry DOI: 10.7270/Q26110M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50022815 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool Curated by ChEMBL | Assay Description Inhibition of Cyclophilin D (unknown origin) activity in absence of detergent | J Med Chem 59: 2596-611 (2016) Article DOI: 10.1021/acs.jmedchem.5b01801 BindingDB Entry DOI: 10.7270/Q2474CR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50339005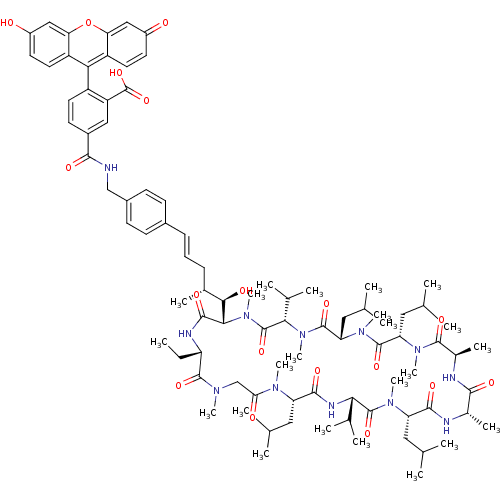 (5-(4-((4R,5R,E)-5-((2S,5S,11S,14S,17S,20S,23R,26S,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Cyclophilin 40 PPIase activity | ACS Med Chem Lett 1: 536-539 (2010) Article DOI: 10.1021/ml1001272 BindingDB Entry DOI: 10.7270/Q26110M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50022815 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Cyclophilin 40 PPIase activity | ACS Med Chem Lett 1: 536-539 (2010) Article DOI: 10.1021/ml1001272 BindingDB Entry DOI: 10.7270/Q26110M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50164666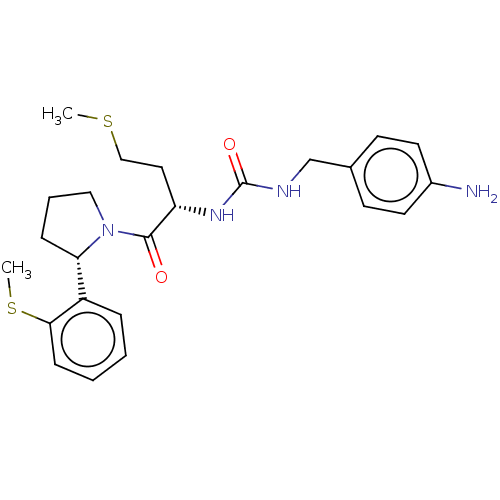 (CHEMBL3799661) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool Curated by ChEMBL | Assay Description Inhibition of Cyclophilin D (unknown origin) activity preincubated for 15 mins followed Suc-AAPF-pNA substrate addition by chymotrypsin coupled based... | J Med Chem 59: 2596-611 (2016) Article DOI: 10.1021/acs.jmedchem.5b01801 BindingDB Entry DOI: 10.7270/Q2474CR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50164670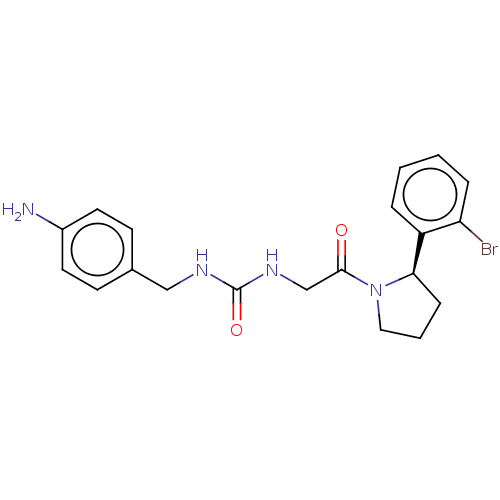 (CHEMBL3799597) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool Curated by ChEMBL | Assay Description Inhibition of Cyclophilin D (unknown origin) activity preincubated for 15 mins followed Suc-AAPF-pNA substrate addition by chymotrypsin coupled based... | J Med Chem 59: 2596-611 (2016) Article DOI: 10.1021/acs.jmedchem.5b01801 BindingDB Entry DOI: 10.7270/Q2474CR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50169545 (CHEMBL3805631) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Binding affinity to human recombinant cyclophilin D by surface plasmon resonance analysis | ACS Med Chem Lett 7: 294-9 (2016) Article DOI: 10.1021/acsmedchemlett.5b00451 BindingDB Entry DOI: 10.7270/Q24T6M8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50495808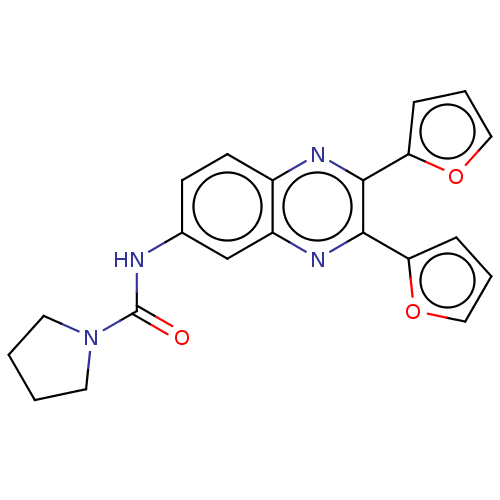 (CHEMBL1557710) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM92912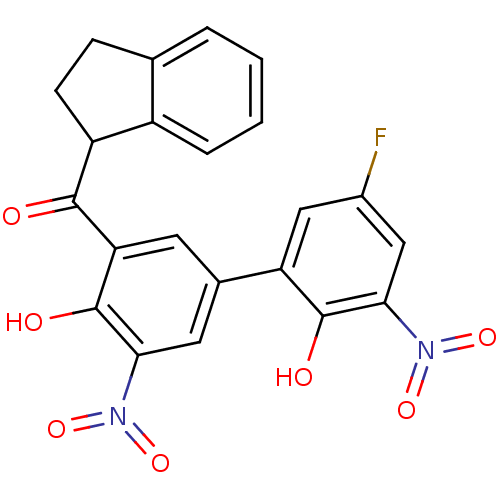 (Aryl 1-indanylketone, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.42E+3 | -29.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50164674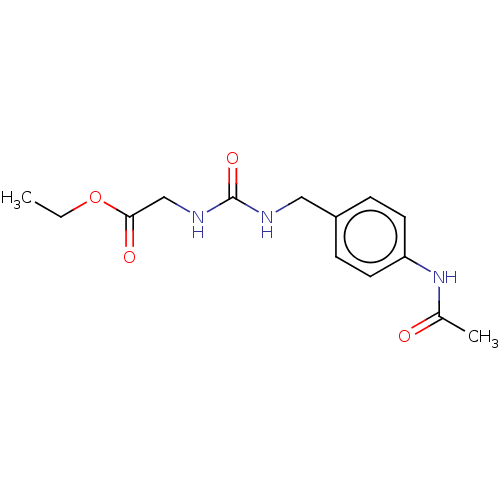 (CHEMBL3799875) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool Curated by ChEMBL | Assay Description Inhibition of Cyclophilin D (unknown origin) activity preincubated for 15 mins followed Suc-AAPF-pNA substrate addition by chymotrypsin coupled based... | J Med Chem 59: 2596-611 (2016) Article DOI: 10.1021/acs.jmedchem.5b01801 BindingDB Entry DOI: 10.7270/Q2474CR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | CHEMBL2006156 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | CHEMBL5275318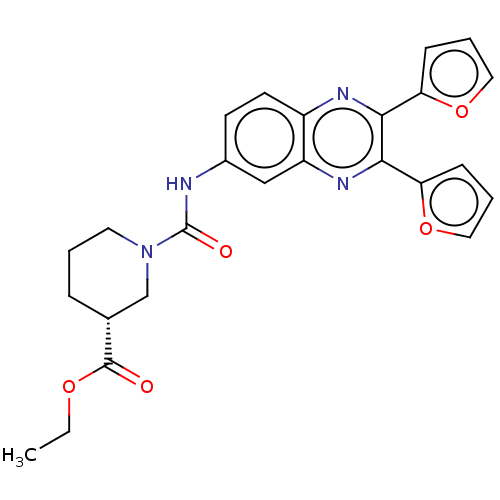 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50164673 (CHEMBL3797905) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool Curated by ChEMBL | Assay Description Inhibition of Cyclophilin D (unknown origin) activity preincubated for 15 mins followed Suc-AAPF-pNA substrate addition by chymotrypsin coupled based... | J Med Chem 59: 2596-611 (2016) Article DOI: 10.1021/acs.jmedchem.5b01801 BindingDB Entry DOI: 10.7270/Q2474CR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM139995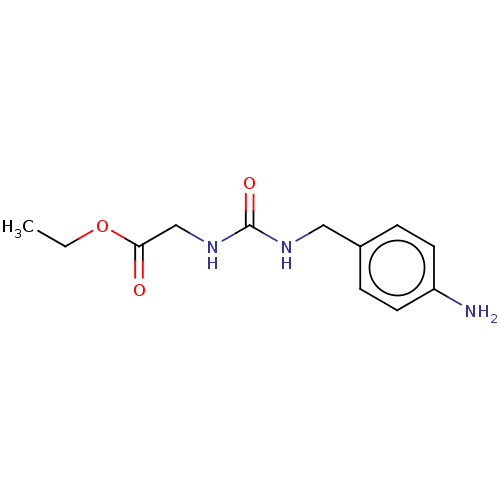 (US8901295, F428) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool Curated by ChEMBL | Assay Description Inhibition of Cyclophilin D (unknown origin) activity in absence of detergent | J Med Chem 59: 2596-611 (2016) Article DOI: 10.1021/acs.jmedchem.5b01801 BindingDB Entry DOI: 10.7270/Q2474CR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM139995 (US8901295, F428) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool Curated by ChEMBL | Assay Description Inhibition of Cyclophilin D (unknown origin) activity preincubated for 15 mins followed Suc-AAPF-pNA substrate addition by chymotrypsin coupled based... | J Med Chem 59: 2596-611 (2016) Article DOI: 10.1021/acs.jmedchem.5b01801 BindingDB Entry DOI: 10.7270/Q2474CR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM92913 (Aryl 1-indanylketone, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.28E+3 | -27.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50164669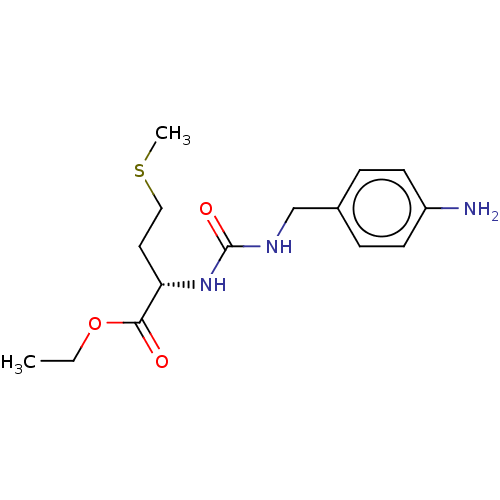 (CHEMBL3798305) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool Curated by ChEMBL | Assay Description Inhibition of Cyclophilin D (unknown origin) activity preincubated for 15 mins followed Suc-AAPF-pNA substrate addition by chymotrypsin coupled based... | J Med Chem 59: 2596-611 (2016) Article DOI: 10.1021/acs.jmedchem.5b01801 BindingDB Entry DOI: 10.7270/Q2474CR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50164671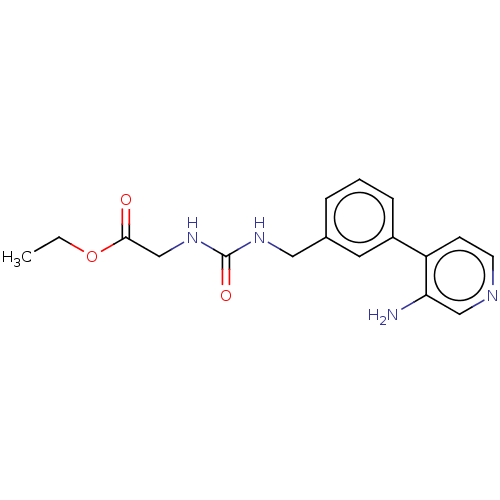 (CHEMBL3798030) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool Curated by ChEMBL | Assay Description Inhibition of Cyclophilin D (unknown origin) activity preincubated for 15 mins followed Suc-AAPF-pNA substrate addition by chymotrypsin coupled based... | J Med Chem 59: 2596-611 (2016) Article DOI: 10.1021/acs.jmedchem.5b01801 BindingDB Entry DOI: 10.7270/Q2474CR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50164665 (CHEMBL3797675) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool Curated by ChEMBL | Assay Description Inhibition of Cyclophilin D (unknown origin) activity preincubated for 15 mins followed Suc-AAPF-pNA substrate addition by chymotrypsin coupled based... | J Med Chem 59: 2596-611 (2016) Article DOI: 10.1021/acs.jmedchem.5b01801 BindingDB Entry DOI: 10.7270/Q2474CR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50022815 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of human recombinant cyclophilin D using Suc-AAPF-MCA as substrate preincubated for 1 hr followed by substrate addition measured per milli... | ACS Med Chem Lett 7: 294-9 (2016) Article DOI: 10.1021/acsmedchemlett.5b00451 BindingDB Entry DOI: 10.7270/Q24T6M8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50516083 (CHEMBL4470490) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of CypD (unknown origin) PPIase activity using N-Suc-AAPF-p-nitroanilide as substrate preincubated with enzyme for 10 mins followed by chy... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126717 BindingDB Entry DOI: 10.7270/Q2JM2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50516083 (CHEMBL4470490) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Displacement of fluorescein labelled cyclosporine A derivative from human recombinant CypD (30 to 207 residues) expressed in Escherichia coli cells i... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126717 BindingDB Entry DOI: 10.7270/Q2JM2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50516100 (CHEMBL4466871) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Displacement of fluorescein labelled cyclosporine A derivative from human recombinant CypD (30 to 207 residues) expressed in Escherichia coli cells i... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126717 BindingDB Entry DOI: 10.7270/Q2JM2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50516080 (CHEMBL4464178) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of CypD (unknown origin) PPIase activity using N-Suc-AAPF-p-nitroanilide as substrate preincubated with enzyme for 10 mins followed by chy... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126717 BindingDB Entry DOI: 10.7270/Q2JM2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM140005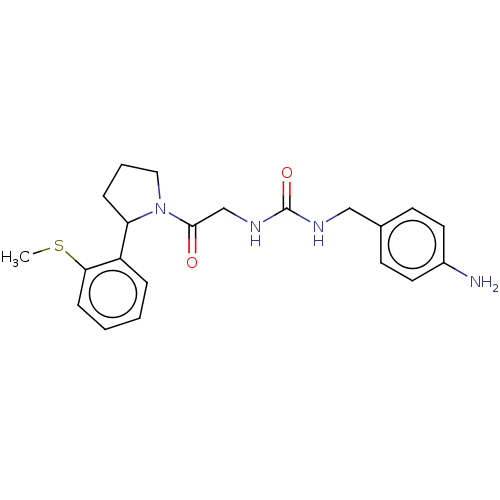 (US8901295, F684) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 640 | n/a | n/a | n/a | n/a | 7.8 | 20 |
Institut National de la Sante et de la Recherche Medicale (INSERM); Universite Montpellier 1; Universite Paris-EST Creteil val de Marne; Assistance Publique-Hopitaux de Paris; Centre National de la Recherche Scientifique US Patent | Assay Description Cyclophilin PPlase activity was measured at 20 C. by using the standard chymotrypsin coupled assay (Kofron J L, Kuzmic P, Kishore V, Colon-Bonilla E,... | US Patent US8901295 (2014) BindingDB Entry DOI: 10.7270/Q2R49PGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM140008 (US8901295, F716) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 660 | n/a | n/a | n/a | n/a | 7.8 | 20 |
Institut National de la Sante et de la Recherche Medicale (INSERM); Universite Montpellier 1; Universite Paris-EST Creteil val de Marne; Assistance Publique-Hopitaux de Paris; Centre National de la Recherche Scientifique US Patent | Assay Description Cyclophilin PPlase activity was measured at 20 C. by using the standard chymotrypsin coupled assay (Kofron J L, Kuzmic P, Kishore V, Colon-Bonilla E,... | US Patent US8901295 (2014) BindingDB Entry DOI: 10.7270/Q2R49PGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50516098 (CHEMBL4547673) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 735 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Displacement of fluorescein labelled cyclosporine A derivative from human recombinant CypD (30 to 207 residues) expressed in Escherichia coli cells i... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126717 BindingDB Entry DOI: 10.7270/Q2JM2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM140007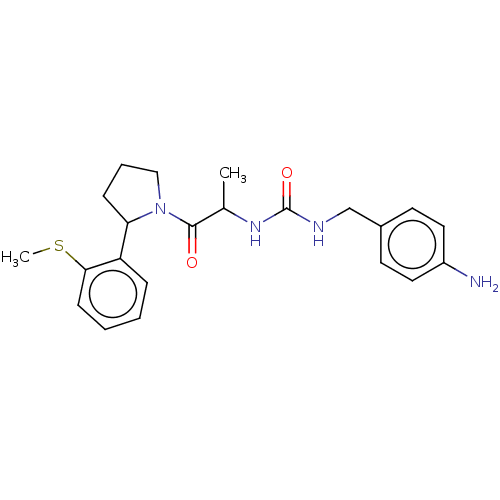 (US8901295, F714) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 7.8 | 20 |
Institut National de la Sante et de la Recherche Medicale (INSERM); Universite Montpellier 1; Universite Paris-EST Creteil val de Marne; Assistance Publique-Hopitaux de Paris; Centre National de la Recherche Scientifique US Patent | Assay Description Cyclophilin PPlase activity was measured at 20 C. by using the standard chymotrypsin coupled assay (Kofron J L, Kuzmic P, Kishore V, Colon-Bonilla E,... | US Patent US8901295 (2014) BindingDB Entry DOI: 10.7270/Q2R49PGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM140004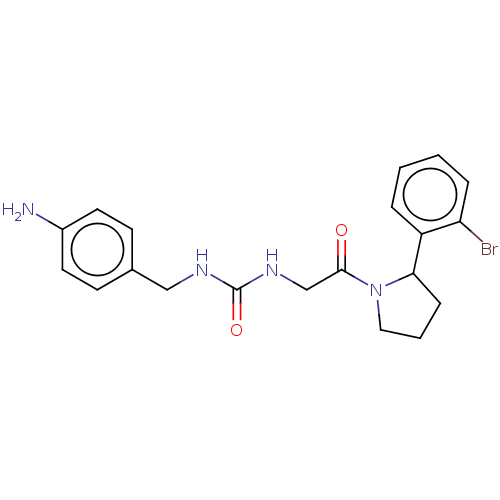 (US8901295, F680) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 7.8 | 20 |
Institut National de la Sante et de la Recherche Medicale (INSERM); Universite Montpellier 1; Universite Paris-EST Creteil val de Marne; Assistance Publique-Hopitaux de Paris; Centre National de la Recherche Scientifique US Patent | Assay Description Cyclophilin PPlase activity was measured at 20 C. by using the standard chymotrypsin coupled assay (Kofron J L, Kuzmic P, Kishore V, Colon-Bonilla E,... | US Patent US8901295 (2014) BindingDB Entry DOI: 10.7270/Q2R49PGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50516080 (CHEMBL4464178) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Displacement of fluorescein labelled cyclosporine A derivative from human recombinant CypD (30 to 207 residues) expressed in Escherichia coli cells i... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126717 BindingDB Entry DOI: 10.7270/Q2JM2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM140002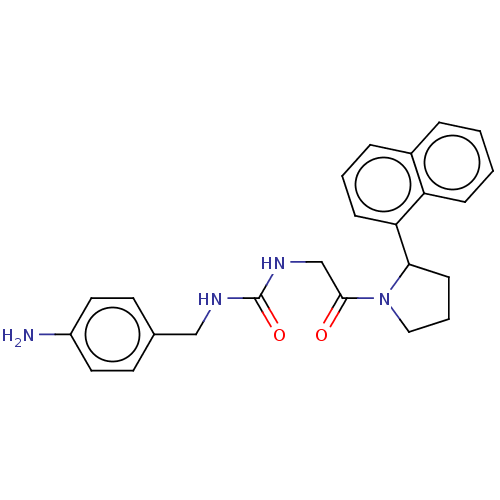 (US8901295, F671) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | 7.8 | 20 |
Institut National de la Sante et de la Recherche Medicale (INSERM); Universite Montpellier 1; Universite Paris-EST Creteil val de Marne; Assistance Publique-Hopitaux de Paris; Centre National de la Recherche Scientifique US Patent | Assay Description Cyclophilin PPlase activity was measured at 20 C. by using the standard chymotrypsin coupled assay (Kofron J L, Kuzmic P, Kishore V, Colon-Bonilla E,... | US Patent US8901295 (2014) BindingDB Entry DOI: 10.7270/Q2R49PGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50169545 (CHEMBL3805631) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of human recombinant cyclophilin D using Suc-AAPF-MCA as substrate preincubated for 1 hr followed by substrate addition measured per milli... | ACS Med Chem Lett 7: 294-9 (2016) Article DOI: 10.1021/acsmedchemlett.5b00451 BindingDB Entry DOI: 10.7270/Q24T6M8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50516082 (CHEMBL4464825) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of CypD (unknown origin) PPIase activity using N-Suc-AAPF-p-nitroanilide as substrate preincubated with enzyme for 10 mins followed by chy... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126717 BindingDB Entry DOI: 10.7270/Q2JM2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50516095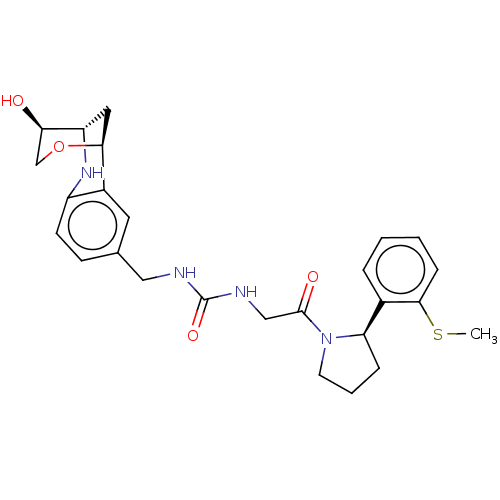 (CHEMBL4451136) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Displacement of fluorescein labelled cyclosporine A derivative from human recombinant CypD (30 to 207 residues) expressed in Escherichia coli cells i... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126717 BindingDB Entry DOI: 10.7270/Q2JM2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50516084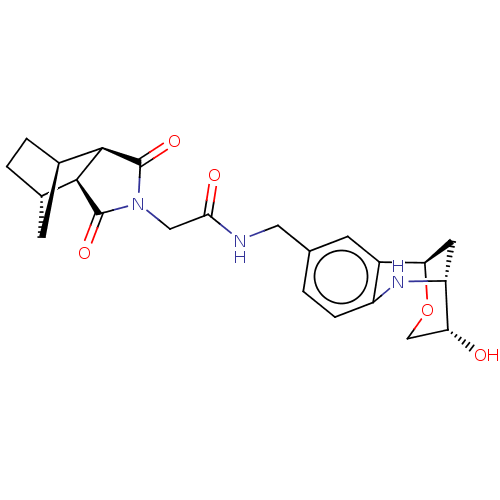 (CHEMBL4520315) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Displacement of fluorescein labelled cyclosporine A derivative from human recombinant CypD (30 to 207 residues) expressed in Escherichia coli cells i... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126717 BindingDB Entry DOI: 10.7270/Q2JM2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50516082 (CHEMBL4464825) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Displacement of fluorescein labelled cyclosporine A derivative from human recombinant CypD (30 to 207 residues) expressed in Escherichia coli cells i... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126717 BindingDB Entry DOI: 10.7270/Q2JM2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50381013 (CHEMBL2017129) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 2.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50516087 (CHEMBL4471175) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Displacement of fluorescein labelled cyclosporine A derivative from human recombinant CypD (30 to 207 residues) expressed in Escherichia coli cells i... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126717 BindingDB Entry DOI: 10.7270/Q2JM2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM140006 (US8901295, F712) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | 7.8 | 20 |
Institut National de la Sante et de la Recherche Medicale (INSERM); Universite Montpellier 1; Universite Paris-EST Creteil val de Marne; Assistance Publique-Hopitaux de Paris; Centre National de la Recherche Scientifique US Patent | Assay Description Cyclophilin PPlase activity was measured at 20 C. by using the standard chymotrypsin coupled assay (Kofron J L, Kuzmic P, Kishore V, Colon-Bonilla E,... | US Patent US8901295 (2014) BindingDB Entry DOI: 10.7270/Q2R49PGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50516096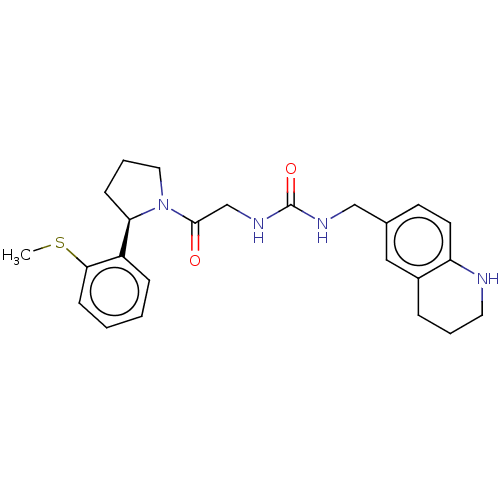 (CHEMBL4540033) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Displacement of fluorescein labelled cyclosporine A derivative from human recombinant CypD (30 to 207 residues) expressed in Escherichia coli cells i... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126717 BindingDB Entry DOI: 10.7270/Q2JM2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50516094 (CHEMBL4453162) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Displacement of fluorescein labelled cyclosporine A derivative from human recombinant CypD (30 to 207 residues) expressed in Escherichia coli cells i... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126717 BindingDB Entry DOI: 10.7270/Q2JM2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50516090 (CHEMBL4563478) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Displacement of fluorescein labelled cyclosporine A derivative from human recombinant CypD (30 to 207 residues) expressed in Escherichia coli cells i... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126717 BindingDB Entry DOI: 10.7270/Q2JM2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM139995 (US8901295, F428) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | 7.8 | 20 |
Institut National de la Sante et de la Recherche Medicale (INSERM); Universite Montpellier 1; Universite Paris-EST Creteil val de Marne; Assistance Publique-Hopitaux de Paris; Centre National de la Recherche Scientifique US Patent | Assay Description Cyclophilin PPlase activity was measured at 20 C. by using the standard chymotrypsin coupled assay (Kofron J L, Kuzmic P, Kishore V, Colon-Bonilla E,... | US Patent US8901295 (2014) BindingDB Entry DOI: 10.7270/Q2R49PGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM140003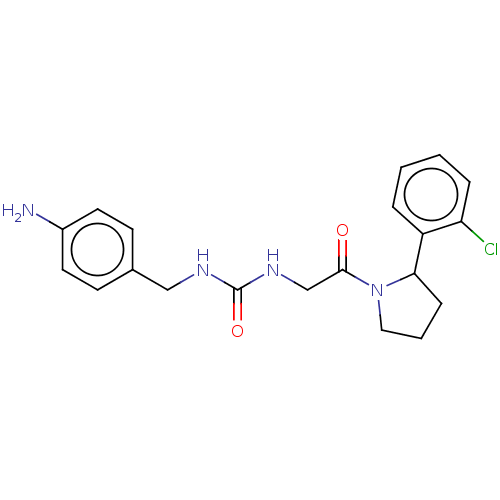 (US8901295, F673) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | 7.8 | 20 |
Institut National de la Sante et de la Recherche Medicale (INSERM); Universite Montpellier 1; Universite Paris-EST Creteil val de Marne; Assistance Publique-Hopitaux de Paris; Centre National de la Recherche Scientifique US Patent | Assay Description Cyclophilin PPlase activity was measured at 20 C. by using the standard chymotrypsin coupled assay (Kofron J L, Kuzmic P, Kishore V, Colon-Bonilla E,... | US Patent US8901295 (2014) BindingDB Entry DOI: 10.7270/Q2R49PGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM92913 (Aryl 1-indanylketone, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 6.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50516096 (CHEMBL4540033) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of CypD (unknown origin) PPIase activity using N-Suc-AAPF-p-nitroanilide as substrate preincubated with enzyme for 10 mins followed by chy... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126717 BindingDB Entry DOI: 10.7270/Q2JM2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM394270 (US9975902, Example 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The binding capacity of the compounds was measured using a competition Fluorescence-Polarisation based assay with fluorescine labelled cyclosporin. | J Med Chem 52: 2724-32 (2009) BindingDB Entry DOI: 10.7270/Q26W9DD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM394272 (US9975902, Example 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The binding capacity of the compounds was measured using a competition Fluorescence-Polarisation based assay with fluorescine labelled cyclosporin. | J Med Chem 52: 2724-32 (2009) BindingDB Entry DOI: 10.7270/Q26W9DD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM394272 (US9975902, Example 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The peptidyl-proline isomerase activity (PPase) was determined by using a PPase-chymotrypsin coupled assay with suc-AAPF-p-NA as substrated and color... | J Med Chem 52: 2724-32 (2009) BindingDB Entry DOI: 10.7270/Q26W9DD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 94 total ) | Next | Last >> |