

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50303326 ((S)-2-{2-Benzyl-3-[((R)-1-benzyloxycarbonylamino-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human somatic ECE1 | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50303328 ((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1{[(benzyloxy)c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human somatic ECE1 | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50303323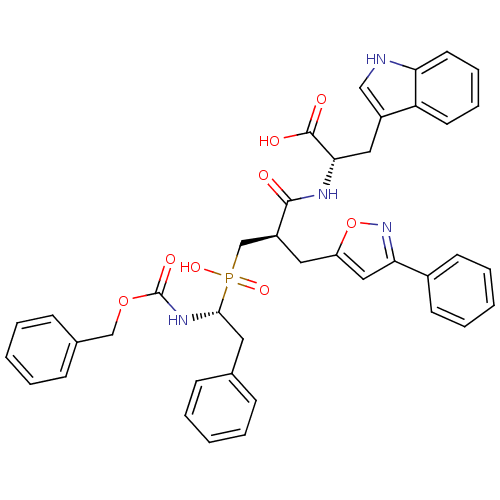 ((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human somatic ECE1 | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50115848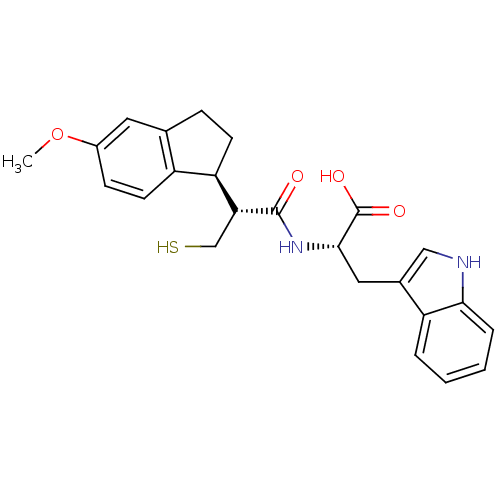 ((S)-3-(1H-Indol-3-yl)-2-[(R)-3-mercapto-2-((S)-5-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Curated by ChEMBL | Assay Description Inhibition constant against endothelin converting enzyme 1 | J Med Chem 48: 6523-43 (2005) Article DOI: 10.1021/jm058225d BindingDB Entry DOI: 10.7270/Q2SF2WZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50115846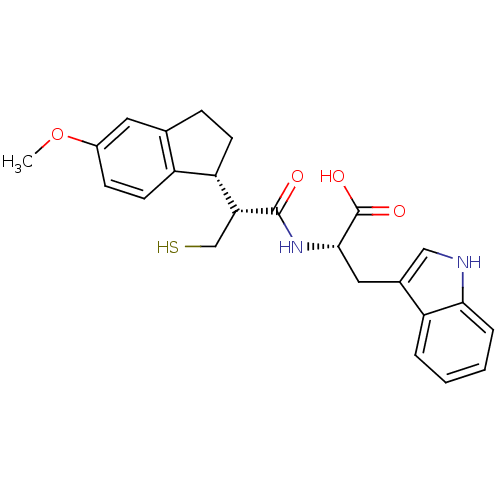 ((S)-3-(1H-Indol-3-yl)-2-[(R)-3-mercapto-2-((R)-5-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of endothelin converting enzyme. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21643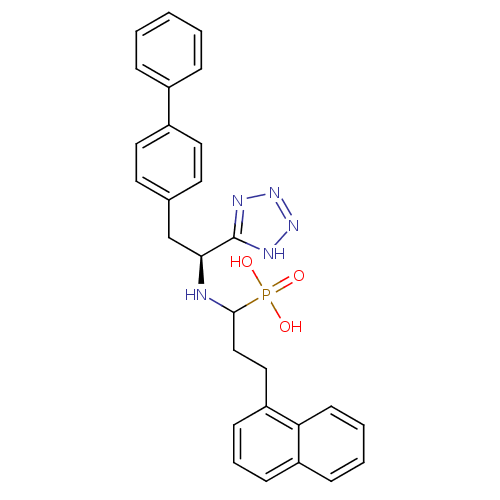 (CGS-31,447 | [3-(naphthalen-1-yl)-1-{[(1S)-2-(4-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 10.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50303322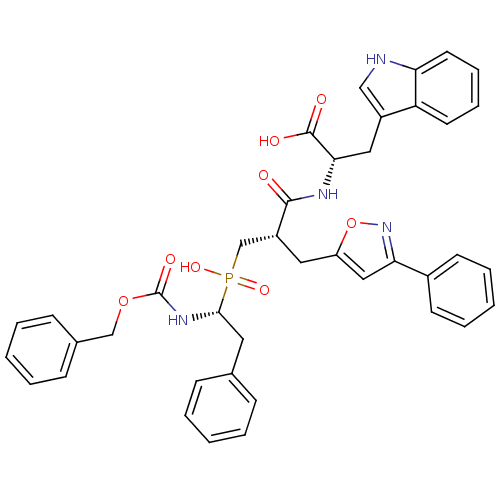 ((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human somatic ECE1 | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50303320 ((S)-2-[(R)-3-[((R)-1-Benzyloxycarbonylamino-2-phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human somatic ECE1 | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50115843 ((2S)-2-[(2R)-2-[(1R)-5-bromo-2,3-dihydro-1H-inden-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of endothelin converting enzyme. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50115841 (3-(1H-Indol-3-yl)-2-[3-mercapto-2-(5-methoxy-indan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of endothelin converting enzyme. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21653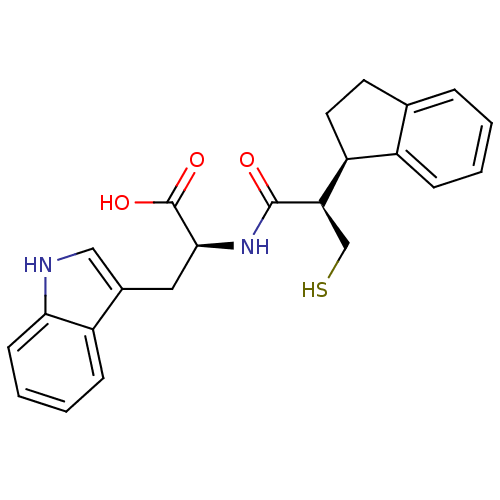 ((2S)-2-[(2R)-2-[(1R)-2,3-dihydro-1H-inden-1-yl]-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21653 ((2S)-2-[(2R)-2-[(1R)-2,3-dihydro-1H-inden-1-yl]-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of endothelin converting enzyme. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50303322 ((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human somatic ECE1 | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50115840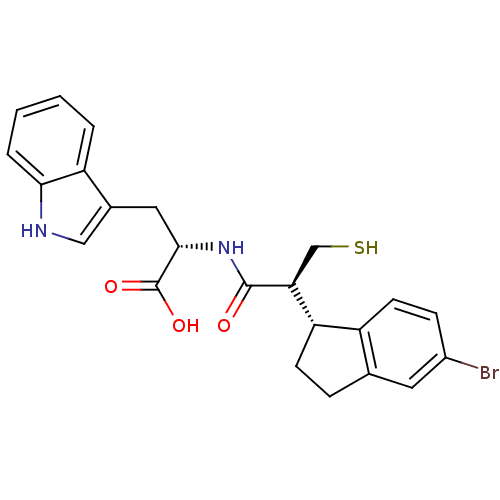 ((S)-2-[(S)-2-(5-Bromo-indan-1-yl)-3-mercapto-propi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of endothelin converting enzyme. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50115845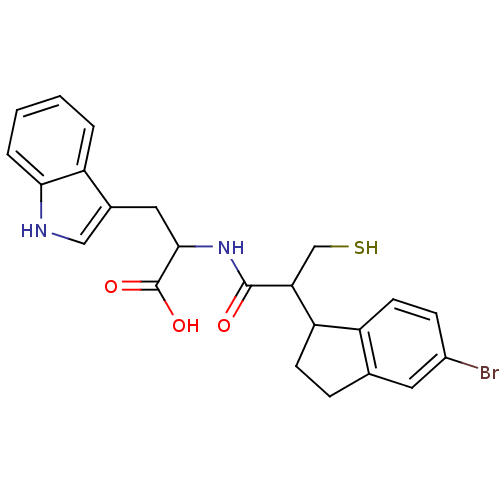 (2-[2-(5-Bromo-indan-1-yl)-3-mercapto-propionylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of endothelin converting enzyme. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50115849 (3-(1H-Indol-3-yl)-2-[3-mercapto-2-(5-methylsulfany...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of endothelin converting enzyme. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50115838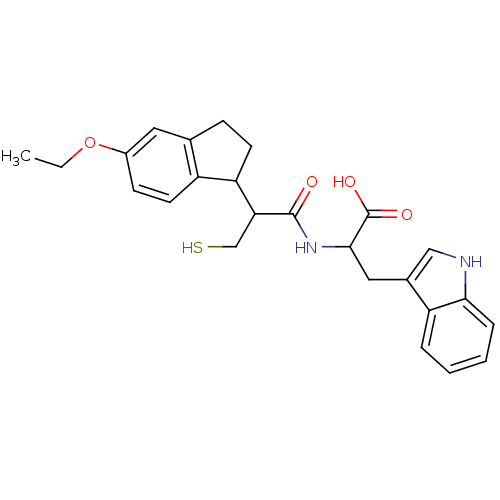 (2-[2-(5-Ethoxy-indan-1-yl)-3-mercapto-propionylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of endothelin converting enzyme. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50115835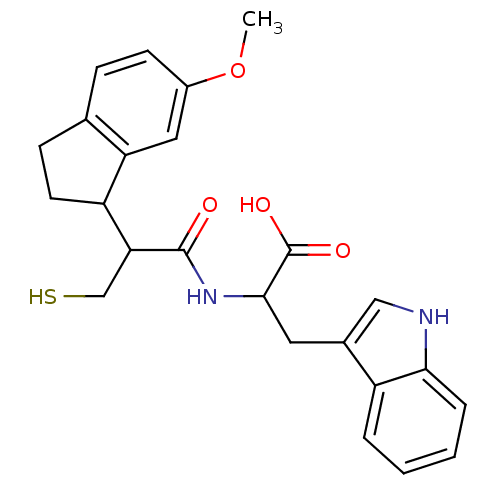 (3-(1H-Indol-3-yl)-2-[3-mercapto-2-(6-methoxy-indan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of endothelin converting enzyme. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50115847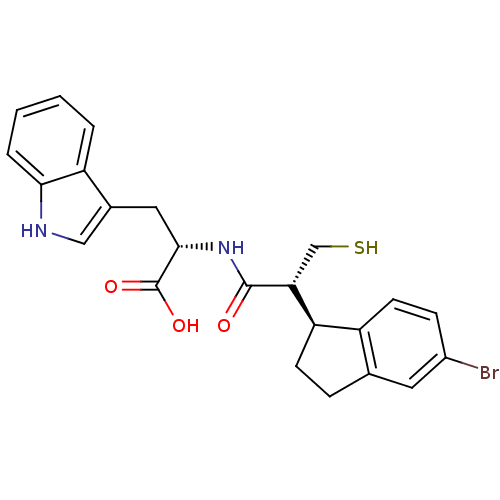 ((2S)-2-[(2R)-2-[(1S)-5-bromo-2,3-dihydro-1H-inden-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of endothelin converting enzyme. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50115839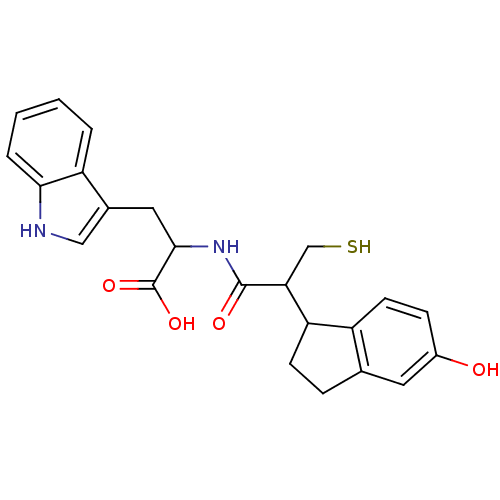 (2-[2-(5-Hydroxy-indan-1-yl)-3-mercapto-propionylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of endothelin converting enzyme. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50115842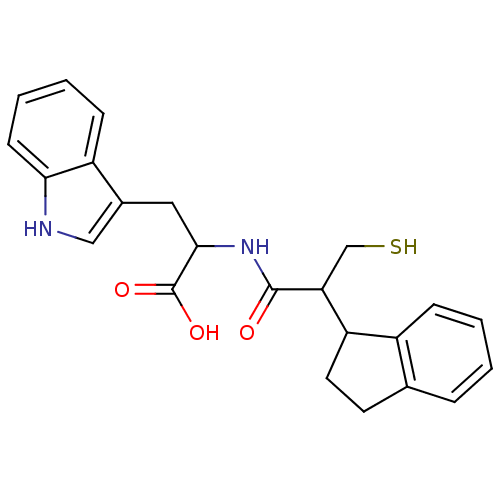 (2-(2-Indan-1-yl-3-mercapto-propionylamino)-3-(1H-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of endothelin converting enzyme. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21644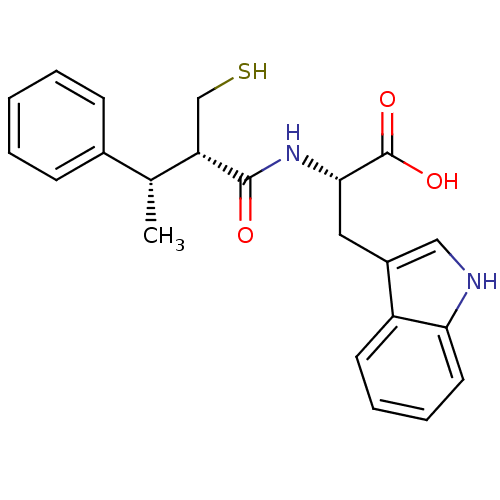 ((2S)-3-(1H-indol-3-yl)-2-[(2S,3S)-3-phenyl-2-(sulf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21639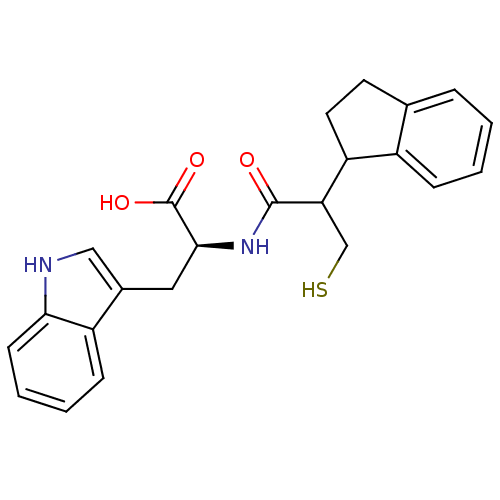 ((2S)-2-[2-(2,3-dihydro-1H-inden-1-yl)-3-sulfanylpr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50115850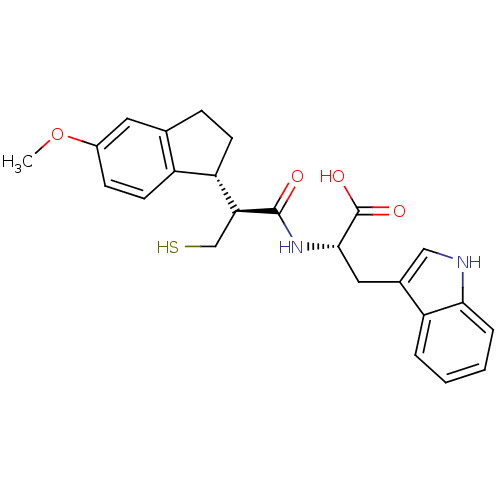 ((S)-3-(1H-Indol-3-yl)-2-[(S)-3-mercapto-2-((R)-5-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of endothelin converting enzyme. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50115851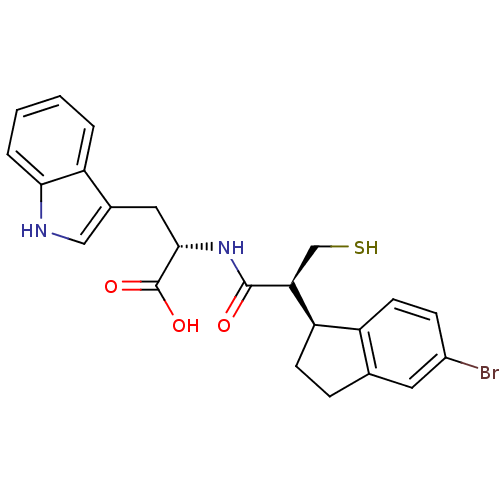 ((S)-2-[(S)-2-((S)-5-Bromo-indan-1-yl)-3-mercapto-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of endothelin converting enzyme. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21648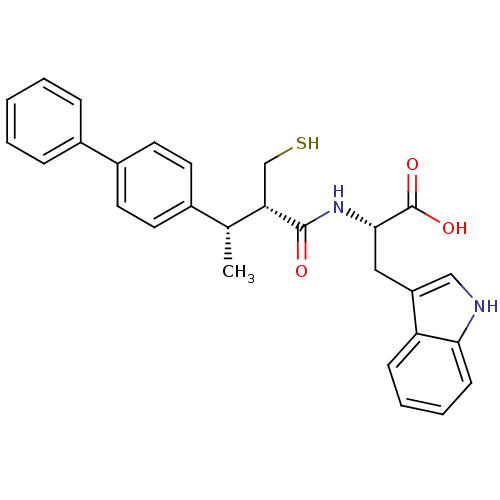 ((2S)-3-(1H-indol-3-yl)-2-[(2S,3S)-3-(4-phenylpheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21649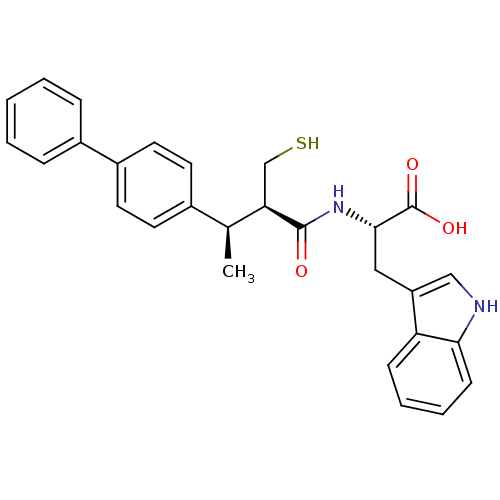 ((2S)-3-(1H-indol-3-yl)-2-[(2R,3R)-3-(4-phenylpheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50115834 (3-(1H-Indol-3-yl)-2-[3-mercapto-2-(5-phenyl-indan-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of endothelin converting enzyme. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50115848 ((S)-3-(1H-Indol-3-yl)-2-[(R)-3-mercapto-2-((S)-5-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of endothelin converting enzyme. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21651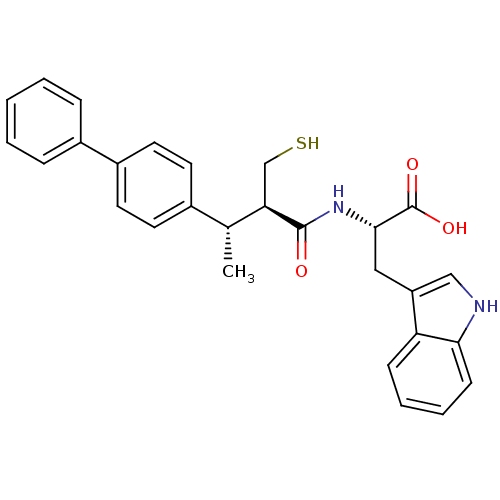 ((2S)-3-(1H-indol-3-yl)-2-[(2R,3S)-3-(4-phenylpheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21658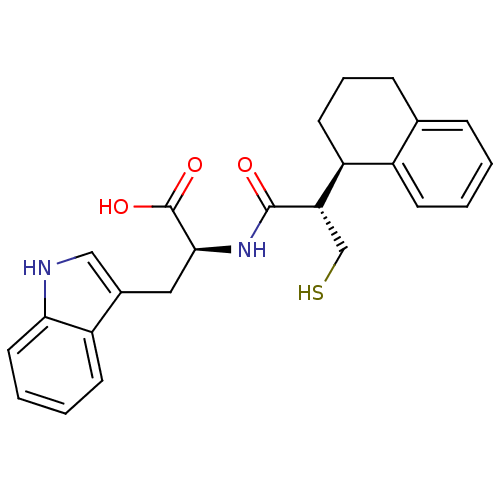 ((2S)-3-(1H-indol-3-yl)-2-[(2S)-3-sulfanyl-2-[(1R)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21645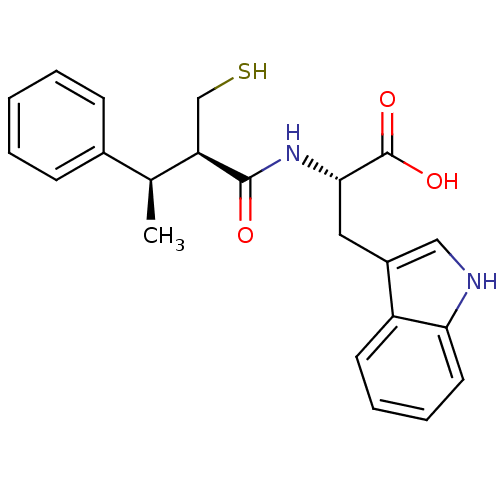 ((2S)-3-(1H-indol-3-yl)-2-[(2R,3R)-3-phenyl-2-(sulf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50115837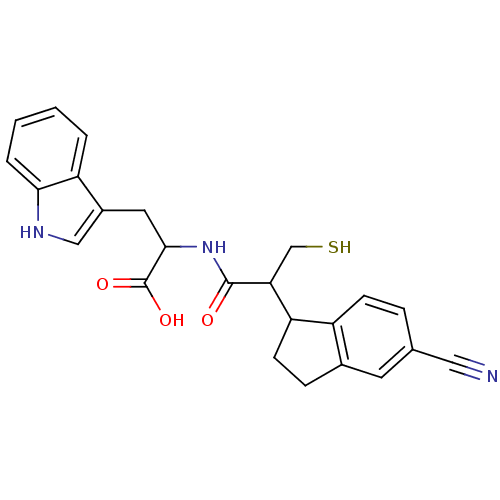 (2-[2-(5-Cyano-indan-1-yl)-3-mercapto-propionylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of endothelin converting enzyme. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21636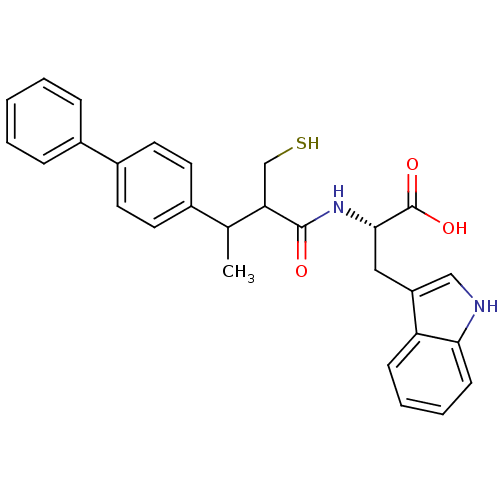 ((2S)-3-(1H-indol-3-yl)-2-[3-(4-phenylphenyl)-2-(su...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50303321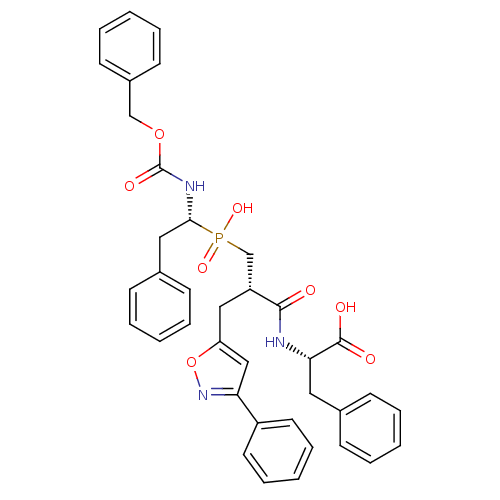 ((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human somatic ECE1 | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21657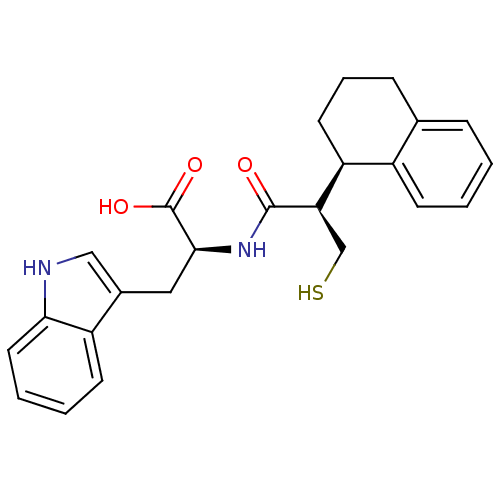 ((2S)-3-(1H-indol-3-yl)-2-[(2R)-3-sulfanyl-2-[(1R)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50303319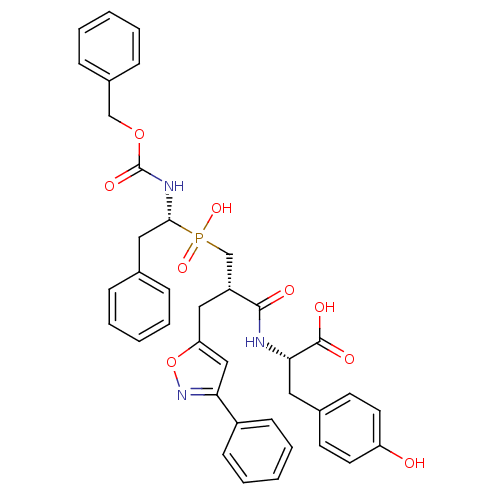 ((S)-2-[(S)-3-[((R)-1-Benzyloxycarbonylamino-2-phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human somatic ECE1 | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21654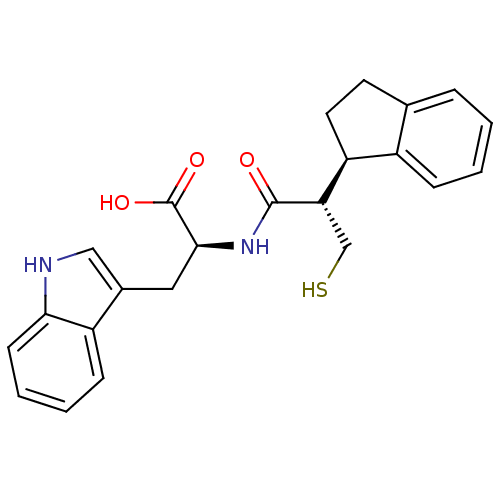 ((2S)-2-[(2S)-2-[(1R)-2,3-dihydro-1H-inden-1-yl]-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21640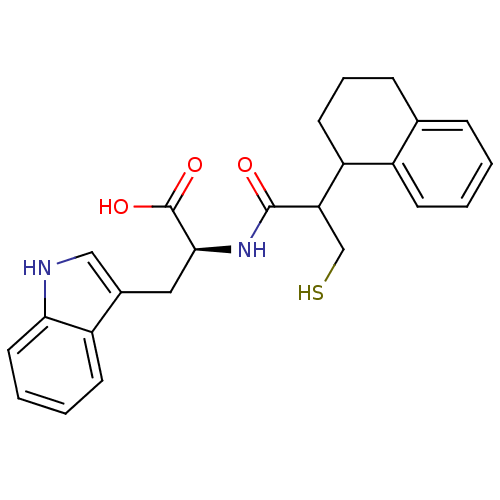 ((2S)-3-(1H-indol-3-yl)-2-[3-sulfanyl-2-(1,2,3,4-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21635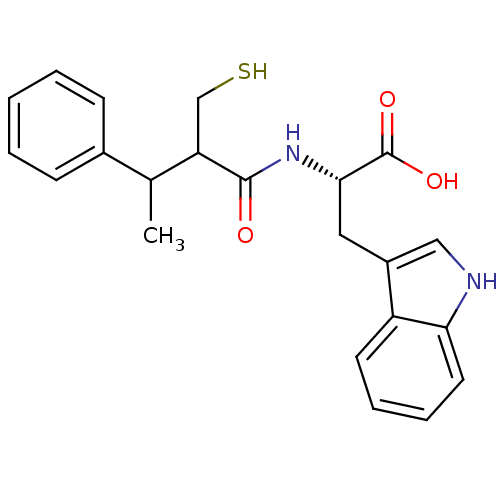 ((2S)-3-(1H-indol-3-yl)-2-[3-phenyl-2-(sulfanylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50115852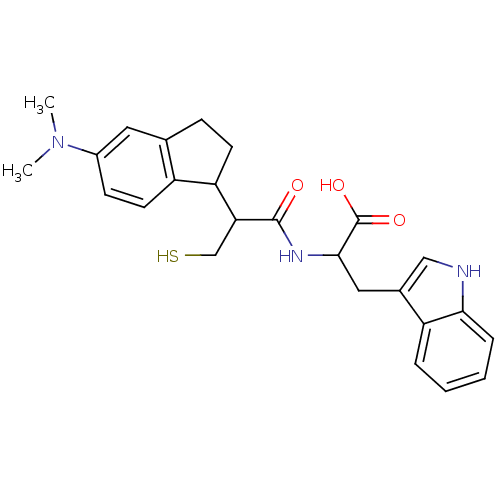 (2-[2-(5-Dimethylamino-indan-1-yl)-3-mercapto-propi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of endothelin converting enzyme. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50115833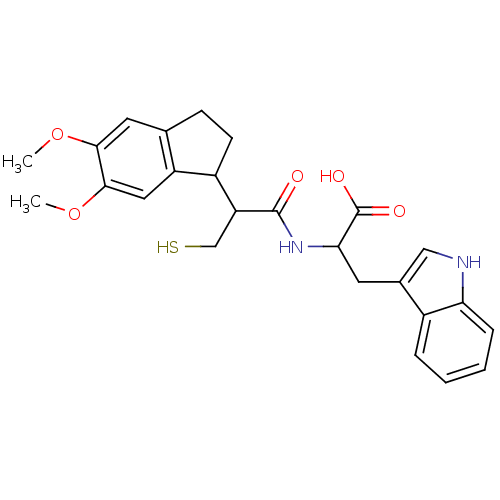 (2-[2-(5,6-Dimethoxy-indan-1-yl)-3-mercapto-propion...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of endothelin converting enzyme. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21647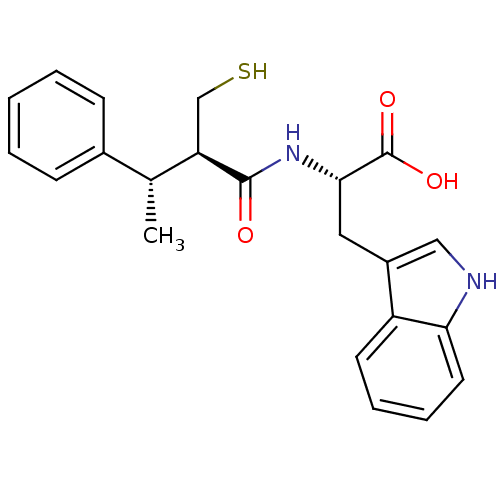 ((2S)-3-(1H-indol-3-yl)-2-[(2R,3S)-3-phenyl-2-(sulf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21638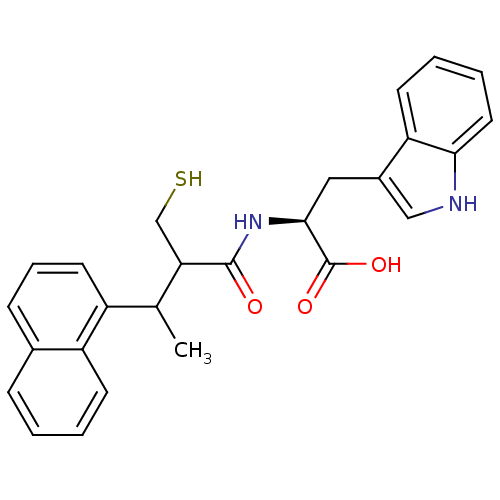 ((2S)-3-(1H-indol-3-yl)-2-[3-(naphthalen-1-yl)-2-(s...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21656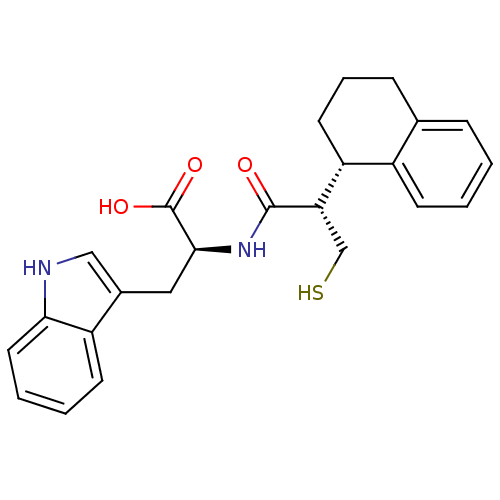 ((2S)-3-(1H-indol-3-yl)-2-[(2S)-3-sulfanyl-2-[(1S)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21655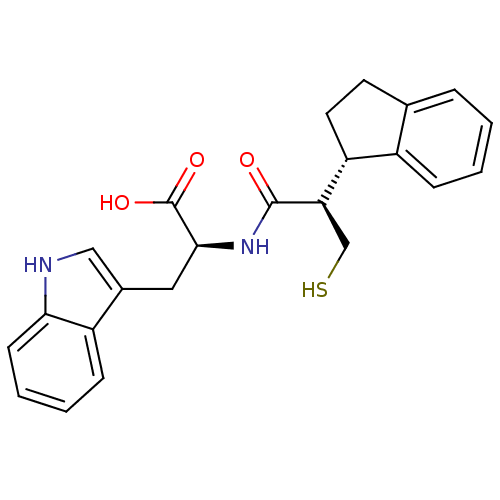 ((2S)-2-[(2R)-2-[(1S)-2,3-dihydro-1H-inden-1-yl]-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50115836 (3-(1H-Indol-3-yl)-2-[3-mercapto-2-(4-methoxy-indan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of endothelin converting enzyme. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21646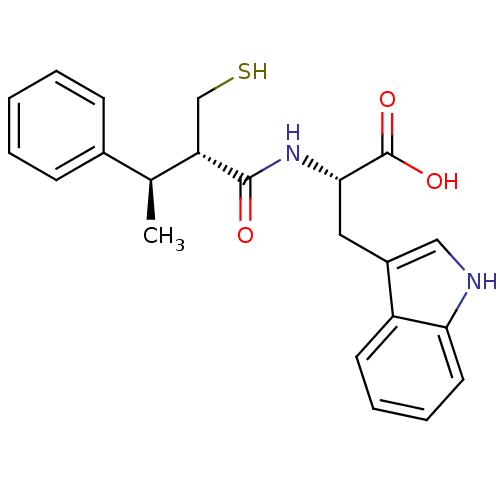 ((2S)-3-(1H-indol-3-yl)-2-[(2S,3R)-3-phenyl-2-(sulf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21632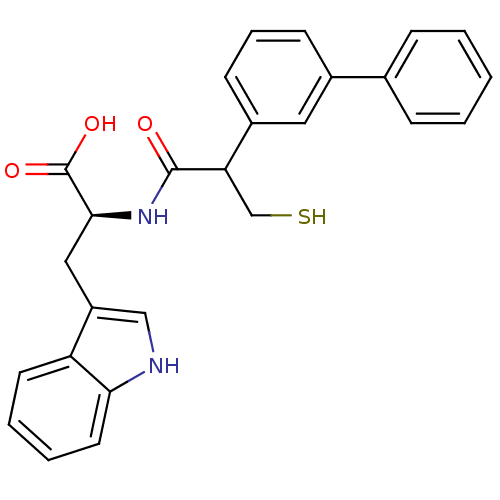 ((2S)-3-(1H-indol-3-yl)-2-[2-(3-phenylphenyl)-3-sul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50303324 ((2S)-2-{[3-(4',5'-Dihydro-3'-phenyl-5'-isoxazolyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human somatic ECE1 | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 589 total ) | Next | Last >> |