

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103118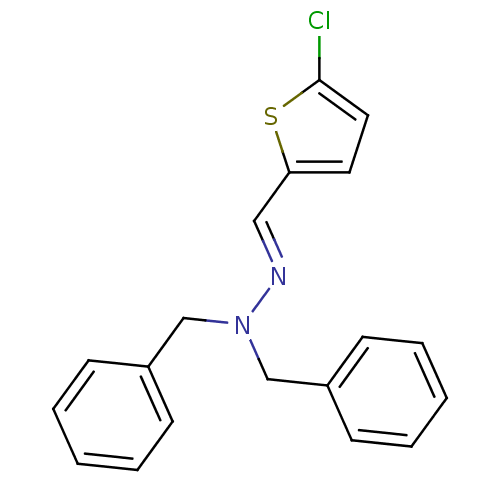 (CHEMBL66059 | N,N-Dibenzyl-N'-[1-(5-chloro-thiophe...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103125 (CHEMBL69115 | N,N-Dibenzyl-N'-[1-(4-methoxy-phenyl...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103111 (CHEMBL69665 | N,N-Dibenzyl-N'-[1-(4-chloro-phenyl)...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103119 (CHEMBL302504 | N,N-Dibenzyl-N'-[1-(5-ethyl-furan-2...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103107 (CHEMBL65842 | N,N-Dibenzyl-N'-[1-(3-methoxy-phenyl...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103128 (CHEMBL68984 | N,N-Dibenzyl-N'-[1-furan-2-yl-meth-(...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103106 (CHEMBL69480 | N,N-Dibenzyl-N'-[1-thiophen-2-yl-met...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103122 (CHEMBL69334 | N,N-Dibenzyl-N'-[1-phenyl-meth-(E)-y...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103110 (CHEMBL305258 | N,N-Dibenzyl-N'-[1-(3,4-dichloro-ph...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103114 (CHEMBL69541 | N,N-Dibenzyl-N'-[1-cyclohexyl-meth-(...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103115 (4-(Dibenzyl-hydrazonomethyl)-phenol | CHEMBL70032) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103126 (CHEMBL68011 | N,N-Dibenzyl-N'-[1-pyridin-3-yl-meth...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Rattus norvegicus) | BDBM50529620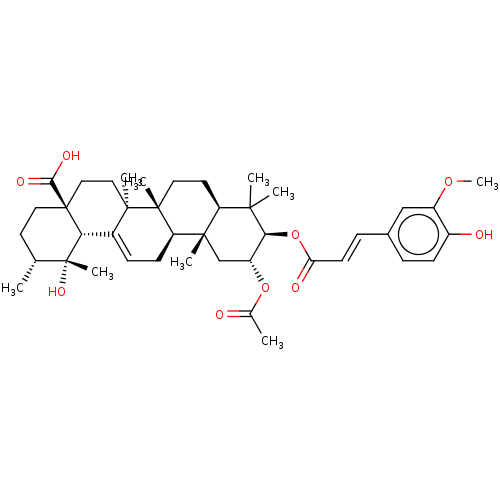 (CHEMBL4558600) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval Curated by ChEMBL | Assay Description Inhibition of glucose-6-phosphatase in rat H42E cells using glucose-6-phosphate as substrate preincubated overnight followed by substrate addition an... | J Nat Prod 81: 2169-2176 (2018) Article DOI: 10.1021/acs.jnatprod.8b00079 BindingDB Entry DOI: 10.7270/Q2VM4GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Rattus norvegicus) | BDBM50529620 (CHEMBL4558600) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval Curated by ChEMBL | Assay Description Inhibition of glucose-6-phosphatase in rat H42E cells using glucose-6-phosphate as substrate preincubated overnight followed by substrate addition an... | J Nat Prod 81: 2169-2176 (2018) Article DOI: 10.1021/acs.jnatprod.8b00079 BindingDB Entry DOI: 10.7270/Q2VM4GRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103120 (CHEMBL69497 | N,N-Dibenzyl-N'-[1-pyridin-2-yl-meth...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Rattus norvegicus) | BDBM50389999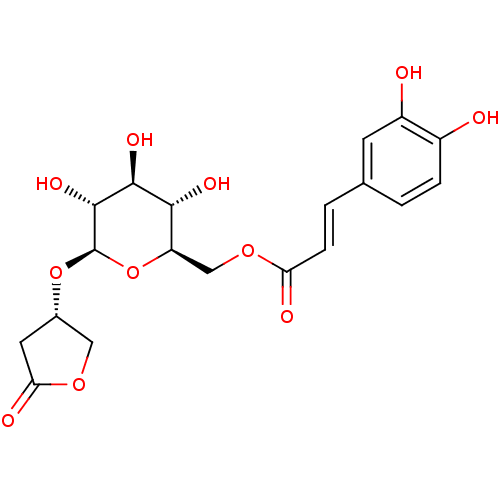 (CHEMBL2071306) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ottawa Curated by ChEMBL | Assay Description Inhibition of glucose-6-phosphatase enzymatic activity in rat H42E cells using G6P substrate incubated for 18 hrs by colorimetric assay | J Nat Prod 75: 1284-8 (2012) Article DOI: 10.1021/np3001317 BindingDB Entry DOI: 10.7270/Q2VM4DBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103124 (CHEMBL67272 | [5-(Dibenzyl-hydrazonomethyl)-furan-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103112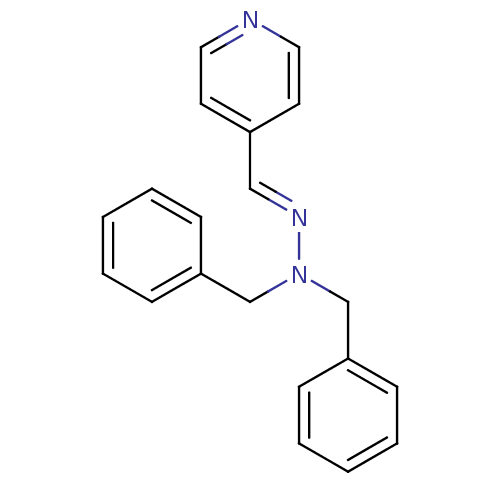 (CHEMBL71650 | N,N-Dibenzyl-N'-[1-pyridin-4-yl-meth...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103121 (CHEMBL419070 | N,N-Dibenzyl-N'-[1-phenyl-eth-(E)-y...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103109 (CHEMBL66774 | N,N-Dibenzyl-N'-[3,4-dihydro-2H-naph...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103116 (CHEMBL68981 | N,N-Dibenzyl-N'-[6-methoxy-3,4-dihyd...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50277824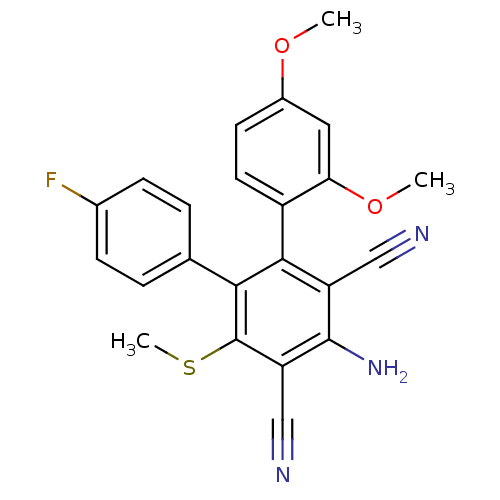 (4'-Amino-4-fluoro-2'',4''-dimethoxy-6'-methylsulfa...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of glucose-6-phosphatase (unknown origin) | Bioorg Med Chem Lett 19: 2158-61 (2009) Article DOI: 10.1016/j.bmcl.2009.02.118 BindingDB Entry DOI: 10.7270/Q28G8KKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Rattus norvegicus) | BDBM50294478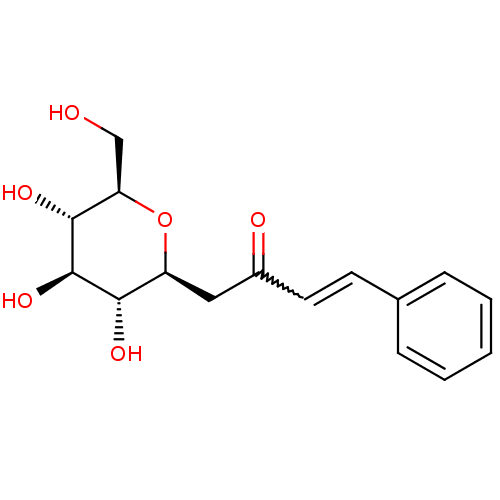 ((E)-4-phenyl-1-((2S,3R,4R,5S,6R)-3,4,5-trihydroxy-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of rat liver glucose-6-phosphatase | Bioorg Med Chem Lett 19: 2699-703 (2009) Article DOI: 10.1016/j.bmcl.2009.03.136 BindingDB Entry DOI: 10.7270/Q20V8CTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Rattus norvegicus) | BDBM50294477 ((E)-1-(beta-D-glucopyranosyl)-4-(4'-chlorophenyl)b...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of rat liver glucose-6-phosphatase | Bioorg Med Chem Lett 19: 2699-703 (2009) Article DOI: 10.1016/j.bmcl.2009.03.136 BindingDB Entry DOI: 10.7270/Q20V8CTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103113 (CHEMBL69758 | [4-(Dibenzyl-hydrazonomethyl)-phenyl...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103123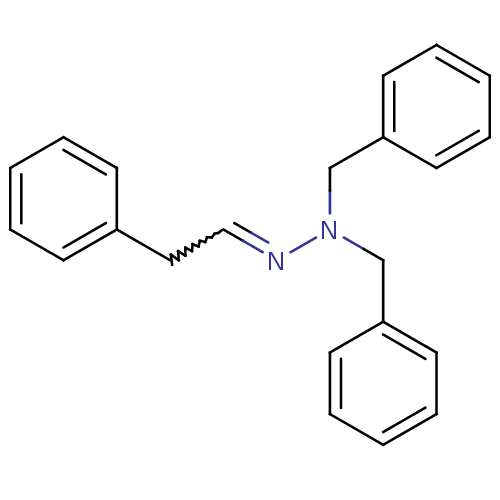 (CHEMBL68153 | N,N-Dibenzyl-N'-[2-phenyl-eth-(E)-yl...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103117 (CHEMBL302496 | N,N-Dibenzyl-N'-[1-(3,5-dichloro-ph...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103127 (CHEMBL69742 | N,N-Dibenzyl-N'-[1-(5-nitro-furan-2-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Homo sapiens (Human)) | BDBM50103108 (CHEMBL304503 | N,N-Dibenzyl-N'-[1-(2-methoxy-pheny...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Glucose-6-Phosphatase from Triton X-100 disrupted pig liver microsomes. | Bioorg Med Chem Lett 11: 2165-7 (2001) BindingDB Entry DOI: 10.7270/Q2M61JJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphatase catalytic subunit 1 (Rattus norvegicus) | BDBM50390000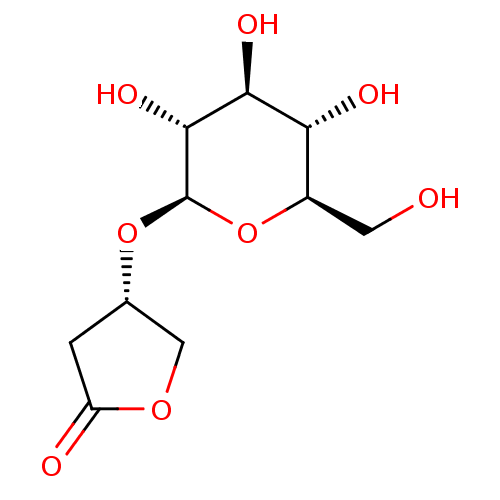 (CHEMBL2071309) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.91E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ottawa Curated by ChEMBL | Assay Description Inhibition of glucose-6-phosphatase enzymatic activity in rat H42E cells using G6P substrate incubated for 18 hrs by colorimetric assay | J Nat Prod 75: 1284-8 (2012) Article DOI: 10.1021/np3001317 BindingDB Entry DOI: 10.7270/Q2VM4DBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||