

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM97445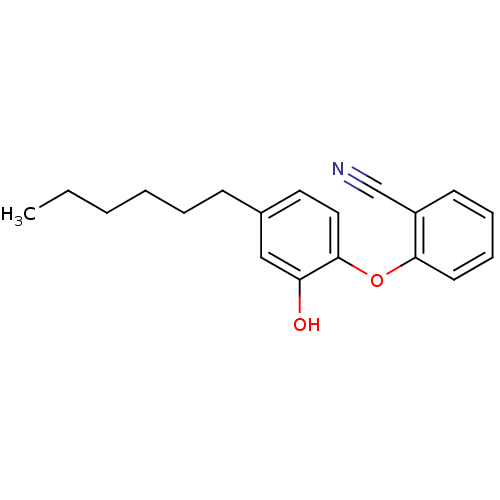 (PT119) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus enoyl ACP reductase | Eur J Med Chem 88: 66-73 (2014) Article DOI: 10.1016/j.ejmech.2014.09.008 BindingDB Entry DOI: 10.7270/Q25T3N3S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452511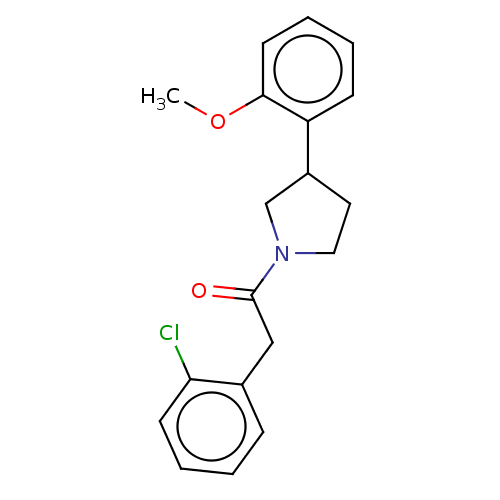 (CHEMBL4204517) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452502 (CHEMBL4209594) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452501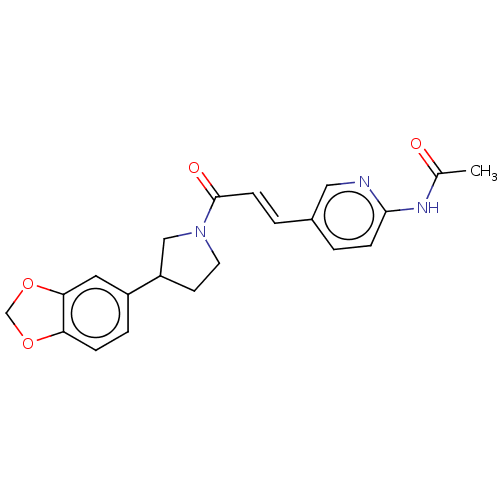 (CHEMBL4204415) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452494 (CHEMBL4204858) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452479 (CHEMBL4210008) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452512 (CHEMBL4205638) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452516 (CHEMBL4211939) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452506 (CHEMBL4216771) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452510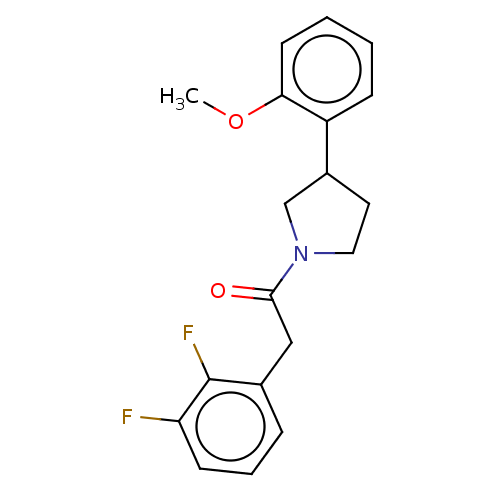 (CHEMBL4210599) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452515 (CHEMBL4214096) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452496 (CHEMBL4217852) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452482 (CHEMBL4208104) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452477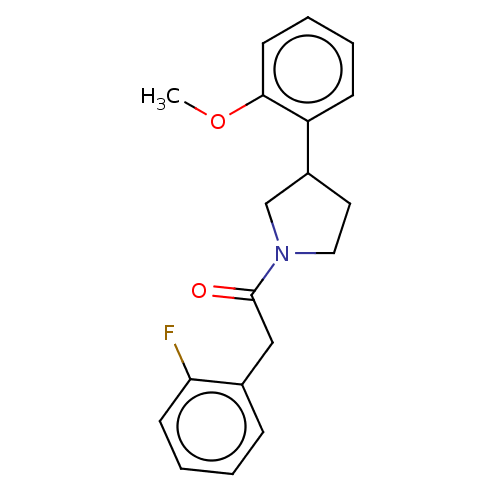 (CHEMBL4209685) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452483 (CHEMBL4210639) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452495 (CHEMBL4218049) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452499 (CHEMBL4213777) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452507 (CHEMBL4205014) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452509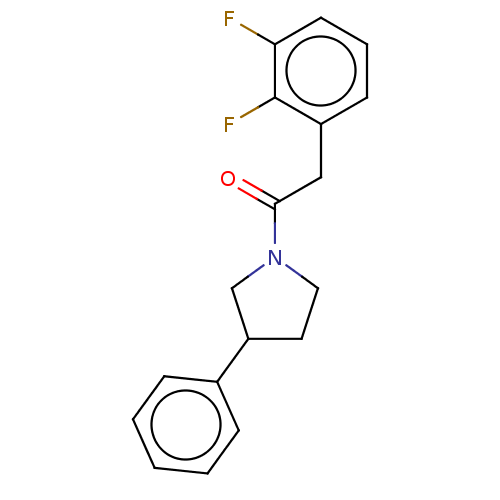 (CHEMBL4217672) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452497 (CHEMBL4205565) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452508 (CHEMBL4209393) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452481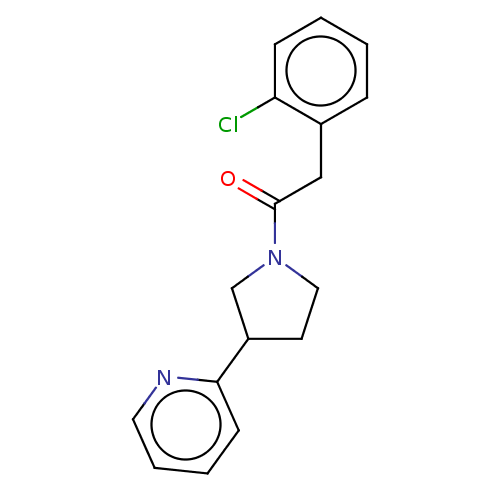 (CHEMBL4216697) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452498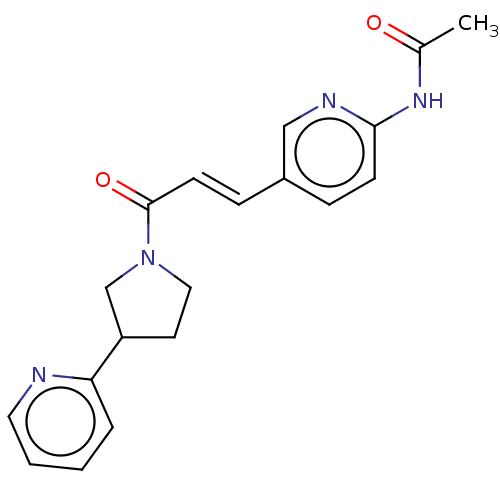 (CHEMBL4217928) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452513 (CHEMBL4213468) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452500 (CHEMBL4213757) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452504 (CHEMBL4218400) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452514 (CHEMBL4217867) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452480 (CHEMBL4206150) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452503 (CHEMBL4204726) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452505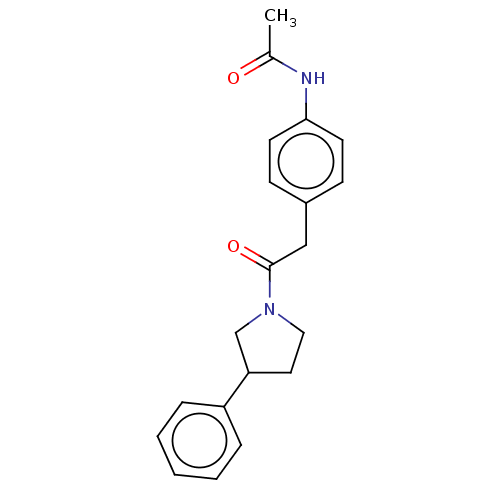 (CHEMBL4214922) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50052244 (CHEMBL1652621) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Ltd Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus FabI-mediated reduction of enoyl-ACP preincubated for 30 mins measured after 2 hrs by spectrophotometry | Eur J Med Chem 84: 382-94 (2014) Article DOI: 10.1016/j.ejmech.2014.07.036 BindingDB Entry DOI: 10.7270/Q2CJ8G45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50052239 (CHEMBL3322726) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Ltd Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus FabI-mediated reduction of enoyl-ACP preincubated for 30 mins measured after 2 hrs by spectrophotometry | Eur J Med Chem 84: 382-94 (2014) Article DOI: 10.1016/j.ejmech.2014.07.036 BindingDB Entry DOI: 10.7270/Q2CJ8G45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50052241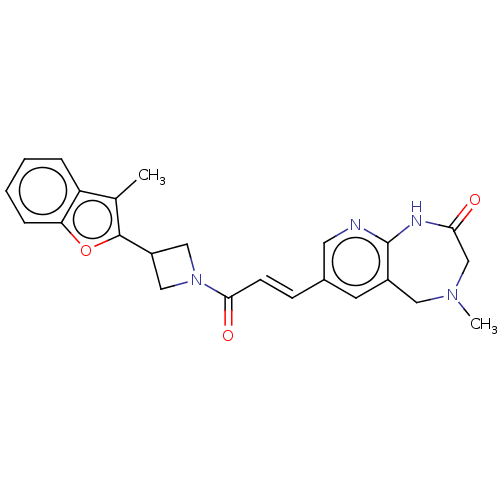 (CHEMBL3322728) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Ltd Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus FabI-mediated reduction of enoyl-ACP preincubated for 30 mins measured after 2 hrs by spectrophotometry | Eur J Med Chem 84: 382-94 (2014) Article DOI: 10.1016/j.ejmech.2014.07.036 BindingDB Entry DOI: 10.7270/Q2CJ8G45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50052232 (CHEMBL3322718) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Ltd Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus FabI-mediated reduction of enoyl-ACP preincubated for 30 mins measured after 2 hrs by spectrophotometry | Eur J Med Chem 84: 382-94 (2014) Article DOI: 10.1016/j.ejmech.2014.07.036 BindingDB Entry DOI: 10.7270/Q2CJ8G45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50052237 (CHEMBL3322724) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Ltd Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus FabI-mediated reduction of enoyl-ACP preincubated for 30 mins measured after 2 hrs by spectrophotometry | Eur J Med Chem 84: 382-94 (2014) Article DOI: 10.1016/j.ejmech.2014.07.036 BindingDB Entry DOI: 10.7270/Q2CJ8G45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50052227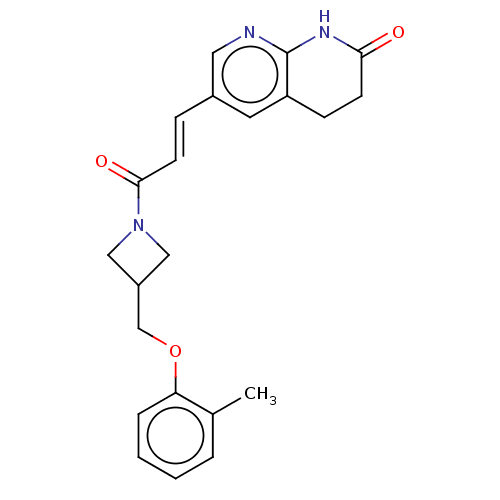 (CHEMBL3322710) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 177 | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Ltd Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus FabI-mediated reduction of enoyl-ACP preincubated for 30 mins measured after 2 hrs by spectrophotometry | Eur J Med Chem 84: 382-94 (2014) Article DOI: 10.1016/j.ejmech.2014.07.036 BindingDB Entry DOI: 10.7270/Q2CJ8G45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50052233 (CHEMBL3322720) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 204 | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Ltd Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus FabI-mediated reduction of enoyl-ACP preincubated for 30 mins measured after 2 hrs by spectrophotometry | Eur J Med Chem 84: 382-94 (2014) Article DOI: 10.1016/j.ejmech.2014.07.036 BindingDB Entry DOI: 10.7270/Q2CJ8G45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50052235 (CHEMBL3322722) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 242 | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Ltd Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus FabI-mediated reduction of enoyl-ACP preincubated for 30 mins measured after 2 hrs by spectrophotometry | Eur J Med Chem 84: 382-94 (2014) Article DOI: 10.1016/j.ejmech.2014.07.036 BindingDB Entry DOI: 10.7270/Q2CJ8G45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50052234 (CHEMBL3322721) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 242 | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Ltd Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus FabI-mediated reduction of enoyl-ACP preincubated for 30 mins measured after 2 hrs by spectrophotometry | Eur J Med Chem 84: 382-94 (2014) Article DOI: 10.1016/j.ejmech.2014.07.036 BindingDB Entry DOI: 10.7270/Q2CJ8G45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50052223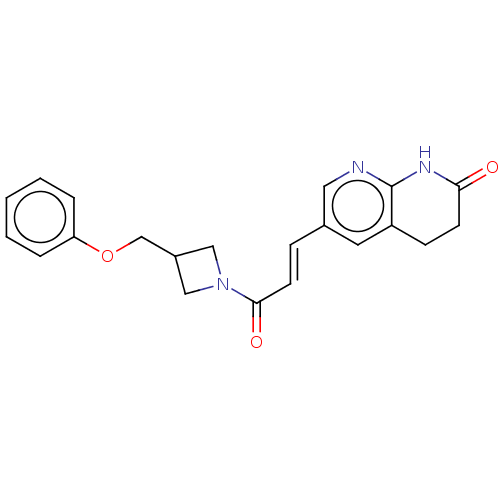 (CHEMBL3322706) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 265 | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Ltd Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus FabI-mediated reduction of enoyl-ACP preincubated for 30 mins measured after 2 hrs by spectrophotometry | Eur J Med Chem 84: 382-94 (2014) Article DOI: 10.1016/j.ejmech.2014.07.036 BindingDB Entry DOI: 10.7270/Q2CJ8G45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50052242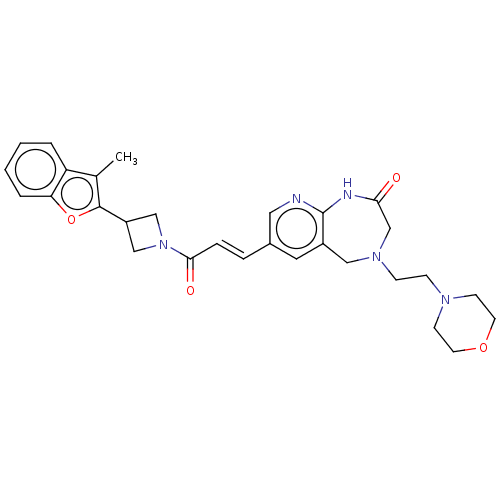 (CHEMBL3322729) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 289 | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Ltd Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus FabI-mediated reduction of enoyl-ACP preincubated for 30 mins measured after 2 hrs by spectrophotometry | Eur J Med Chem 84: 382-94 (2014) Article DOI: 10.1016/j.ejmech.2014.07.036 BindingDB Entry DOI: 10.7270/Q2CJ8G45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50052243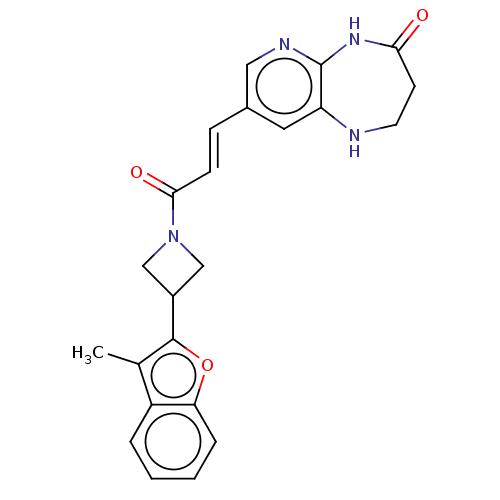 (CHEMBL3322730) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Ltd Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus FabI-mediated reduction of enoyl-ACP preincubated for 30 mins measured after 2 hrs by spectrophotometry | Eur J Med Chem 84: 382-94 (2014) Article DOI: 10.1016/j.ejmech.2014.07.036 BindingDB Entry DOI: 10.7270/Q2CJ8G45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50052226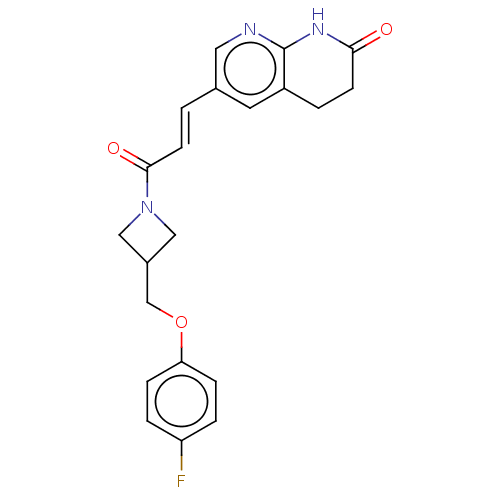 (CHEMBL3322709) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Ltd Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus FabI-mediated reduction of enoyl-ACP preincubated for 30 mins measured after 2 hrs by spectrophotometry | Eur J Med Chem 84: 382-94 (2014) Article DOI: 10.1016/j.ejmech.2014.07.036 BindingDB Entry DOI: 10.7270/Q2CJ8G45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50052230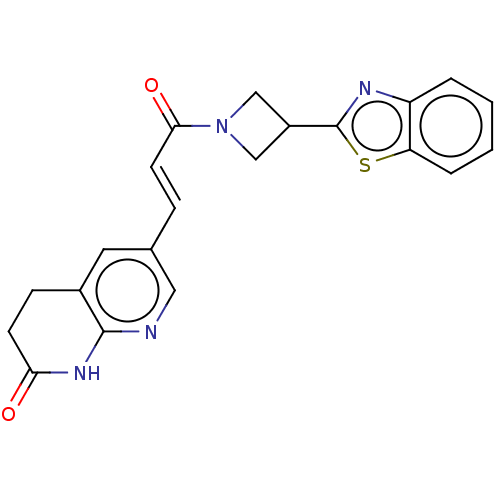 (CHEMBL3322716) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 373 | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Ltd Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus FabI-mediated reduction of enoyl-ACP preincubated for 30 mins measured after 2 hrs by spectrophotometry | Eur J Med Chem 84: 382-94 (2014) Article DOI: 10.1016/j.ejmech.2014.07.036 BindingDB Entry DOI: 10.7270/Q2CJ8G45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM133673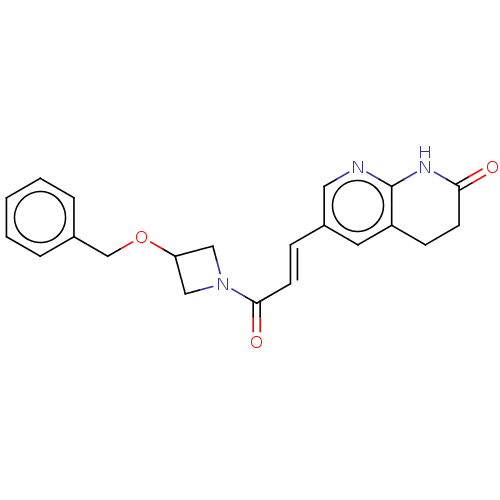 (US8846711, 17 | US9051321, 17) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 376 | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Ltd Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus FabI-mediated reduction of enoyl-ACP preincubated for 30 mins measured after 2 hrs by spectrophotometry | Eur J Med Chem 84: 382-94 (2014) Article DOI: 10.1016/j.ejmech.2014.07.036 BindingDB Entry DOI: 10.7270/Q2CJ8G45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50052224 (CHEMBL3322707) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 378 | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Ltd Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus FabI-mediated reduction of enoyl-ACP preincubated for 30 mins measured after 2 hrs by spectrophotometry | Eur J Med Chem 84: 382-94 (2014) Article DOI: 10.1016/j.ejmech.2014.07.036 BindingDB Entry DOI: 10.7270/Q2CJ8G45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50052240 (CHEMBL3322727) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 384 | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Ltd Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus FabI-mediated reduction of enoyl-ACP preincubated for 30 mins measured after 2 hrs by spectrophotometry | Eur J Med Chem 84: 382-94 (2014) Article DOI: 10.1016/j.ejmech.2014.07.036 BindingDB Entry DOI: 10.7270/Q2CJ8G45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50052225 (CHEMBL3322708) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 438 | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Ltd Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus FabI-mediated reduction of enoyl-ACP preincubated for 30 mins measured after 2 hrs by spectrophotometry | Eur J Med Chem 84: 382-94 (2014) Article DOI: 10.1016/j.ejmech.2014.07.036 BindingDB Entry DOI: 10.7270/Q2CJ8G45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50052236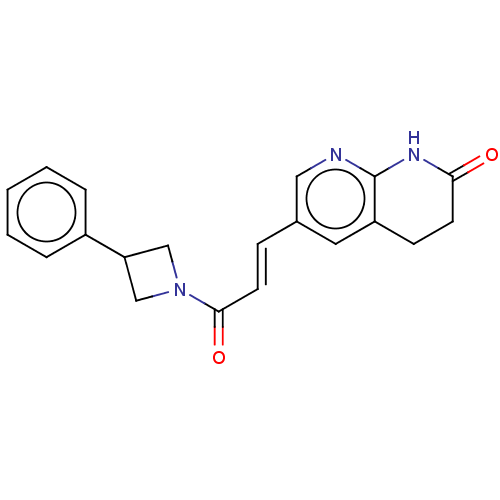 (CHEMBL3322723) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 488 | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Ltd Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus FabI-mediated reduction of enoyl-ACP preincubated for 30 mins measured after 2 hrs by spectrophotometry | Eur J Med Chem 84: 382-94 (2014) Article DOI: 10.1016/j.ejmech.2014.07.036 BindingDB Entry DOI: 10.7270/Q2CJ8G45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452511 (CHEMBL4204517) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH after 10 mins b... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 85 total ) | Next | Last >> |