 Found 10231 hits Enz. Inhib. hit(s) with Target = 'Dipeptidyl peptidase IV'
Found 10231 hits Enz. Inhib. hit(s) with Target = 'Dipeptidyl peptidase IV' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050525
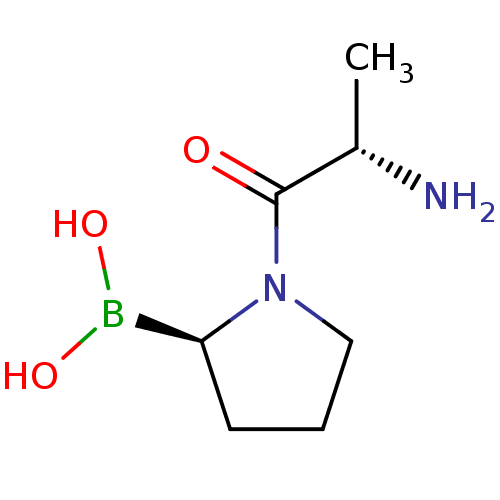
((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...)Show InChI InChI=1S/C7H15BN2O3/c1-5(9)7(11)10-4-2-3-6(10)8(12)13/h5-6,12-13H,2-4,9H2,1H3/t5-,6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 50: 2391-8 (2007)
Article DOI: 10.1021/jm061321+
BindingDB Entry DOI: 10.7270/Q2NC6407 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50146972
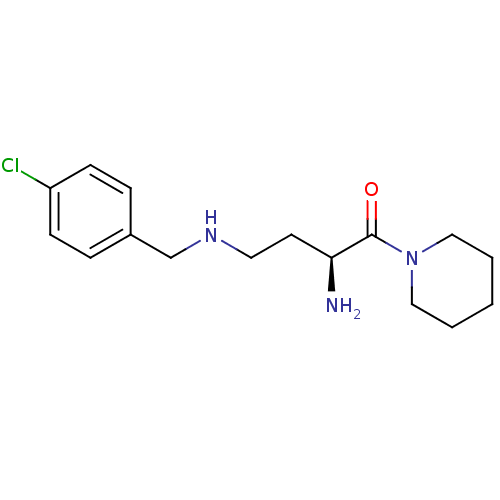
((S)-4-(4-chlorobenzylamino)-2-amino-1-(piperidin-1...)Show InChI InChI=1S/C16H24ClN3O/c17-14-6-4-13(5-7-14)12-19-9-8-15(18)16(21)20-10-2-1-3-11-20/h4-7,15,19H,1-3,8-12,18H2/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 16: 4777-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.082
BindingDB Entry DOI: 10.7270/Q2GM86XS |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324510
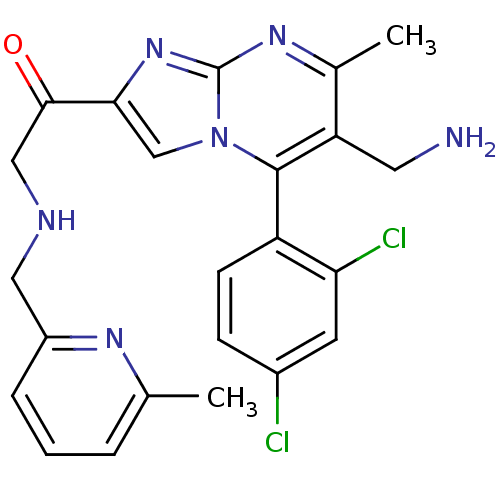
(1-(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl...)Show SMILES Cc1cccc(CNCC(=O)c2cn3c(c(CN)c(C)nc3n2)-c2ccc(Cl)cc2Cl)n1 |(-4.59,-8.35,;-3.87,-6.99,;-4.69,-5.68,;-3.96,-4.31,;-2.42,-4.28,;-1.62,-5.59,;-.08,-5.54,;.65,-4.18,;2.19,-4.14,;2.92,-2.78,;2.11,-1.48,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.17,-3.27,;9.45,-1.81,;10.76,-1,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.48,-6.48,;10.83,-5.74,;-2.34,-6.93,)| Show InChI InChI=1S/C23H22Cl2N6O/c1-13-4-3-5-16(28-13)10-27-11-21(32)20-12-31-22(17-7-6-15(24)8-19(17)25)18(9-26)14(2)29-23(31)30-20/h3-8,12,27H,9-11,26H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050513
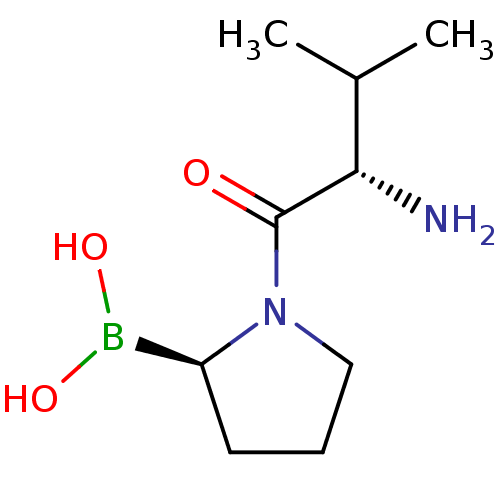
((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116748
BindingDB Entry DOI: 10.7270/Q2MG7TGW |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050513

((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 50: 2391-8 (2007)
Article DOI: 10.1021/jm061321+
BindingDB Entry DOI: 10.7270/Q2NC6407 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050513

((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324512
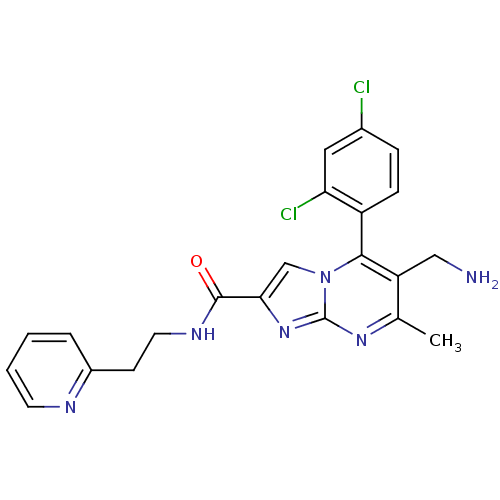
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl-N-...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)NCCc1ccccn1 |(10.76,-1,;9.45,-1.81,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.17,-3.27,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.48,-6.48,;10.83,-5.74,;2.92,-2.78,;2.11,-1.48,;2.19,-4.14,;.65,-4.18,;-.08,-5.54,;-1.62,-5.59,;-2.34,-6.93,;-3.87,-6.99,;-4.69,-5.68,;-3.96,-4.31,;-2.42,-4.28,)| Show InChI InChI=1S/C22H20Cl2N6O/c1-13-17(11-25)20(16-6-5-14(23)10-18(16)24)30-12-19(29-22(30)28-13)21(31)27-9-7-15-4-2-3-8-26-15/h2-6,8,10,12H,7,9,11,25H2,1H3,(H,27,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324523
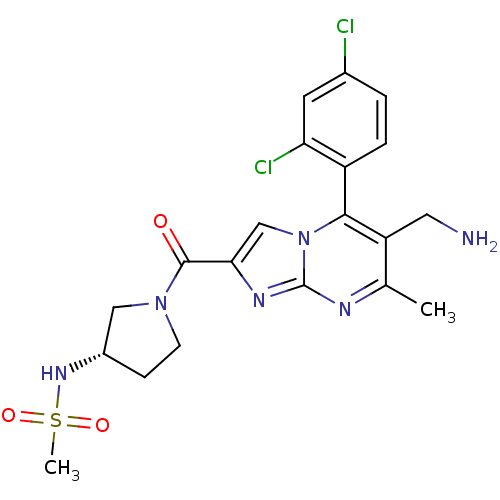
(CHEMBL1215018 | N-((3S)-1-(6-(aminomethyl)-5-(2,4-...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)N1CC[C@@H](C1)NS(C)(=O)=O |r,wD:25.30,(7.23,-5.85,;5.92,-6.66,;4.56,-5.95,;3.26,-6.75,;1.79,-6.32,;.92,-7.59,;1.87,-8.81,;3.31,-8.29,;4.66,-9.02,;5.97,-8.21,;7.33,-8.93,;8.64,-8.12,;4.63,-10.55,;3.28,-11.29,;3.25,-12.83,;4.57,-13.63,;4.54,-15.17,;5.92,-12.87,;5.95,-11.34,;7.29,-10.6,;-.62,-7.63,;-1.43,-6.33,;-1.34,-8.99,;-.88,-10.46,;-2.12,-11.36,;-3.37,-10.46,;-2.89,-8.99,;-4.91,-10.48,;-5.99,-9.37,;-7.4,-10.02,;-6.68,-7.99,;-4.84,-8.35,)| Show InChI InChI=1S/C20H22Cl2N6O3S/c1-11-15(8-23)18(14-4-3-12(21)7-16(14)22)28-10-17(25-20(28)24-11)19(29)27-6-5-13(9-27)26-32(2,30)31/h3-4,7,10,13,26H,5-6,8-9,23H2,1-2H3/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356582
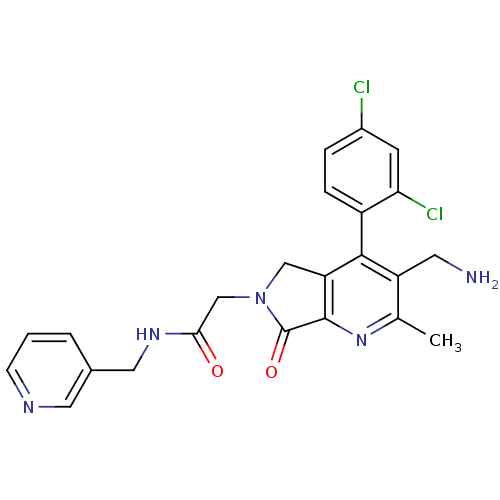
(CHEMBL1910126)Show SMILES Cc1nc2C(=O)N(CC(=O)NCc3cccnc3)Cc2c(c1CN)-c1ccc(Cl)cc1Cl |(14.01,-46.7,;12.68,-47.48,;11.34,-46.71,;10.02,-47.49,;8.55,-47.01,;8.08,-45.55,;7.65,-48.25,;6.11,-48.26,;5.34,-49.59,;6.12,-50.92,;3.8,-49.59,;3.04,-50.93,;1.5,-50.93,;.74,-52.26,;-.8,-52.27,;-1.58,-50.93,;-.81,-49.6,;.73,-49.59,;8.55,-49.5,;10.01,-49.03,;11.35,-49.8,;12.68,-49.03,;14.02,-49.79,;15.35,-49.02,;11.35,-51.33,;10.01,-52.1,;10.01,-53.64,;11.35,-54.41,;11.35,-55.95,;12.69,-53.64,;12.68,-52.1,;14.01,-51.33,)| Show InChI InChI=1S/C23H21Cl2N5O2/c1-13-17(8-26)21(16-5-4-15(24)7-19(16)25)18-11-30(23(32)22(18)29-13)12-20(31)28-10-14-3-2-6-27-9-14/h2-7,9H,8,10-12,26H2,1H3,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356591

(CHEMBL1910117)Show SMILES CN(C)C(=O)CN1Cc2c(nc(C)c(CN)c2-c2ccc(Cl)cc2Cl)C1=O |(23.14,-19.79,;23.91,-21.12,;23.14,-22.45,;25.45,-21.12,;26.22,-22.45,;26.22,-19.78,;27.75,-19.78,;28.65,-21.03,;30.12,-20.55,;30.12,-19.01,;31.45,-18.24,;32.78,-19,;34.12,-18.23,;32.79,-20.55,;34.13,-21.32,;35.46,-20.55,;31.45,-21.32,;31.46,-22.86,;30.12,-23.63,;30.12,-25.17,;31.45,-25.94,;31.45,-27.48,;32.79,-25.16,;32.79,-23.62,;34.12,-22.85,;28.66,-18.54,;28.18,-17.07,)| Show InChI InChI=1S/C19H20Cl2N4O2/c1-10-13(7-22)17(12-5-4-11(20)6-15(12)21)14-8-25(9-16(26)24(2)3)19(27)18(14)23-10/h4-6H,7-9,22H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324512

(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl-N-...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)NCCc1ccccn1 |(10.76,-1,;9.45,-1.81,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.17,-3.27,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.48,-6.48,;10.83,-5.74,;2.92,-2.78,;2.11,-1.48,;2.19,-4.14,;.65,-4.18,;-.08,-5.54,;-1.62,-5.59,;-2.34,-6.93,;-3.87,-6.99,;-4.69,-5.68,;-3.96,-4.31,;-2.42,-4.28,)| Show InChI InChI=1S/C22H20Cl2N6O/c1-13-17(11-25)20(16-6-5-14(23)10-18(16)24)30-12-19(29-22(30)28-13)21(31)27-9-7-15-4-2-3-8-26-15/h2-6,8,10,12H,7,9,11,25H2,1H3,(H,27,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050525

((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...)Show InChI InChI=1S/C7H15BN2O3/c1-5(9)7(11)10-4-2-3-6(10)8(12)13/h5-6,12-13H,2-4,9H2,1H3/t5-,6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324510

(1-(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl...)Show SMILES Cc1cccc(CNCC(=O)c2cn3c(c(CN)c(C)nc3n2)-c2ccc(Cl)cc2Cl)n1 |(-4.59,-8.35,;-3.87,-6.99,;-4.69,-5.68,;-3.96,-4.31,;-2.42,-4.28,;-1.62,-5.59,;-.08,-5.54,;.65,-4.18,;2.19,-4.14,;2.92,-2.78,;2.11,-1.48,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.17,-3.27,;9.45,-1.81,;10.76,-1,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.48,-6.48,;10.83,-5.74,;-2.34,-6.93,)| Show InChI InChI=1S/C23H22Cl2N6O/c1-13-4-3-5-16(28-13)10-27-11-21(32)20-12-31-22(17-7-6-15(24)8-19(17)25)18(9-26)14(2)29-23(31)30-20/h3-8,12,27H,9-11,26H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356589
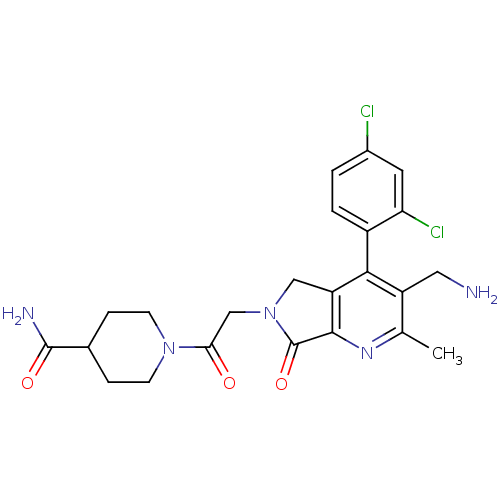
(CHEMBL1910119)Show SMILES Cc1nc2C(=O)N(CC(=O)N3CCC(CC3)C(N)=O)Cc2c(c1CN)-c1ccc(Cl)cc1Cl |(6.41,-30.16,;5.08,-30.93,;3.75,-30.17,;2.42,-30.95,;.95,-30.47,;.48,-29,;.05,-31.71,;-1.49,-31.71,;-2.25,-33.05,;-1.48,-34.38,;-3.79,-33.05,;-4.56,-31.72,;-6.09,-31.72,;-6.87,-33.05,;-6.1,-34.38,;-4.56,-34.39,;-8.41,-33.04,;-9.18,-31.71,;-9.18,-34.38,;.95,-32.96,;2.41,-32.48,;3.75,-33.26,;5.09,-32.48,;6.42,-33.25,;7.75,-32.48,;3.75,-34.79,;2.41,-35.56,;2.41,-37.1,;3.75,-37.87,;3.75,-39.41,;5.09,-37.09,;5.08,-35.56,;6.41,-34.78,)| Show InChI InChI=1S/C23H25Cl2N5O3/c1-12-16(9-26)20(15-3-2-14(24)8-18(15)25)17-10-30(23(33)21(17)28-12)11-19(31)29-6-4-13(5-7-29)22(27)32/h2-3,8,13H,4-7,9-11,26H2,1H3,(H2,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324511
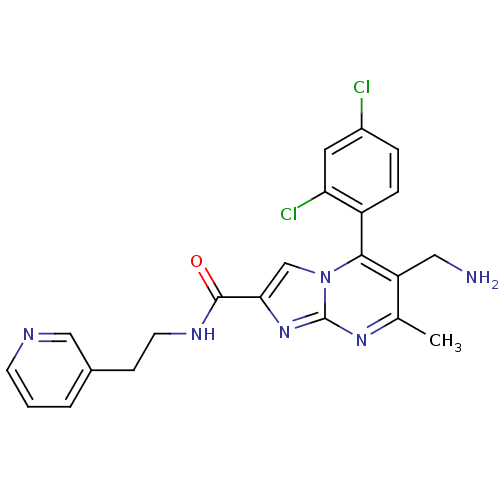
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl-N-...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)NCCc1cccnc1 |(10.76,-1,;9.45,-1.81,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.18,-3.27,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.49,-6.48,;10.83,-5.74,;2.92,-2.78,;2.11,-1.48,;2.19,-4.14,;.65,-4.18,;-.08,-5.54,;-1.62,-5.59,;-2.34,-6.94,;-3.87,-6.99,;-4.69,-5.68,;-3.96,-4.32,;-2.42,-4.28,)| Show InChI InChI=1S/C22H20Cl2N6O/c1-13-17(10-25)20(16-5-4-15(23)9-18(16)24)30-12-19(29-22(30)28-13)21(31)27-8-6-14-3-2-7-26-11-14/h2-5,7,9,11-12H,6,8,10,25H2,1H3,(H,27,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356585

(CHEMBL1910123)Show SMILES Cc1nc2C(=O)N(CC(=O)N3CCCC3)Cc2c(c1CN)-c1ccc(Cl)cc1Cl |(22.17,-39.13,;20.84,-39.9,;19.5,-39.14,;18.17,-39.91,;16.71,-39.44,;16.24,-37.97,;15.8,-40.68,;14.27,-40.68,;13.5,-42.02,;14.28,-43.35,;11.96,-42.02,;11.05,-40.78,;9.59,-41.26,;9.59,-42.8,;11.06,-43.27,;16.71,-41.93,;18.17,-41.45,;19.51,-42.22,;20.84,-41.45,;22.18,-42.22,;23.51,-41.45,;19.51,-43.76,;18.17,-44.53,;18.17,-46.07,;19.51,-46.84,;19.51,-48.38,;20.84,-46.06,;20.84,-44.52,;22.17,-43.75,)| Show InChI InChI=1S/C21H22Cl2N4O2/c1-12-15(9-24)19(14-5-4-13(22)8-17(14)23)16-10-27(21(29)20(16)25-12)11-18(28)26-6-2-3-7-26/h4-5,8H,2-3,6-7,9-11,24H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324525
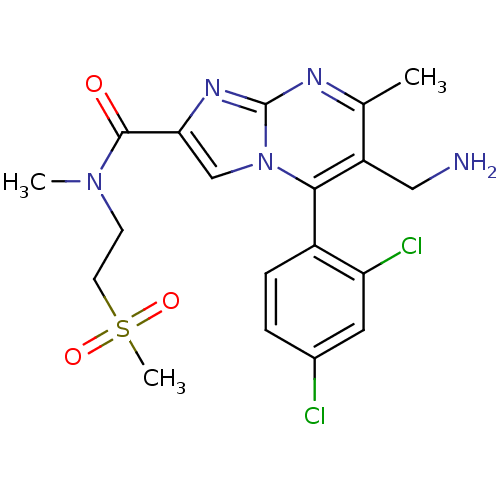
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-N,7-dimethy...)Show SMILES CN(CCS(C)(=O)=O)C(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(2.96,-5.01,;2.15,-3.7,;.61,-3.75,;-.19,-2.43,;-1.73,-2.48,;-2.54,-1.17,;-3.23,-2.88,;-1.74,-4.02,;2.88,-2.34,;2.07,-1.03,;4.42,-2.29,;5.36,-3.51,;6.81,-2.99,;8.16,-3.72,;9.47,-2.91,;10.83,-3.64,;12.13,-2.83,;9.42,-1.37,;10.73,-.55,;8.06,-.65,;6.76,-1.46,;5.29,-1.03,;8.13,-5.25,;6.78,-5.99,;6.75,-7.53,;8.07,-8.33,;8.04,-9.87,;9.42,-7.58,;9.44,-6.05,;10.79,-5.3,)| Show InChI InChI=1S/C19H21Cl2N5O3S/c1-11-14(9-22)17(13-5-4-12(20)8-15(13)21)26-10-16(24-19(26)23-11)18(27)25(2)6-7-30(3,28)29/h4-5,8,10H,6-7,9,22H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50442192

(CHEMBL2441845)Show SMILES Cc1[nH]c2cn(CCC(O)=O)c(=O)c2c(c1CN)-c1ccc(Cl)cc1Cl |(11.05,-4.3,;9.72,-5.08,;8.38,-4.32,;7.05,-5.08,;5.58,-4.61,;4.67,-5.87,;3.13,-5.88,;2.36,-4.55,;3.12,-3.21,;2.34,-1.88,;4.66,-3.2,;5.59,-7.12,;5.12,-8.58,;7.06,-6.63,;8.39,-7.4,;9.72,-6.63,;11.06,-7.4,;12.39,-6.62,;8.38,-8.94,;7.05,-9.7,;7.04,-11.24,;8.37,-12.01,;8.37,-13.55,;9.71,-11.24,;9.71,-9.71,;11.05,-8.93,)| Show InChI InChI=1S/C18H17Cl2N3O3/c1-9-12(7-21)16(11-3-2-10(19)6-13(11)20)17-14(22-9)8-23(18(17)26)5-4-15(24)25/h2-3,6,8,22H,4-5,7,21H2,1H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 (unknown origin) |
J Med Chem 56: 7343-57 (2013)
Article DOI: 10.1021/jm4008906
BindingDB Entry DOI: 10.7270/Q2N01806 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356584
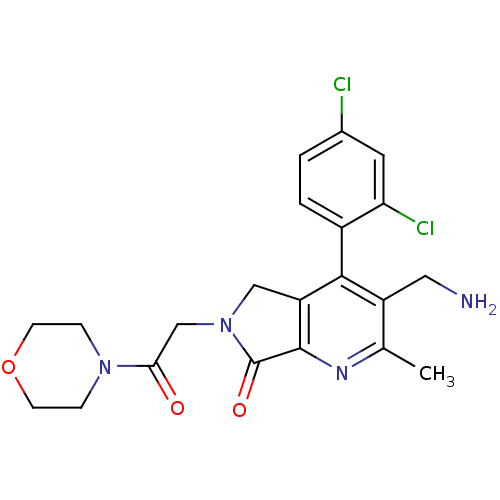
(CHEMBL1910124)Show SMILES Cc1nc2C(=O)N(CC(=O)N3CCOCC3)Cc2c(c1CN)-c1ccc(Cl)cc1Cl |(23.9,-37.72,;22.57,-38.49,;21.24,-37.73,;19.91,-38.51,;18.45,-38.03,;17.97,-36.56,;17.54,-39.27,;16.01,-39.27,;15.24,-40.61,;16.01,-41.94,;13.7,-40.61,;12.93,-39.28,;11.4,-39.28,;10.62,-40.61,;11.39,-41.94,;12.94,-41.95,;18.44,-40.52,;19.91,-40.04,;21.24,-40.82,;22.58,-40.04,;23.91,-40.81,;25.25,-40.04,;21.24,-42.35,;19.91,-43.12,;19.91,-44.66,;21.24,-45.43,;21.24,-46.97,;22.58,-44.65,;22.57,-43.12,;23.9,-42.34,)| Show InChI InChI=1S/C21H22Cl2N4O3/c1-12-15(9-24)19(14-3-2-13(22)8-17(14)23)16-10-27(21(29)20(16)25-12)11-18(28)26-4-6-30-7-5-26/h2-3,8H,4-7,9-11,24H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356592

(CHEMBL1910116)Show SMILES CNC(=O)CN1Cc2c(nc(C)c(CN)c2-c2ccc(Cl)cc2Cl)C1=O |(7.18,-19.79,;7.95,-21.12,;9.49,-21.12,;10.26,-22.45,;10.26,-19.78,;11.79,-19.78,;12.69,-21.03,;14.16,-20.55,;14.16,-19.01,;15.49,-18.24,;16.82,-19,;18.16,-18.23,;16.83,-20.55,;18.17,-21.32,;19.5,-20.55,;15.49,-21.32,;15.5,-22.86,;14.16,-23.63,;14.16,-25.17,;15.49,-25.94,;15.49,-27.48,;16.83,-25.16,;16.83,-23.62,;18.16,-22.85,;12.7,-18.54,;12.22,-17.07,)| Show InChI InChI=1S/C18H18Cl2N4O2/c1-9-12(6-21)16(11-4-3-10(19)5-14(11)20)13-7-24(8-15(25)22-2)18(26)17(13)23-9/h3-5H,6-8,21H2,1-2H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050511
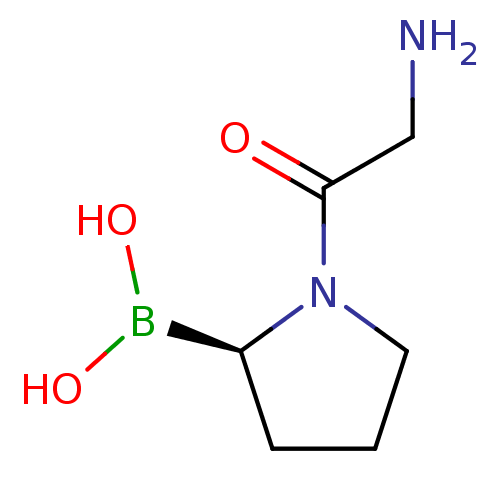
((R)-1-(2-aminoacetyl)pyrrolidin-2-ylboronic acid |...)Show InChI InChI=1S/C6H13BN2O3/c8-4-6(10)9-3-1-2-5(9)7(11)12/h5,11-12H,1-4,8H2/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356583

(CHEMBL1910125)Show SMILES Cc1nc2C(=O)N(CC(=O)Nc3ccnn3C)Cc2c(c1CN)-c1ccc(Cl)cc1Cl |(39.71,-37.55,;38.38,-38.32,;37.04,-37.56,;35.71,-38.33,;34.25,-37.86,;33.78,-36.39,;33.34,-39.1,;31.81,-39.1,;31.04,-40.44,;31.81,-41.77,;29.5,-40.44,;28.73,-41.77,;27.21,-41.94,;26.89,-43.44,;28.22,-44.21,;29.37,-43.18,;30.87,-43.5,;34.25,-40.35,;35.71,-39.87,;37.05,-40.64,;38.38,-39.87,;39.72,-40.64,;41.05,-39.87,;37.05,-42.18,;35.71,-42.95,;35.71,-44.49,;37.04,-45.26,;37.05,-46.8,;38.38,-44.48,;38.38,-42.94,;39.71,-42.17,)| Show InChI InChI=1S/C21H20Cl2N6O2/c1-11-14(8-24)19(13-4-3-12(22)7-16(13)23)15-9-29(21(31)20(15)26-11)10-18(30)27-17-5-6-25-28(17)2/h3-7H,8-10,24H2,1-2H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324500
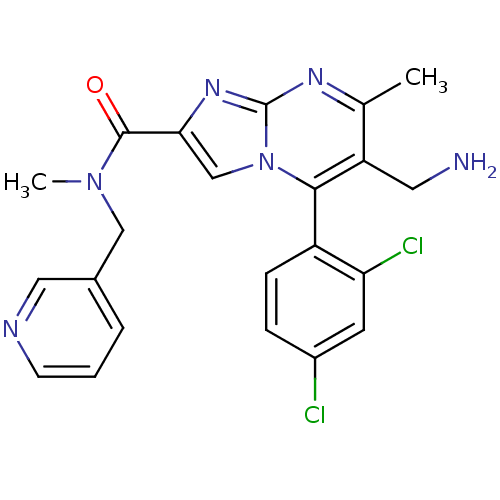
(1-(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl...)Show SMILES CN(Cc1cccnc1)C(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(4.9,-13.73,;4.13,-12.4,;2.59,-12.4,;1.82,-13.73,;.28,-13.72,;-.49,-15.05,;.28,-16.39,;1.83,-16.4,;2.59,-15.06,;4.91,-11.07,;4.14,-9.74,;6.44,-11.06,;7.34,-12.29,;8.8,-11.82,;10.13,-12.59,;11.47,-11.82,;12.8,-12.6,;14.14,-11.85,;11.46,-10.28,;12.8,-9.51,;10.13,-9.51,;8.8,-10.28,;7.34,-9.8,;10.13,-14.13,;8.79,-14.9,;8.8,-16.44,;10.13,-17.21,;10.14,-18.75,;11.45,-16.44,;11.45,-14.9,;12.8,-14.11,)| Show InChI InChI=1S/C22H20Cl2N6O/c1-13-17(9-25)20(16-6-5-15(23)8-18(16)24)30-12-19(28-22(30)27-13)21(31)29(2)11-14-4-3-7-26-10-14/h3-8,10,12H,9,11,25H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11108
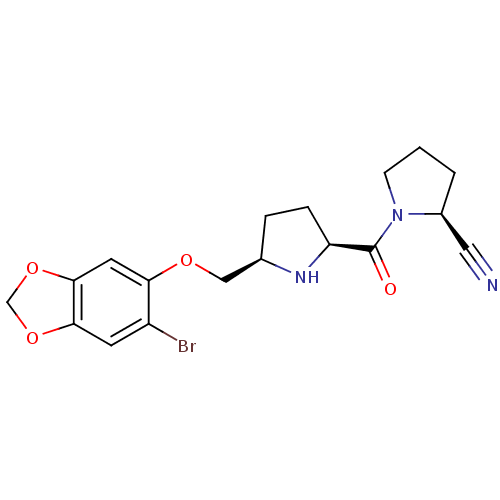
((2S)-1-{[(2S,5R)-5-{[(6-bromo-2H-1,3-benzodioxol-5...)Show SMILES Brc1cc2OCOc2cc1OC[C@H]1CC[C@H](N1)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C18H20BrN3O4/c19-13-6-16-17(26-10-25-16)7-15(13)24-9-11-3-4-14(21-11)18(23)22-5-1-2-12(22)8-20/h6-7,11-12,14,21H,1-5,9-10H2/t11-,12+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... |
J Med Chem 49: 3520-35 (2006)
Article DOI: 10.1021/jm051283e
BindingDB Entry DOI: 10.7270/Q2WS8RG1 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50288303

((R)-3-((S)-2-Amino-3-methyl-pentanoyl)-thiazolidin...)Show InChI InChI=1S/C10H17N3OS/c1-3-7(2)9(12)10(14)13-6-15-5-8(13)4-11/h7-9H,3,5-6,12H2,1-2H3/t7?,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human Dipeptidyl peptidase IV |
Bioorg Med Chem Lett 6: 2745-2748 (1996)
Article DOI: 10.1016/S0960-894X(96)00491-X
BindingDB Entry DOI: 10.7270/Q2MP537C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50050511

((R)-1-(2-aminoacetyl)pyrrolidin-2-ylboronic acid |...)Show InChI InChI=1S/C6H13BN2O3/c8-4-6(10)9-3-1-2-5(9)7(11)12/h5,11-12H,1-4,8H2/t5-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP9 expressed in HEK293T cells |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324524
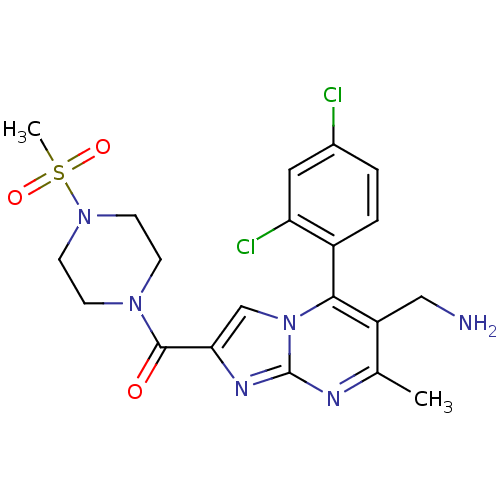
((6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methylim...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)N1CCN(CC1)S(C)(=O)=O |(11.6,.54,;10.29,-.27,;8.93,.44,;7.63,-.37,;6.16,.07,;5.29,-1.2,;6.24,-2.42,;7.68,-1.9,;9.03,-2.63,;10.34,-1.82,;11.7,-2.54,;13.01,-1.73,;9,-4.16,;7.65,-4.9,;7.62,-6.44,;8.94,-7.24,;8.91,-8.78,;10.29,-6.48,;10.32,-4.95,;11.66,-4.21,;3.75,-1.24,;2.94,.06,;3.02,-2.6,;3.82,-3.91,;3.09,-5.26,;1.55,-5.3,;.75,-3.99,;1.48,-2.64,;.82,-6.65,;-.72,-6.7,;.4,-8.14,;2.15,-7.42,)| Show InChI InChI=1S/C20H22Cl2N6O3S/c1-12-15(10-23)18(14-4-3-13(21)9-16(14)22)28-11-17(25-20(28)24-12)19(29)26-5-7-27(8-6-26)32(2,30)31/h3-4,9,11H,5-8,10,23H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356590

(CHEMBL1910118)Show SMILES CCN(CC)C(=O)CN1Cc2c(nc(C)c(CN)c2-c2ccc(Cl)cc2Cl)C1=O |(39.88,-17.76,;39.11,-19.1,;39.89,-20.43,;39.12,-21.76,;39.89,-23.09,;41.43,-20.43,;42.2,-21.76,;42.19,-19.09,;43.73,-19.09,;44.63,-20.33,;46.09,-19.86,;46.1,-18.32,;47.43,-17.55,;48.76,-18.31,;50.09,-17.53,;48.77,-19.86,;50.1,-20.63,;51.43,-19.86,;47.43,-20.63,;47.43,-22.17,;46.09,-22.94,;46.09,-24.47,;47.43,-25.25,;47.43,-26.79,;48.77,-24.47,;48.76,-22.93,;50.09,-22.16,;44.63,-17.84,;44.16,-16.38,)| Show InChI InChI=1S/C21H24Cl2N4O2/c1-4-26(5-2)18(28)11-27-10-16-19(14-7-6-13(22)8-17(14)23)15(9-24)12(3)25-20(16)21(27)29/h6-8H,4-5,9-11,24H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11103
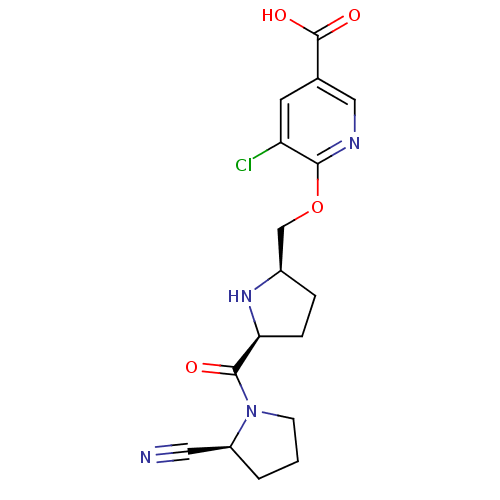
(2-cyanopyrrolidine 21aj | 5-chloro-6-{[(2R,5S)-5-{...)Show SMILES OC(=O)c1cnc(OC[C@H]2CC[C@H](N2)C(=O)N2CCC[C@H]2C#N)c(Cl)c1 |r| Show InChI InChI=1S/C17H19ClN4O4/c18-13-6-10(17(24)25)8-20-15(13)26-9-11-3-4-14(21-11)16(23)22-5-1-2-12(22)7-19/h6,8,11-12,14,21H,1-5,9H2,(H,24,25)/t11-,12+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... |
J Med Chem 49: 3520-35 (2006)
Article DOI: 10.1021/jm051283e
BindingDB Entry DOI: 10.7270/Q2WS8RG1 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356587
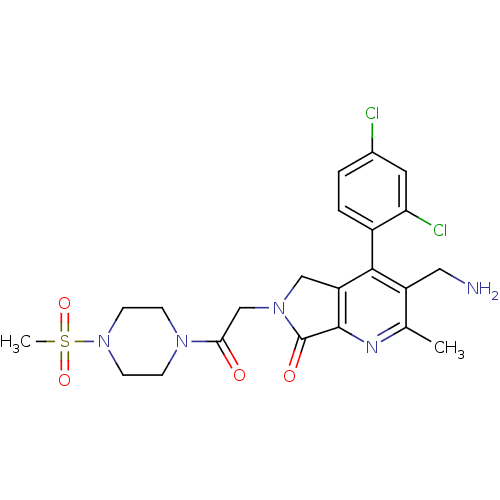
(CHEMBL1910121)Show SMILES Cc1nc2C(=O)N(CC(=O)N3CCN(CC3)S(C)(=O)=O)Cc2c(c1CN)-c1ccc(Cl)cc1Cl |(48.61,-29.07,;47.28,-29.84,;45.95,-29.08,;44.62,-29.86,;43.16,-29.38,;42.68,-27.91,;42.25,-30.62,;40.72,-30.62,;39.95,-31.96,;40.72,-33.29,;38.41,-31.96,;37.64,-30.63,;36.11,-30.63,;35.33,-31.96,;36.1,-33.29,;37.65,-33.3,;33.79,-31.95,;33.03,-30.62,;32.45,-32.72,;33.78,-33.49,;43.15,-31.87,;44.62,-31.4,;45.95,-32.17,;47.29,-31.39,;48.62,-32.16,;49.96,-31.39,;45.95,-33.7,;44.62,-34.47,;44.62,-36.01,;45.95,-36.78,;45.95,-38.32,;47.29,-36,;47.28,-34.47,;48.61,-33.69,)| Show InChI InChI=1S/C22H25Cl2N5O4S/c1-13-16(10-25)20(15-4-3-14(23)9-18(15)24)17-11-28(22(31)21(17)26-13)12-19(30)27-5-7-29(8-6-27)34(2,32)33/h3-4,9H,5-8,10-12,25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50442198
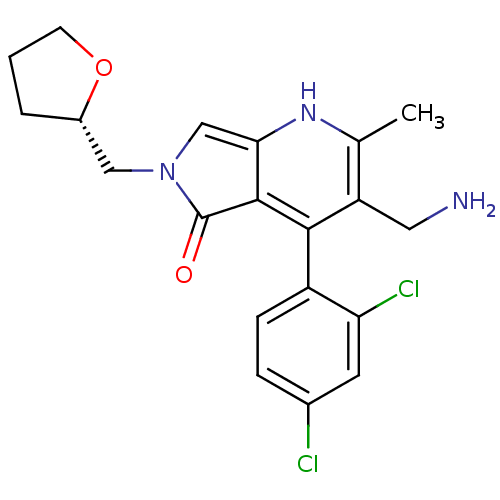
(CHEMBL2441839)Show SMILES Cc1[nH]c2cn(C[C@@H]3CCCO3)c(=O)c2c(c1CN)-c1ccc(Cl)cc1Cl |r,wD:7.6,(24.71,-38.82,;23.38,-39.6,;22.05,-38.83,;20.72,-39.6,;19.24,-39.13,;18.34,-40.39,;16.8,-40.39,;16.02,-39.06,;14.47,-39,;14.06,-37.52,;15.35,-36.67,;16.55,-37.63,;19.25,-41.63,;18.79,-43.1,;20.72,-41.15,;22.05,-41.92,;23.39,-41.15,;24.72,-41.91,;26.05,-41.14,;22.05,-43.46,;20.71,-44.22,;20.71,-45.76,;22.04,-46.53,;22.04,-48.07,;23.38,-45.76,;23.38,-44.22,;24.71,-43.45,)| Show InChI InChI=1S/C20H21Cl2N3O2/c1-11-15(8-23)18(14-5-4-12(21)7-16(14)22)19-17(24-11)10-25(20(19)26)9-13-3-2-6-27-13/h4-5,7,10,13,24H,2-3,6,8-9,23H2,1H3/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 (unknown origin) |
J Med Chem 56: 7343-57 (2013)
Article DOI: 10.1021/jm4008906
BindingDB Entry DOI: 10.7270/Q2N01806 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50288308

((R)-3-(2-Amino-2-cyclopentyl-acetyl)-thiazolidine-...)Show InChI InChI=1S/C11H17N3OS/c12-5-9-6-16-7-14(9)11(15)10(13)8-3-1-2-4-8/h8-10H,1-4,6-7,13H2/t9-,10?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human Dipeptidyl peptidase IV |
Bioorg Med Chem Lett 6: 2745-2748 (1996)
Article DOI: 10.1016/S0960-894X(96)00491-X
BindingDB Entry DOI: 10.7270/Q2MP537C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324524

((6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methylim...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)N1CCN(CC1)S(C)(=O)=O |(11.6,.54,;10.29,-.27,;8.93,.44,;7.63,-.37,;6.16,.07,;5.29,-1.2,;6.24,-2.42,;7.68,-1.9,;9.03,-2.63,;10.34,-1.82,;11.7,-2.54,;13.01,-1.73,;9,-4.16,;7.65,-4.9,;7.62,-6.44,;8.94,-7.24,;8.91,-8.78,;10.29,-6.48,;10.32,-4.95,;11.66,-4.21,;3.75,-1.24,;2.94,.06,;3.02,-2.6,;3.82,-3.91,;3.09,-5.26,;1.55,-5.3,;.75,-3.99,;1.48,-2.64,;.82,-6.65,;-.72,-6.7,;.4,-8.14,;2.15,-7.42,)| Show InChI InChI=1S/C20H22Cl2N6O3S/c1-12-15(10-23)18(14-4-3-13(21)9-16(14)22)28-11-17(25-20(28)24-12)19(29)26-5-7-27(8-6-26)32(2,30)31/h3-4,9,11H,5-8,10,23H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50050525

((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...)Show InChI InChI=1S/C7H15BN2O3/c1-5(9)7(11)10-4-2-3-6(10)8(12)13/h5-6,12-13H,2-4,9H2,1H3/t5-,6-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP9 expressed in HEK293T cells |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324504
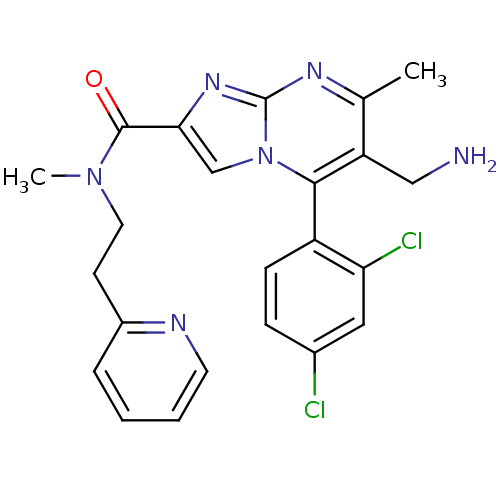
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-N,7-dimethy...)Show SMILES CN(CCc1ccccn1)C(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(7.39,-9.52,;6.58,-8.21,;5.04,-8.26,;4.23,-6.94,;2.69,-6.99,;1.89,-5.67,;.35,-5.72,;-.38,-7.08,;.44,-8.4,;1.97,-8.34,;7.3,-6.85,;6.49,-5.54,;8.85,-6.8,;9.79,-8.02,;11.23,-7.5,;12.59,-8.23,;13.89,-7.42,;15.25,-8.15,;16.56,-7.34,;13.85,-5.88,;15.15,-5.06,;12.48,-5.16,;11.19,-5.97,;9.71,-5.54,;12.55,-9.76,;11.2,-10.5,;11.17,-12.04,;12.49,-12.84,;12.58,-14.44,;13.85,-12.09,;13.87,-10.56,;15.21,-9.81,)| Show InChI InChI=1S/C23H22Cl2N6O/c1-14-18(12-26)21(17-7-6-15(24)11-19(17)25)31-13-20(29-23(31)28-14)22(32)30(2)10-8-16-5-3-4-9-27-16/h3-7,9,11,13H,8,10,12,26H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356588

(CHEMBL1910120)Show SMILES CC(=O)N1CCN(CC1)C(=O)CN1Cc2c(nc(C)c(CN)c2-c2ccc(Cl)cc2Cl)C1=O |(11.15,-31.36,;11.92,-32.69,;11.15,-34.03,;13.46,-32.7,;14.24,-31.37,;15.77,-31.37,;16.54,-32.7,;15.78,-34.04,;14.23,-34.03,;18.08,-32.7,;18.85,-34.03,;18.84,-31.36,;20.38,-31.36,;21.28,-32.61,;22.75,-32.13,;22.75,-30.6,;24.08,-29.82,;25.41,-30.58,;26.74,-29.81,;25.42,-32.13,;26.75,-32.9,;28.08,-32.13,;24.08,-32.91,;24.08,-34.44,;22.75,-35.21,;22.74,-36.75,;24.08,-37.52,;24.08,-39.06,;25.42,-36.74,;25.41,-35.21,;26.74,-34.43,;21.28,-30.12,;20.81,-28.65,)| Show InChI InChI=1S/C23H25Cl2N5O3/c1-13-17(10-26)21(16-4-3-15(24)9-19(16)25)18-11-30(23(33)22(18)27-13)12-20(32)29-7-5-28(6-8-29)14(2)31/h3-4,9H,5-8,10-12,26H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324513
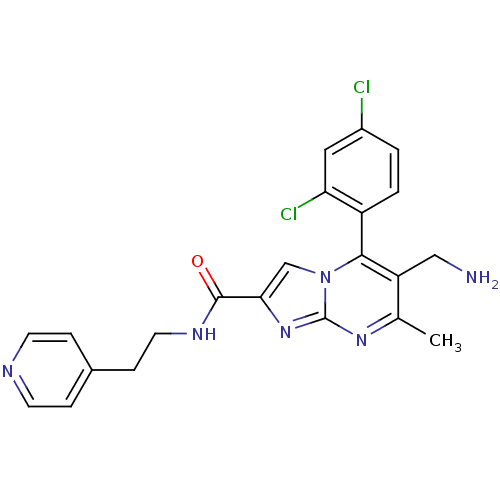
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl-N-...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)NCCc1ccncc1 |(10.76,-1,;9.45,-1.81,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.18,-3.27,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.49,-6.48,;10.83,-5.74,;2.92,-2.78,;2.11,-1.48,;2.19,-4.14,;.65,-4.18,;-.08,-5.54,;-1.62,-5.59,;-2.42,-4.28,;-3.96,-4.32,;-4.69,-5.68,;-3.87,-6.99,;-2.34,-6.94,)| Show InChI InChI=1S/C22H20Cl2N6O/c1-13-17(11-25)20(16-3-2-15(23)10-18(16)24)30-12-19(29-22(30)28-13)21(31)27-9-6-14-4-7-26-8-5-14/h2-5,7-8,10,12H,6,9,11,25H2,1H3,(H,27,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11087
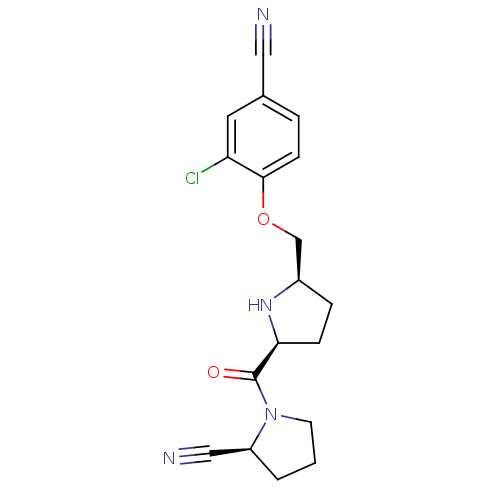
((2S)-1-{[(2S,5R)-5-(2-chloro-4-cyanophenoxymethyl)...)Show SMILES Clc1cc(ccc1OC[C@H]1CC[C@H](N1)C(=O)N1CCC[C@H]1C#N)C#N |r| Show InChI InChI=1S/C18H19ClN4O2/c19-15-8-12(9-20)3-6-17(15)25-11-13-4-5-16(22-13)18(24)23-7-1-2-14(23)10-21/h3,6,8,13-14,16,22H,1-2,4-5,7,11H2/t13-,14+,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... |
J Med Chem 49: 3520-35 (2006)
Article DOI: 10.1021/jm051283e
BindingDB Entry DOI: 10.7270/Q2WS8RG1 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11542
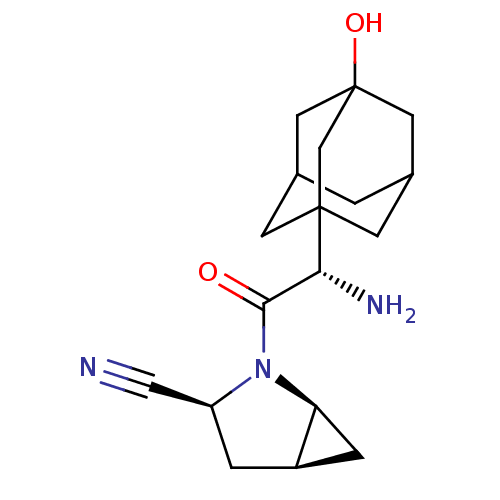
((1S,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxyadamantan-1...)Show SMILES N[C@H](C(=O)N1[C@H]2C[C@H]2C[C@H]1C#N)C12CC3CC(CC(O)(C3)C1)C2 |r,TLB:15:16:21:20.13.14,THB:17:16:13:20.21.18,17:18:16.15.22:13,19:18:16.15.22:13,15:14:16.22.17:21| Show InChI InChI=1S/C18H25N3O2/c19-8-13-2-12-3-14(12)21(13)16(22)15(20)17-4-10-1-11(5-17)7-18(23,6-10)9-17/h10-15,23H,1-7,9,20H2/t10?,11?,12-,13+,14+,15-,17?,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.600 | -52.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... |
J Med Chem 48: 5025-37 (2005)
Article DOI: 10.1021/jm050261p
BindingDB Entry DOI: 10.7270/Q2FN14DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50225074
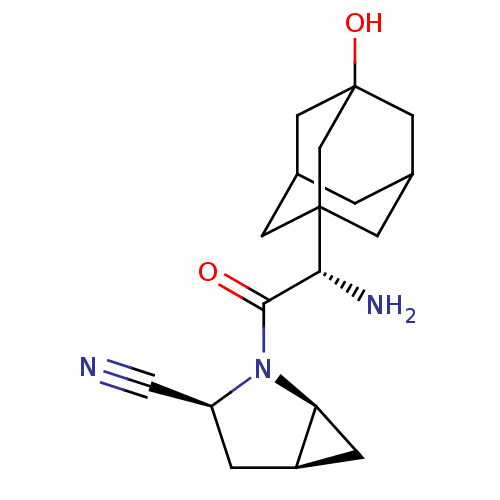
((1S,3S,5S)-2-[(S)-2-amino-2-(3-hydroxy-adamantan-1...)Show SMILES N[C@H](C(=O)N1[C@H]2C[C@H]2C[C@H]1C#N)C12CC3CC(CC(O)(C3)C1)C2 |TLB:1:12:15:20.18.17,THB:13:14:17:22.12.21,13:12:15.14.20:17,21:12:15:20.18.17,21:18:15:22.13.12,19:18:15:22.13.12| Show InChI InChI=1S/C18H25N3O2/c19-8-13-2-12-3-14(12)21(13)16(22)15(20)17-4-10-1-11(5-17)7-18(23,6-10)9-17/h10-15,23H,1-7,9,20H2/t10?,11?,12-,13+,14+,15-,17?,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50225074

((1S,3S,5S)-2-[(S)-2-amino-2-(3-hydroxy-adamantan-1...)Show SMILES N[C@H](C(=O)N1[C@H]2C[C@H]2C[C@H]1C#N)C12CC3CC(CC(O)(C3)C1)C2 |TLB:1:12:15:20.18.17,THB:13:14:17:22.12.21,13:12:15.14.20:17,21:12:15:20.18.17,21:18:15:22.13.12,19:18:15:22.13.12| Show InChI InChI=1S/C18H25N3O2/c19-8-13-2-12-3-14(12)21(13)16(22)15(20)17-4-10-1-11(5-17)7-18(23,6-10)9-17/h10-15,23H,1-7,9,20H2/t10?,11?,12-,13+,14+,15-,17?,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Eur J Med Chem 44: 3318-22 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.021
BindingDB Entry DOI: 10.7270/Q2DR2WGQ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324497
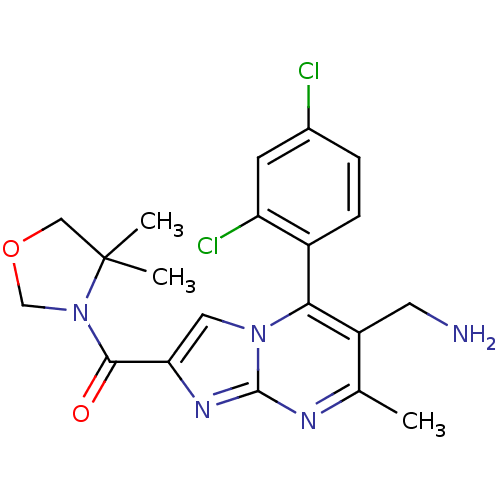
((6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methylim...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)N1COCC1(C)C |(10.72,-.55,;9.42,-1.37,;8.06,-.65,;6.76,-1.46,;5.28,-1.03,;4.42,-2.29,;5.36,-3.51,;6.81,-2.99,;8.16,-3.72,;9.46,-2.91,;10.82,-3.64,;12.13,-2.83,;8.13,-5.25,;6.78,-5.99,;6.74,-7.53,;8.07,-8.33,;8.03,-9.87,;9.42,-7.58,;9.44,-6.05,;10.79,-5.3,;2.88,-2.34,;2.07,-1.03,;2.15,-3.7,;2.66,-5.14,;1.44,-6.08,;.17,-5.2,;.61,-3.72,;-.99,-3.83,;-.16,-2.39,)| Show InChI InChI=1S/C20H21Cl2N5O2/c1-11-14(7-23)17(13-5-4-12(21)6-15(13)22)26-8-16(25-19(26)24-11)18(28)27-10-29-9-20(27,2)3/h4-6,8H,7,9-10,23H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324500

(1-(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl...)Show SMILES CN(Cc1cccnc1)C(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(4.9,-13.73,;4.13,-12.4,;2.59,-12.4,;1.82,-13.73,;.28,-13.72,;-.49,-15.05,;.28,-16.39,;1.83,-16.4,;2.59,-15.06,;4.91,-11.07,;4.14,-9.74,;6.44,-11.06,;7.34,-12.29,;8.8,-11.82,;10.13,-12.59,;11.47,-11.82,;12.8,-12.6,;14.14,-11.85,;11.46,-10.28,;12.8,-9.51,;10.13,-9.51,;8.8,-10.28,;7.34,-9.8,;10.13,-14.13,;8.79,-14.9,;8.8,-16.44,;10.13,-17.21,;10.14,-18.75,;11.45,-16.44,;11.45,-14.9,;12.8,-14.11,)| Show InChI InChI=1S/C22H20Cl2N6O/c1-13-17(9-25)20(16-6-5-15(23)8-18(16)24)30-12-19(28-22(30)27-13)21(31)29(2)11-14-4-3-7-26-10-14/h3-8,10,12H,9,11,25H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324511

(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl-N-...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)NCCc1cccnc1 |(10.76,-1,;9.45,-1.81,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.18,-3.27,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.49,-6.48,;10.83,-5.74,;2.92,-2.78,;2.11,-1.48,;2.19,-4.14,;.65,-4.18,;-.08,-5.54,;-1.62,-5.59,;-2.34,-6.94,;-3.87,-6.99,;-4.69,-5.68,;-3.96,-4.32,;-2.42,-4.28,)| Show InChI InChI=1S/C22H20Cl2N6O/c1-13-17(10-25)20(16-5-4-15(23)9-18(16)24)30-12-19(29-22(30)28-13)21(31)27-8-6-14-3-2-7-26-11-14/h2-5,7,9,11-12H,6,8,10,25H2,1H3,(H,27,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50225074

((1S,3S,5S)-2-[(S)-2-amino-2-(3-hydroxy-adamantan-1...)Show SMILES N[C@H](C(=O)N1[C@H]2C[C@H]2C[C@H]1C#N)C12CC3CC(CC(O)(C3)C1)C2 |TLB:1:12:15:20.18.17,THB:13:14:17:22.12.21,13:12:15.14.20:17,21:12:15:20.18.17,21:18:15:22.13.12,19:18:15:22.13.12| Show InChI InChI=1S/C18H25N3O2/c19-8-13-2-12-3-14(12)21(13)16(22)15(20)17-4-10-1-11(5-17)7-18(23,6-10)9-17/h10-15,23H,1-7,9,20H2/t10?,11?,12-,13+,14+,15-,17?,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 17: 6476-80 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.090
BindingDB Entry DOI: 10.7270/Q2B56JGM |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11110
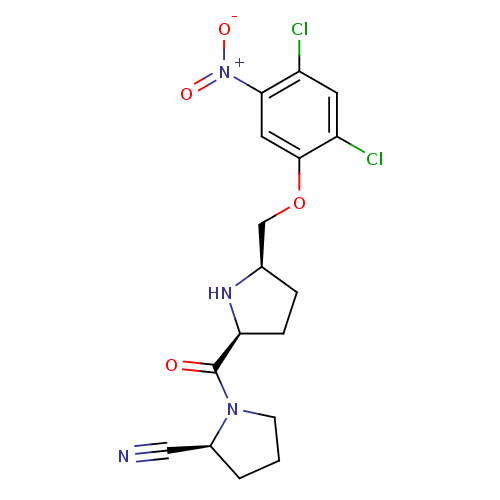
((2S)-1-{[(2S,5R)-5-(2,4-dichloro-5-nitrophenoxymet...)Show SMILES [O-][N+](=O)c1cc(OC[C@H]2CC[C@H](N2)C(=O)N2CCC[C@H]2C#N)c(Cl)cc1Cl |r| Show InChI InChI=1S/C17H18Cl2N4O4/c18-12-6-13(19)16(7-15(12)23(25)26)27-9-10-3-4-14(21-10)17(24)22-5-1-2-11(22)8-20/h6-7,10-11,14,21H,1-5,9H2/t10-,11+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... |
J Med Chem 49: 3520-35 (2006)
Article DOI: 10.1021/jm051283e
BindingDB Entry DOI: 10.7270/Q2WS8RG1 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324508
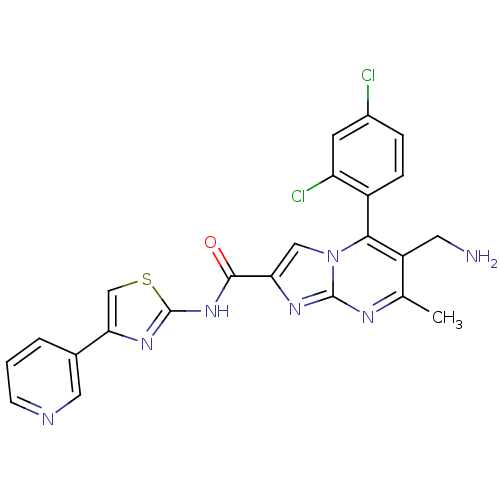
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl-N-...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)Nc1nc(cs1)-c1cccnc1 |(10.76,-1,;9.46,-1.81,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.18,-3.27,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.49,-6.49,;10.83,-5.74,;2.92,-2.78,;2.11,-1.48,;2.19,-4.14,;.65,-4.18,;-.23,-5.46,;-1.7,-5.02,;-1.75,-3.48,;-.3,-2.97,;-2.84,-6.06,;-2.83,-7.6,;-4.15,-8.37,;-5.5,-7.61,;-5.51,-6.06,;-4.17,-5.29,)| Show InChI InChI=1S/C23H17Cl2N7OS/c1-12-16(8-26)20(15-5-4-14(24)7-17(15)25)32-10-18(29-22(32)28-12)21(33)31-23-30-19(11-34-23)13-3-2-6-27-9-13/h2-7,9-11H,8,26H2,1H3,(H,30,31,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11112
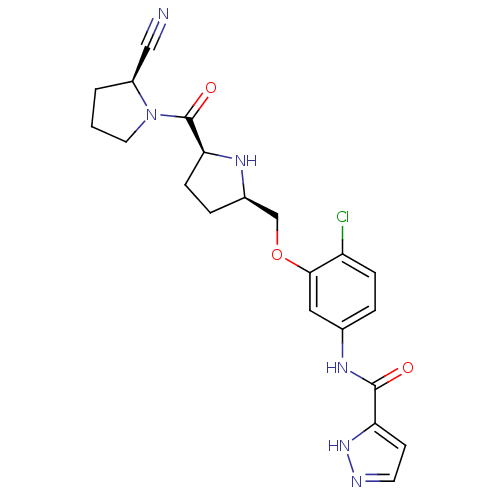
(2-cyanopyrrolidine 21as | N-(4-chloro-3-{[(2R,5S)-...)Show SMILES Clc1ccc(NC(=O)c2ccn[nH]2)cc1OC[C@H]1CC[C@H](N1)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C21H23ClN6O3/c22-16-5-3-13(26-20(29)17-7-8-24-27-17)10-19(16)31-12-14-4-6-18(25-14)21(30)28-9-1-2-15(28)11-23/h3,5,7-8,10,14-15,18,25H,1-2,4,6,9,12H2,(H,24,27)(H,26,29)/t14-,15+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... |
J Med Chem 49: 3520-35 (2006)
Article DOI: 10.1021/jm051283e
BindingDB Entry DOI: 10.7270/Q2WS8RG1 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11101
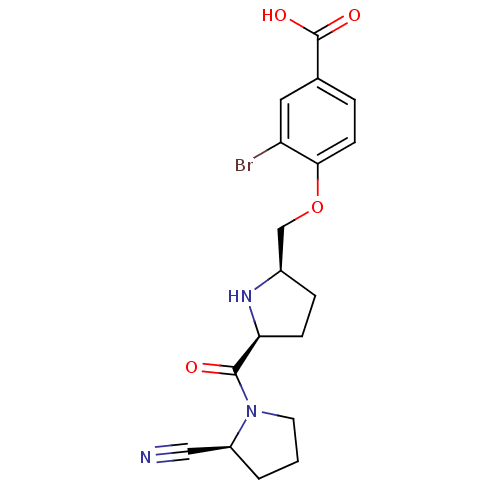
(2-cyanopyrrolidine 21ah | 3-bromo-4-{[(2R,5S)-5-{[...)Show SMILES OC(=O)c1ccc(OC[C@H]2CC[C@H](N2)C(=O)N2CCC[C@H]2C#N)c(Br)c1 |r| Show InChI InChI=1S/C18H20BrN3O4/c19-14-8-11(18(24)25)3-6-16(14)26-10-12-4-5-15(21-12)17(23)22-7-1-2-13(22)9-20/h3,6,8,12-13,15,21H,1-2,4-5,7,10H2,(H,24,25)/t12-,13+,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... |
J Med Chem 49: 3520-35 (2006)
Article DOI: 10.1021/jm051283e
BindingDB Entry DOI: 10.7270/Q2WS8RG1 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356586

(CHEMBL1910122)Show SMILES CN1CCN(CC1)C(=O)CN1Cc2c(nc(C)c(CN)c2-c2ccc(Cl)cc2Cl)C1=O |(-10,-42.49,;-8.46,-42.49,;-7.69,-41.16,;-6.16,-41.16,;-5.39,-42.49,;-6.15,-43.83,;-7.69,-43.83,;-3.85,-42.49,;-3.08,-43.82,;-3.08,-41.16,;-1.55,-41.15,;-.64,-42.4,;.82,-41.93,;.82,-40.39,;2.15,-39.61,;3.49,-40.38,;4.82,-39.6,;3.49,-41.93,;4.83,-42.69,;6.16,-41.92,;2.15,-42.7,;2.16,-44.23,;.82,-45,;.82,-46.54,;2.15,-47.31,;2.16,-48.85,;3.49,-46.53,;3.49,-45,;4.82,-44.22,;-.64,-39.91,;-1.11,-38.44,)| Show InChI InChI=1S/C22H25Cl2N5O2/c1-13-16(10-25)20(15-4-3-14(23)9-18(15)24)17-11-29(22(31)21(17)26-13)12-19(30)28-7-5-27(2)6-8-28/h3-4,9H,5-8,10-12,25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data