

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50071058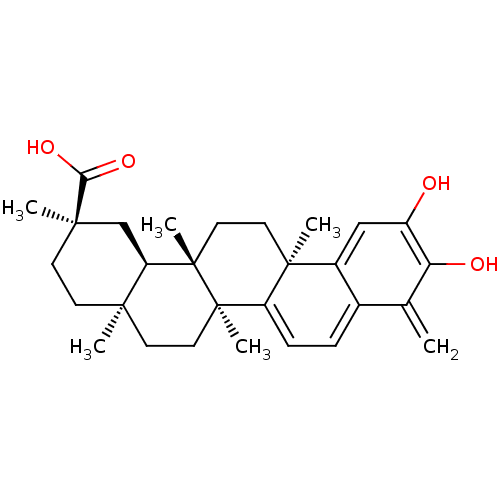 ((2R,4aS,6aS,12bR,14aS,14bR)-10-Hydroxy-2,4a,6a,9,1...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | 7.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50318484 (2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of CYP2C11 in rat liver microsomes | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00724 BindingDB Entry DOI: 10.7270/Q2697778 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50505787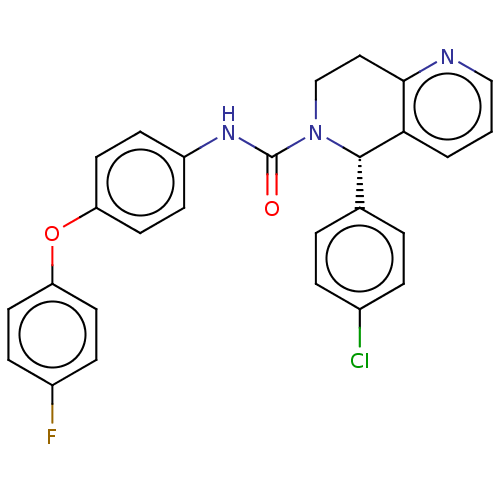 (CHEMBL4537998) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human thyroid stimulating hormone receptor expressed in rat HTC133 cells assessed as reduction in agonist-induced cAMP product... | J Med Chem 62: 10321-10341 (2019) Article DOI: 10.1021/acs.jmedchem.9b01382 BindingDB Entry DOI: 10.7270/Q29P34X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50071058 ((2R,4aS,6aS,12bR,14aS,14bR)-10-Hydroxy-2,4a,6a,9,1...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50004441 ((2R,4R)-ketoconazole | 1-acetyl-4-(4-{[(2R,4R)-2-(...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Inhibition of cytochrome P450 progesterone 2-alpha-hydroxylase | J Med Chem 35: 2818-25 (1992) BindingDB Entry DOI: 10.7270/Q24Q7SZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50505783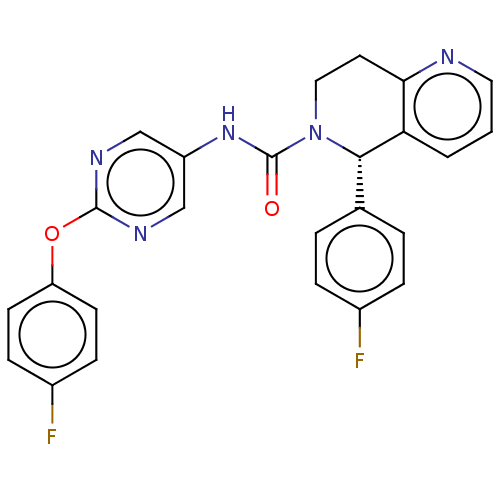 (CHEMBL4458424) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human thyroid stimulating hormone receptor expressed in rat HTC133 cells assessed as reduction in agonist-induced cAMP product... | J Med Chem 62: 10321-10341 (2019) Article DOI: 10.1021/acs.jmedchem.9b01382 BindingDB Entry DOI: 10.7270/Q29P34X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50505789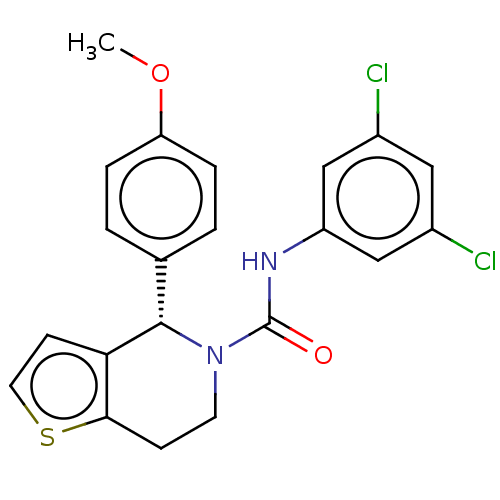 (CHEMBL4471621) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human thyroid stimulating hormone receptor expressed in rat HTC133 cells assessed as reduction in agonist-induced cAMP product... | J Med Chem 62: 10321-10341 (2019) Article DOI: 10.1021/acs.jmedchem.9b01382 BindingDB Entry DOI: 10.7270/Q29P34X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50505793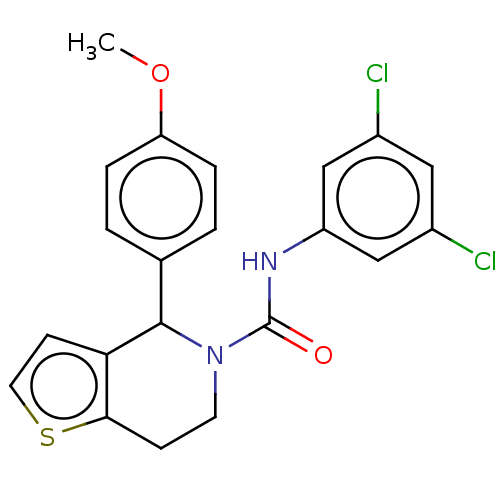 (CHEMBL4593222) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human thyroid stimulating hormone receptor expressed in rat HTC133 cells assessed as reduction in agonist-induced cAMP product... | J Med Chem 62: 10321-10341 (2019) Article DOI: 10.1021/acs.jmedchem.9b01382 BindingDB Entry DOI: 10.7270/Q29P34X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50505788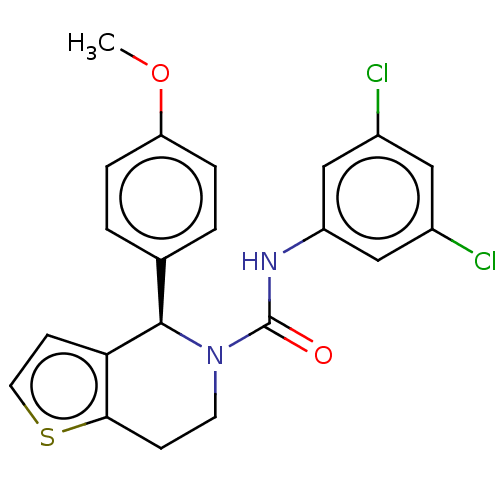 (CHEMBL4449797) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human thyroid stimulating hormone receptor expressed in rat HTC133 cells assessed as reduction in agonist-induced cAMP product... | J Med Chem 62: 10321-10341 (2019) Article DOI: 10.1021/acs.jmedchem.9b01382 BindingDB Entry DOI: 10.7270/Q29P34X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50004442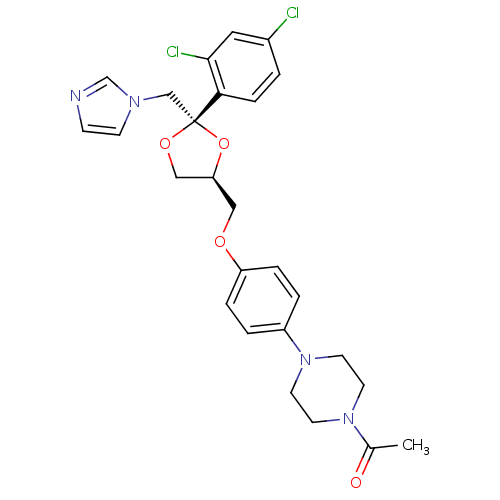 ((2S,4S)-ketoconazole | 1-acetyl-4-(4-{[(2S,4S)-2-(...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 3.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Inhibition of cytochrome P450 progesterone 2-alpha-hydroxylase | J Med Chem 35: 2818-25 (1992) BindingDB Entry DOI: 10.7270/Q24Q7SZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM31768 (CHEMBL295698 | Ketoconazole | Nizoral | Panfungol) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 8.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Inhibition of cytochrome P450 progesterone 15-alpha hydroxylase | J Med Chem 35: 2818-25 (1992) BindingDB Entry DOI: 10.7270/Q24Q7SZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50426305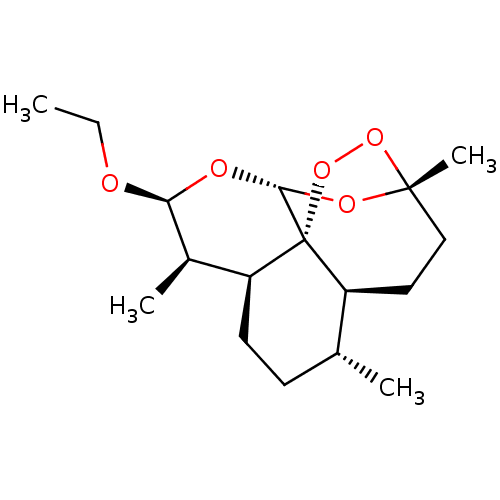 (ARTEMOTIL) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Medicinal& Process Chemistry, Division of Parasitology, Division of Pharmacokinetics and Metabolism, and Sophisticated Analytical Instrument Facility, Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2C11 in rat liver microsome using diclofenac as substrate by HPLC-PDA analysis | ACS Med Chem Lett 4: 165-9 (2013) Article DOI: 10.1021/ml300188t BindingDB Entry DOI: 10.7270/Q2V1264R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM31768 (CHEMBL295698 | Ketoconazole | Nizoral | Panfungol) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Inhibition of cytochrome P450 progesterone 2-alpha-hydroxylase | J Med Chem 35: 2818-25 (1992) BindingDB Entry DOI: 10.7270/Q24Q7SZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496708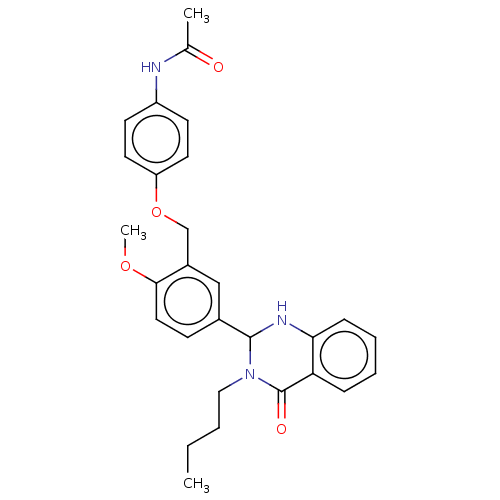 (CHEMBL1315387) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 830 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496707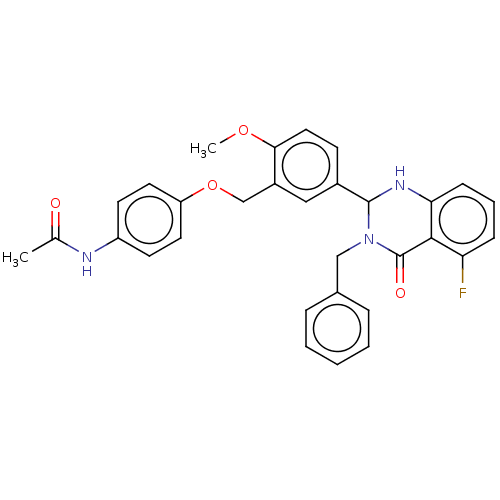 (CHEMBL1708839) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 280 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496710 (CHEMBL1563670) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.16E+3 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496711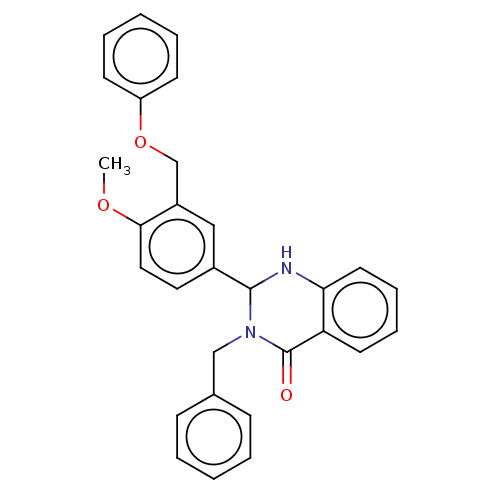 (CHEMBL1729165) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.57E+3 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496712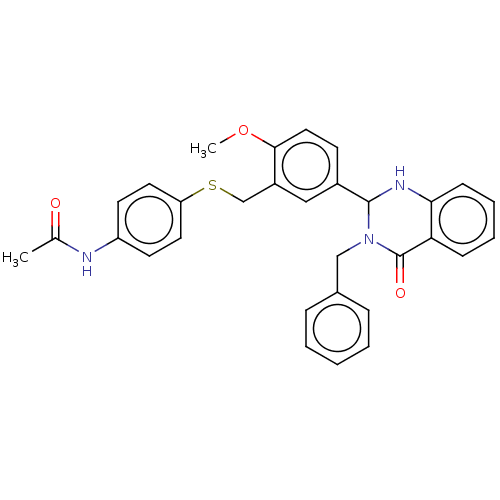 (CHEMBL1719708) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496713 (CHEMBL1715624) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496714 (CHEMBL1718442) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 550 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496715 (CHEMBL1705547) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496716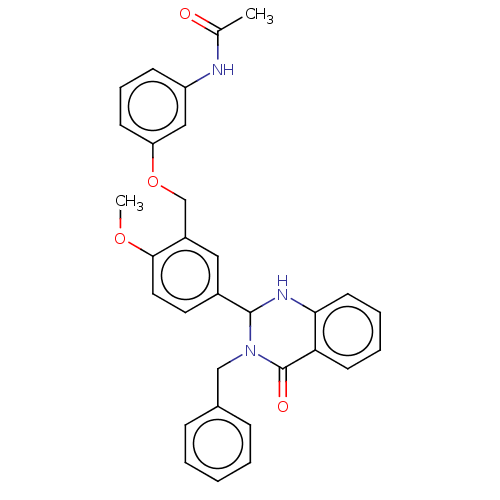 (CHEMBL1706950) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.34E+3 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496717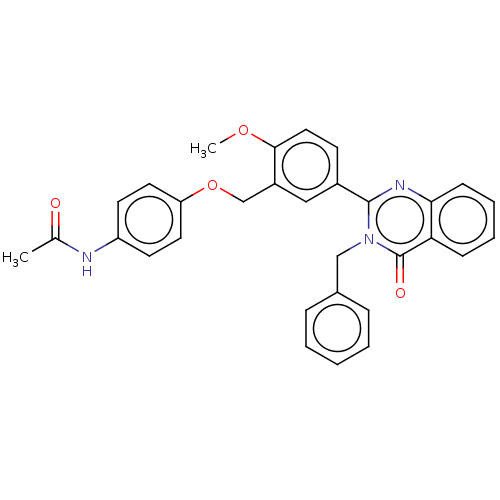 (CHEMBL1357440) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 960 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496718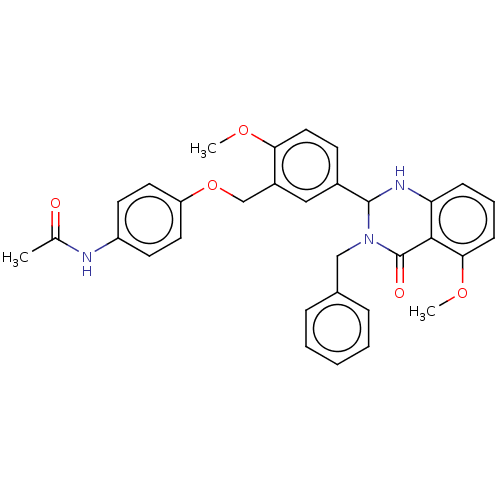 (CHEMBL1473939) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.48E+3 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496719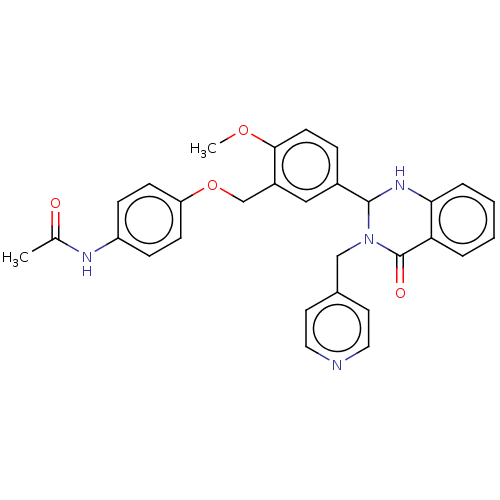 (CHEMBL1571865) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496706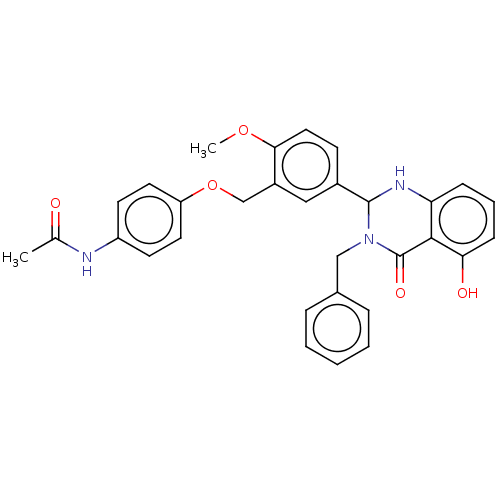 (CHEMBL1515891) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496705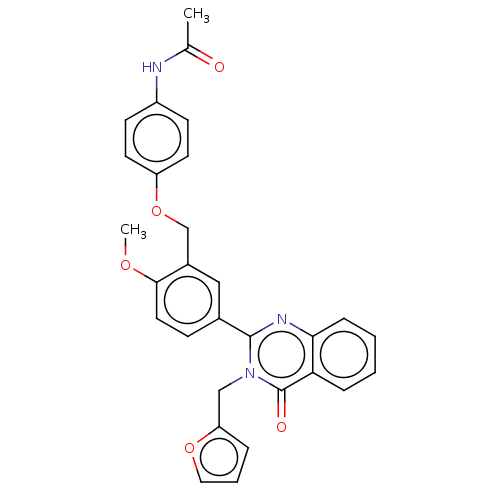 (CHEMBL1370580) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 930 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496704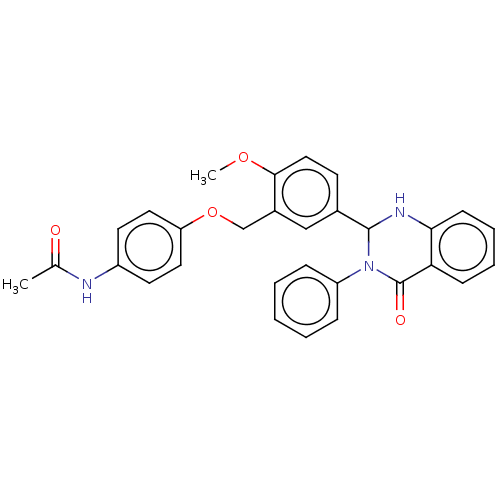 (CHEMBL1701447) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 5.09E+3 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50189778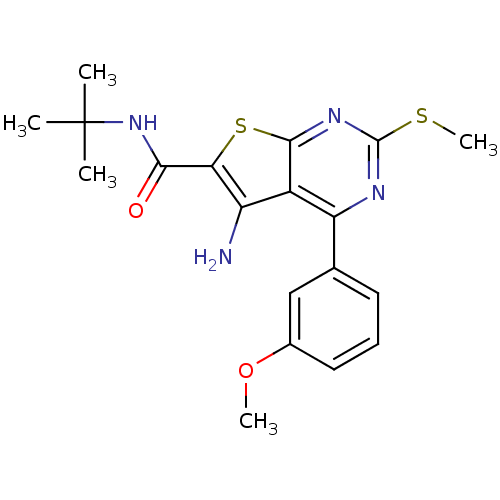 (CHEMBL211405 | N-tert-butyl-5-amino-4-(3-methoxyph...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496703 (CHEMBL1589735) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 430 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496702 (CHEMBL1331194) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM83388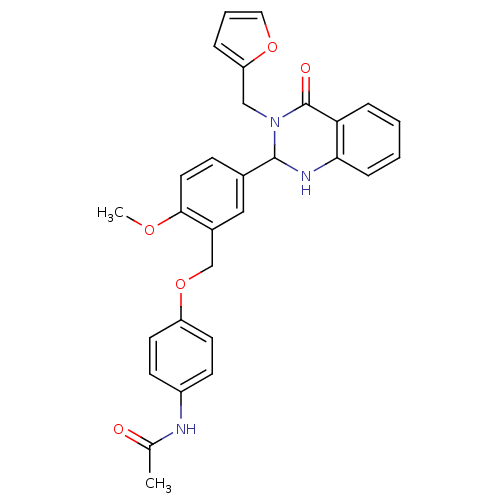 (MLS001073997 | N-[4-[5-[3-(2-furfuryl)-4-keto-1,2-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496709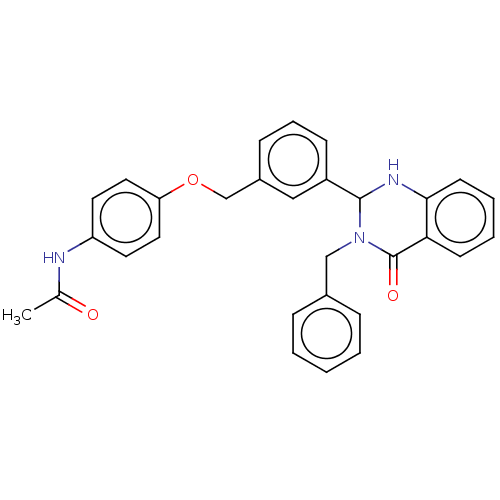 (CHEMBL1720417) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||