

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Macaca mulatta (Rhesus macaque)) | BDBM108297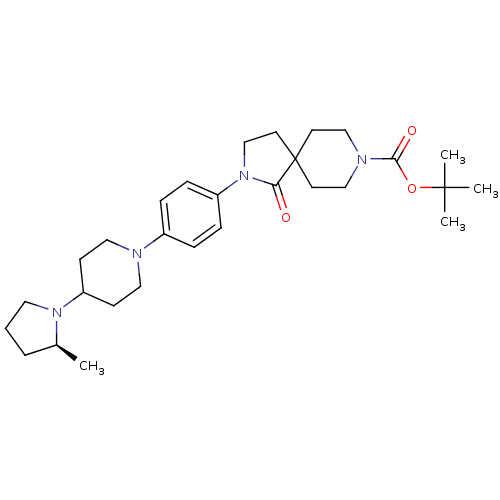 (US8604046, 77) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description The compounds of this invention have been demonstrated to displace [3H]- methylhistamine radioligand binding to mammalian cell membranes expressing r... | US Patent US8604046 (2013) BindingDB Entry DOI: 10.7270/Q2W957TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50434335 (CHEMBL2386729 | US9181275, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0200 | -61.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc. US Patent | Assay Description To determine the effectiveness of representative compounds of this invention as histamine-3 receptor ligands (H3 receptor ligands), the following tes... | US Patent US9181275 (2015) BindingDB Entry DOI: 10.7270/Q2R49PJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50434335 (CHEMBL2386729 | US9181275, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dart Neuroscience LLC Curated by ChEMBL | Assay Description Binding affinity to histamine H3 receptor in rat cortical membranes | ACS Med Chem Lett 4: (2013) Article DOI: 10.1021/ml400105b BindingDB Entry DOI: 10.7270/Q2S75HQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50434337 (CHEMBL2386727 | US9181275, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dart Neuroscience LLC Curated by ChEMBL | Assay Description Binding affinity to histamine H3 receptor in rat cortical membranes | ACS Med Chem Lett 4: (2013) Article DOI: 10.1021/ml400105b BindingDB Entry DOI: 10.7270/Q2S75HQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50138119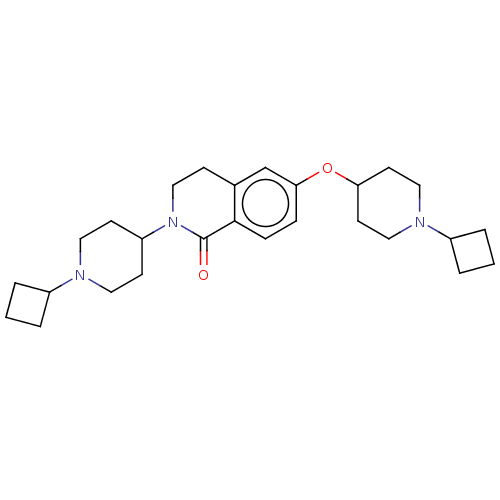 (CHEMBL3753475) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Binding affinity to recombinant human H3 receptor | Eur J Med Chem 108: 655-62 (2016) Article DOI: 10.1016/j.ejmech.2015.12.005 BindingDB Entry DOI: 10.7270/Q2F191KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Macaca mulatta (Rhesus macaque)) | BDBM108281 (US8604046, 61) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description The compounds of this invention have been demonstrated to displace [3H]- methylhistamine radioligand binding to mammalian cell membranes expressing r... | US Patent US8604046 (2013) BindingDB Entry DOI: 10.7270/Q2W957TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50246381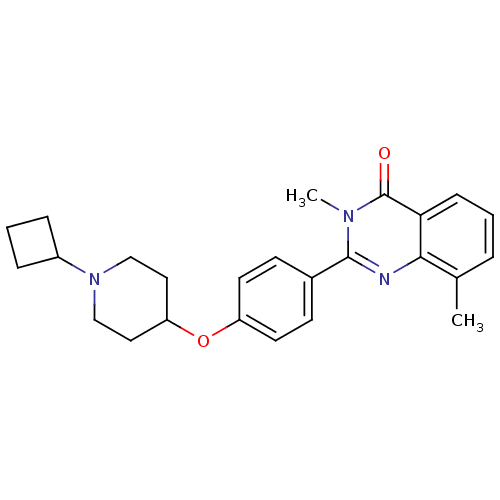 (2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-3,8-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0313 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50434337 (CHEMBL2386727 | US9181275, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0320 | -59.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc. US Patent | Assay Description To determine the effectiveness of representative compounds of this invention as histamine-3 receptor ligands (H3 receptor ligands), the following tes... | US Patent US9181275 (2015) BindingDB Entry DOI: 10.7270/Q2R49PJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Macaca mulatta (Rhesus macaque)) | BDBM108294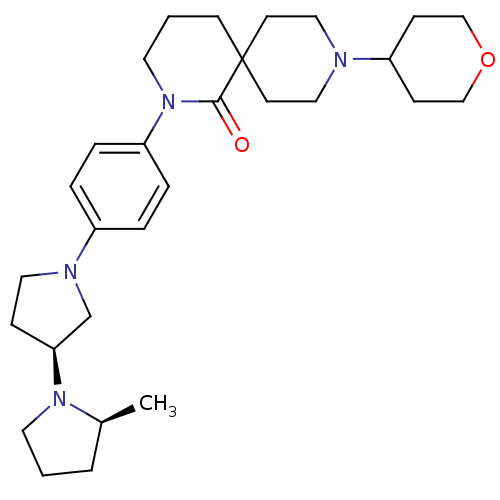 (US8604046, 74) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description The compounds of this invention have been demonstrated to displace [3H]- methylhistamine radioligand binding to mammalian cell membranes expressing r... | US Patent US8604046 (2013) BindingDB Entry DOI: 10.7270/Q2W957TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Macaca mulatta (Rhesus macaque)) | BDBM108315 (US8604046, 95) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description The compounds of this invention have been demonstrated to displace [3H]- methylhistamine radioligand binding to mammalian cell membranes expressing r... | US Patent US8604046 (2013) BindingDB Entry DOI: 10.7270/Q2W957TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50277100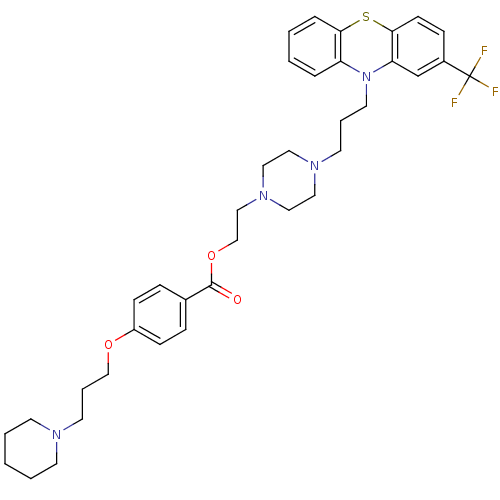 (4-(3-Piperidin-1-yl-propoxy)-benzoic acid 2-{4-[3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe Universit£t Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human histamine H3 receptor expressed in CHO/HEK293 cells | Bioorg Med Chem Lett 19: 538-42 (2008) Article DOI: 10.1016/j.bmcl.2008.09.012 BindingDB Entry DOI: 10.7270/Q25H7H6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50492784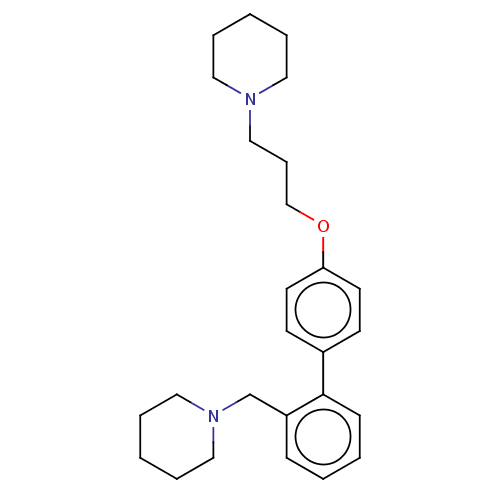 (CHEMBL2413837) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from histamine H3 receptor (unknown origin) expressed in CHO cells | Bioorg Med Chem 21: 4526-9 (2013) Article DOI: 10.1016/j.bmc.2013.05.035 BindingDB Entry DOI: 10.7270/Q2J9699Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50434336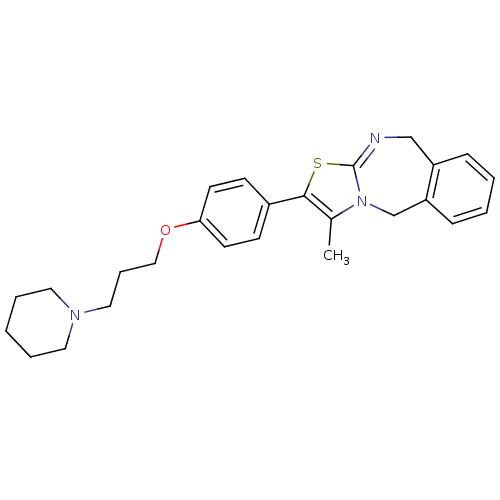 (CHEMBL2386728 | US9181275, 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dart Neuroscience LLC Curated by ChEMBL | Assay Description Binding affinity to histamine H3 receptor in rat cortical membranes | ACS Med Chem Lett 4: (2013) Article DOI: 10.1021/ml400105b BindingDB Entry DOI: 10.7270/Q2S75HQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50374104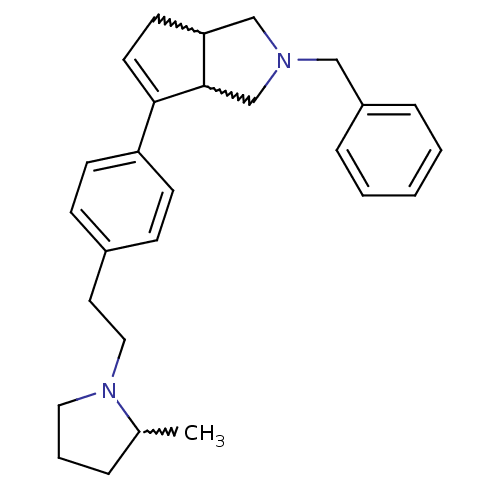 (CHEMBL255962) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex | Bioorg Med Chem Lett 18: 1490-4 (2008) Article DOI: 10.1016/j.bmcl.2007.12.059 BindingDB Entry DOI: 10.7270/Q2Q81DZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50374100 (CHEMBL270011) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex | Bioorg Med Chem Lett 18: 1490-4 (2008) Article DOI: 10.1016/j.bmcl.2007.12.059 BindingDB Entry DOI: 10.7270/Q2Q81DZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50434336 (CHEMBL2386728 | US9181275, 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0500 | -58.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc. US Patent | Assay Description To determine the effectiveness of representative compounds of this invention as histamine-3 receptor ligands (H3 receptor ligands), the following tes... | US Patent US9181275 (2015) BindingDB Entry DOI: 10.7270/Q2R49PJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50319536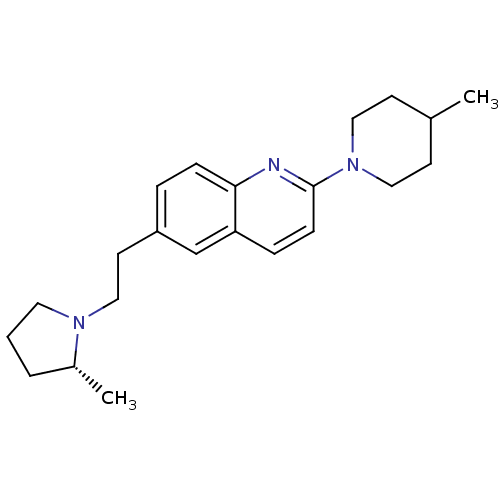 ((R)-2-(4-methylpiperidin-1-yl)-6-(2-(2-methylpyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50319536 ((R)-2-(4-methylpiperidin-1-yl)-6-(2-(2-methylpyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor | Bioorg Med Chem Lett 20: 3295-300 (2010) Article DOI: 10.1016/j.bmcl.2010.04.045 BindingDB Entry DOI: 10.7270/Q2XW4JZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174619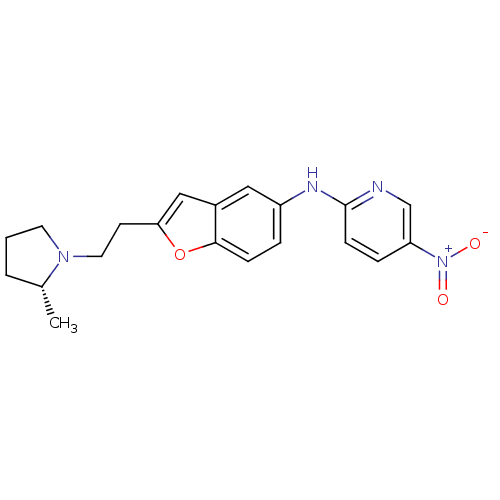 (CHEMBL197747 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50492781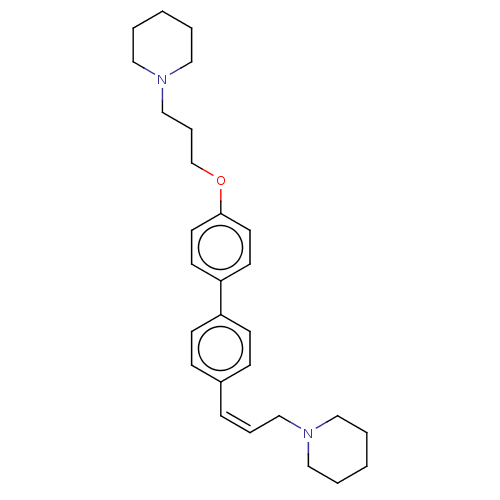 (CHEMBL2413835) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from histamine H3 receptor (unknown origin) expressed in CHO cells | Bioorg Med Chem 21: 4526-9 (2013) Article DOI: 10.1016/j.bmc.2013.05.035 BindingDB Entry DOI: 10.7270/Q2J9699Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Macaca mulatta (Rhesus macaque)) | BDBM108295 (US8604046, 75) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description The compounds of this invention have been demonstrated to displace [3H]- methylhistamine radioligand binding to mammalian cell membranes expressing r... | US Patent US8604046 (2013) BindingDB Entry DOI: 10.7270/Q2W957TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Macaca mulatta (Rhesus macaque)) | BDBM108284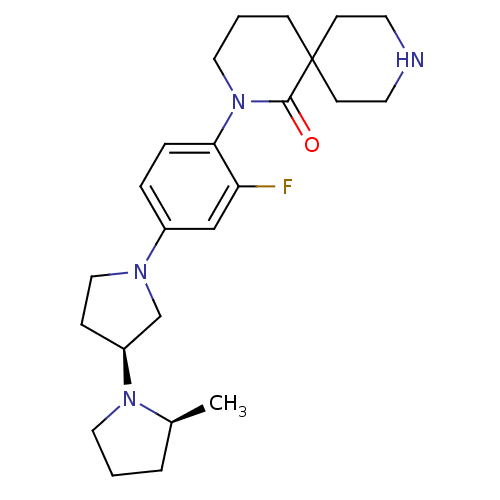 (US8604046, 64) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description The compounds of this invention have been demonstrated to displace [3H]- methylhistamine radioligand binding to mammalian cell membranes expressing r... | US Patent US8604046 (2013) BindingDB Entry DOI: 10.7270/Q2W957TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159110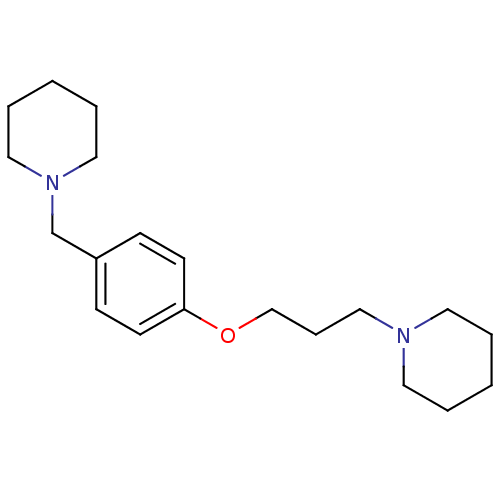 (1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0603 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity in Nluc-hH3R assessed in HEK293 cells by NanoBRET binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01089 BindingDB Entry DOI: 10.7270/Q2TB1BTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50246382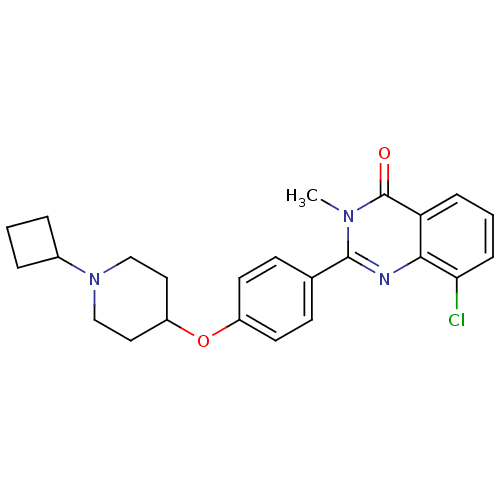 (8-chloro-2-(4-(1-cyclobutylpiperidin-4-yloxy)pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0625 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50278495 (4-chloro-1-(4-(3-(piperidin-1-yl)propoxy)benzyl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human histamine H3 receptor expressed in HEL293 cells | Bioorg Med Chem Lett 19: 2172-5 (2009) Article DOI: 10.1016/j.bmcl.2009.02.110 BindingDB Entry DOI: 10.7270/Q2H41RBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50246290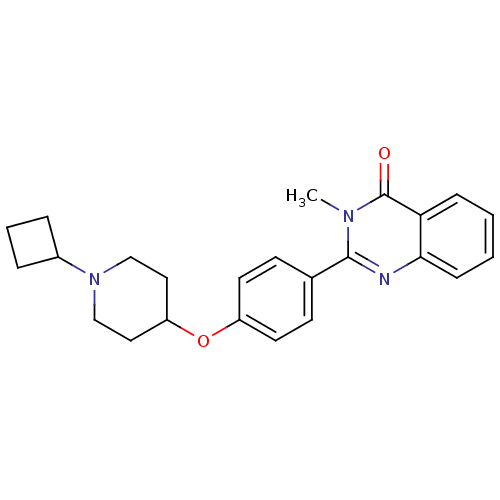 (2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-3-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0688 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50232355 ((3aR,6aR)-2-(cyclopropylmethyl)-6-(4-(2-(pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex | Bioorg Med Chem Lett 18: 1490-4 (2008) Article DOI: 10.1016/j.bmcl.2007.12.059 BindingDB Entry DOI: 10.7270/Q2Q81DZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Macaca mulatta (Rhesus macaque)) | BDBM108282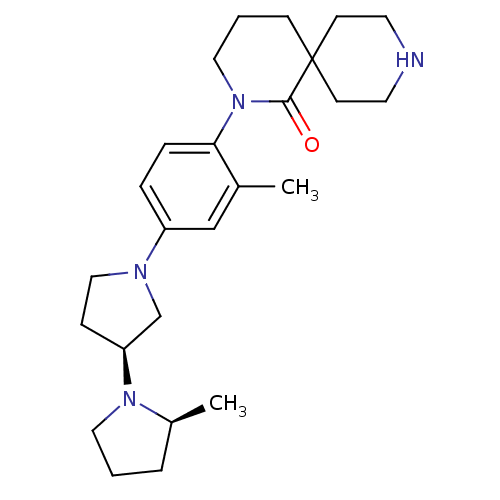 (US8604046, 62) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description The compounds of this invention have been demonstrated to displace [3H]- methylhistamine radioligand binding to mammalian cell membranes expressing r... | US Patent US8604046 (2013) BindingDB Entry DOI: 10.7270/Q2W957TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50232355 ((3aR,6aR)-2-(cyclopropylmethyl)-6-(4-(2-(pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | Bioorg Med Chem Lett 18: 4133-6 (2008) Article DOI: 10.1016/j.bmcl.2008.05.086 BindingDB Entry DOI: 10.7270/Q2C82942 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50246434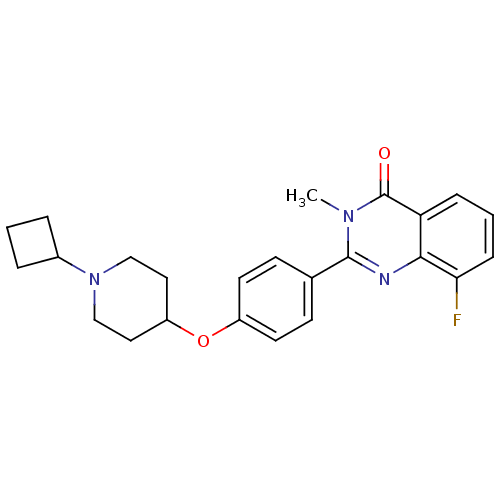 (2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-8-fluo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0781 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50374110 (CHEMBL401954) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex | Bioorg Med Chem Lett 18: 1490-4 (2008) Article DOI: 10.1016/j.bmcl.2007.12.059 BindingDB Entry DOI: 10.7270/Q2Q81DZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50158590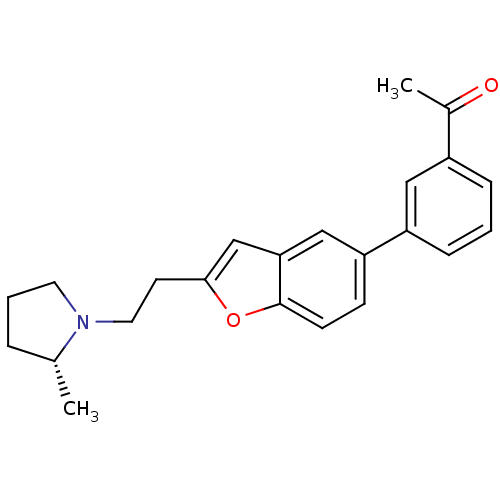 (1-(3-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor | J Med Chem 48: 38-55 (2005) Article DOI: 10.1021/jm040118g BindingDB Entry DOI: 10.7270/Q2571CST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50074627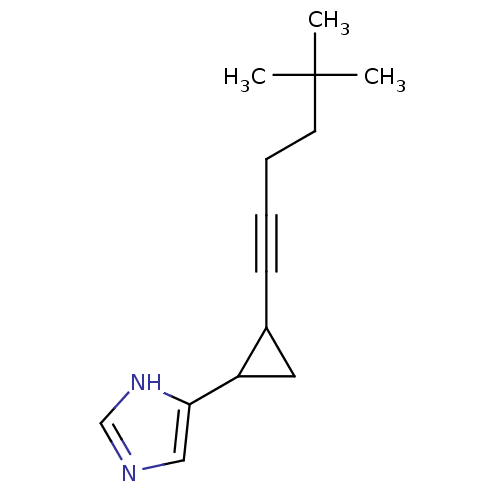 (4-[2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl]-1H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing rat histamine H3 receptor | J Med Chem 48: 38-55 (2005) Article DOI: 10.1021/jm040118g BindingDB Entry DOI: 10.7270/Q2571CST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Macaca mulatta (Rhesus macaque)) | BDBM108310 (US8604046, 90) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description The compounds of this invention have been demonstrated to displace [3H]- methylhistamine radioligand binding to mammalian cell membranes expressing r... | US Patent US8604046 (2013) BindingDB Entry DOI: 10.7270/Q2W957TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50319536 ((R)-2-(4-methylpiperidin-1-yl)-6-(2-(2-methylpyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from rat cloned histamine H3 receptor | Bioorg Med Chem Lett 20: 3295-300 (2010) Article DOI: 10.1016/j.bmcl.2010.04.045 BindingDB Entry DOI: 10.7270/Q2XW4JZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174627 (CHEMBL199245 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50215536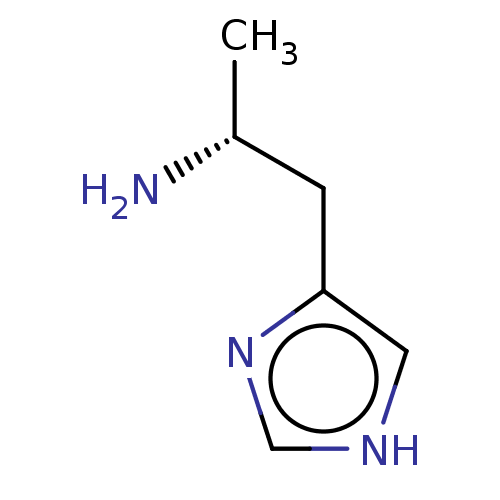 ((R)-Alpha-Methylhistamine | CHEBI:73337 | CHEMBL26...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.0851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Binding affinity towards histamine H3 receptor using [3H](R)-alpha-methylhistamine as radioligand in guinea pig cortical homogenates | Bioorg Med Chem Lett 9: 1825-30 (1999) BindingDB Entry DOI: 10.7270/Q24T6MKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50110291 ((7-Chloro-quinolin-4-yl)-[4-(3-piperidin-1-yl-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Affinity for displacement of [125I]-iodoproxyfan from human histamine H3 receptors stably expressed in CHO cells | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Macaca mulatta (Rhesus macaque)) | BDBM108328 (US8604046, 108) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description The compounds of this invention have been demonstrated to displace [3H]- methylhistamine radioligand binding to mammalian cell membranes expressing r... | US Patent US8604046 (2013) BindingDB Entry DOI: 10.7270/Q2W957TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50158588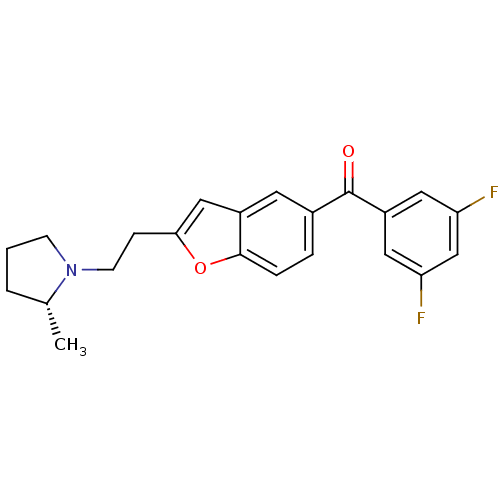 ((3,5-Difluoro-phenyl)-{2-[2-((R)-2-methyl-pyrrolid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor | J Med Chem 48: 38-55 (2005) Article DOI: 10.1021/jm040118g BindingDB Entry DOI: 10.7270/Q2571CST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50110288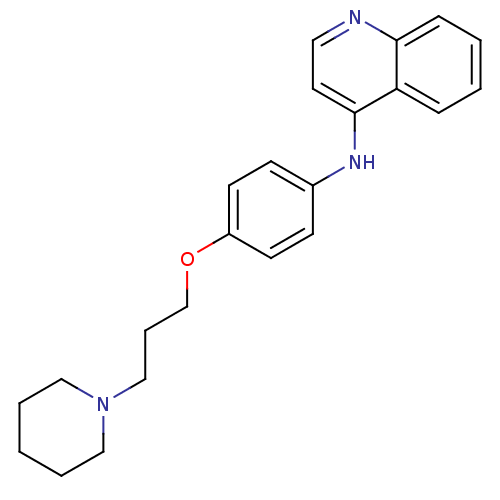 (CHEMBL15153 | N-(4-(3-(piperidin-1-yl)propoxy)phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Affinity for displacement of [125I]-iodoproxyfan from human histamine H3 receptors stably expressed in CHO cells | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50278350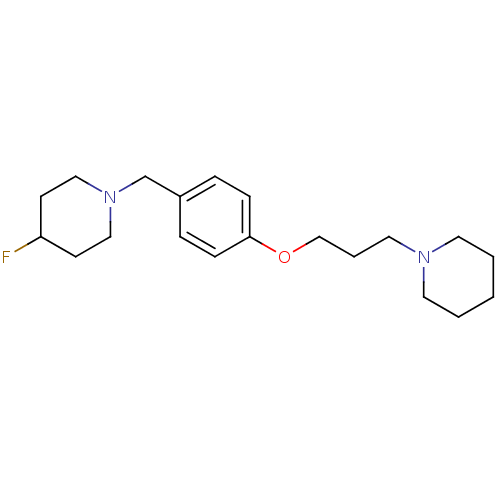 (4-fluoro-1-(4-(3-(piperidin-1-yl)propoxy)benzyl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0933 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe University Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant human histamine H3 receptor expressed in HEK293 cells after 90 mins | Bioorg Med Chem Lett 24: 2236-9 (2014) Article DOI: 10.1016/j.bmcl.2014.03.098 BindingDB Entry DOI: 10.7270/Q2MP54TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50246333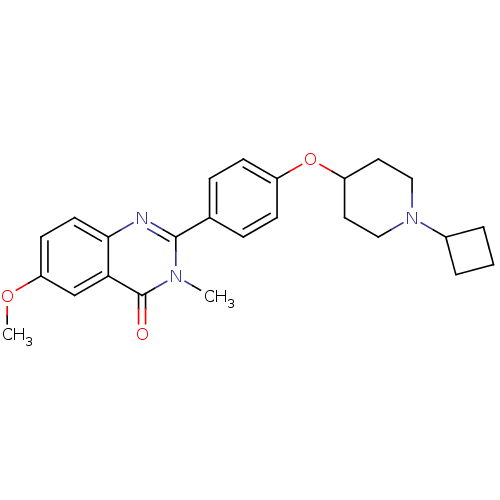 (2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-6-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0938 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50246334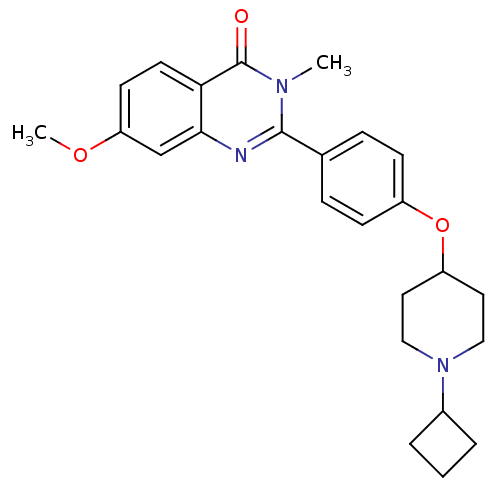 (2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-7-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0938 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50246435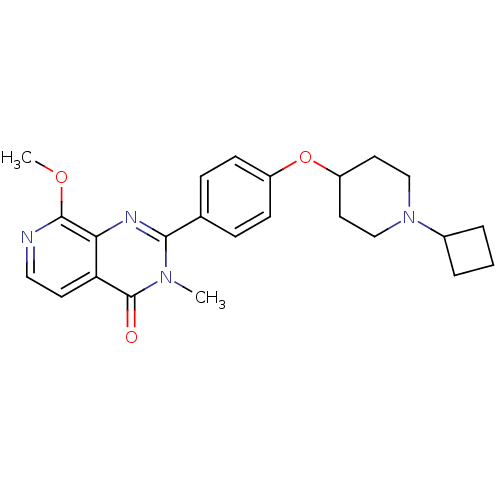 (2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-8-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0938 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50278350 (4-fluoro-1-(4-(3-(piperidin-1-yl)propoxy)benzyl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe University Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant human histamine H3 receptor expressed in HEK293 cells after 90 mins | Bioorg Med Chem Lett 24: 2236-9 (2014) Article DOI: 10.1016/j.bmcl.2014.03.098 BindingDB Entry DOI: 10.7270/Q2MP54TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50278350 (4-fluoro-1-(4-(3-(piperidin-1-yl)propoxy)benzyl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human histamine H3 receptor expressed in HEL293 cells | Bioorg Med Chem Lett 19: 2172-5 (2009) Article DOI: 10.1016/j.bmcl.2009.02.110 BindingDB Entry DOI: 10.7270/Q2H41RBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50246287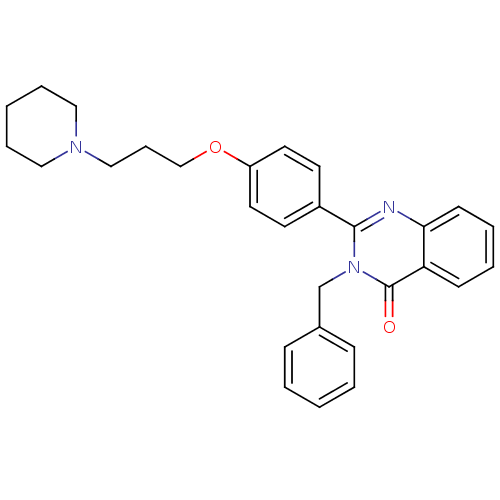 (3-benzyl-2-(4-(3-(piperidin-1-yl)propoxy)phenyl)qu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0969 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50492783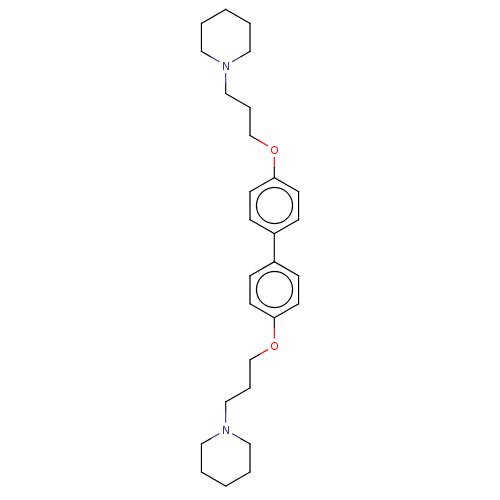 (CHEMBL2413824) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from histamine H3 receptor (unknown origin) expressed in CHO cells | Bioorg Med Chem 21: 4526-9 (2013) Article DOI: 10.1016/j.bmc.2013.05.035 BindingDB Entry DOI: 10.7270/Q2J9699Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Macaca mulatta (Rhesus macaque)) | BDBM108316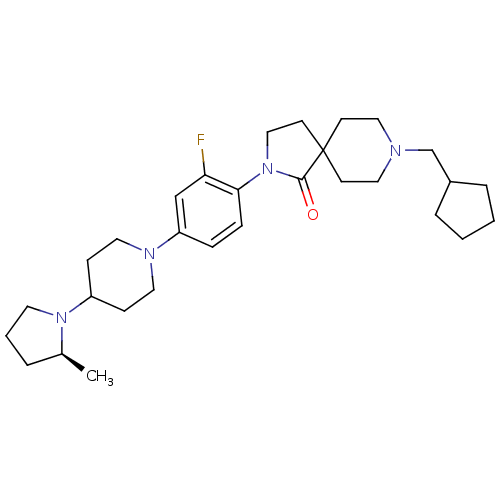 (US8604046, 96) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description The compounds of this invention have been demonstrated to displace [3H]- methylhistamine radioligand binding to mammalian cell membranes expressing r... | US Patent US8604046 (2013) BindingDB Entry DOI: 10.7270/Q2W957TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 8532 total ) | Next | Last >> |