

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine N-methyltransferase (Homo sapiens (Human)) | BDBM50041457 (4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University Curated by ChEMBL | Assay Description Inhibition of histamine N-methyltransferase by radiochemical assay | Bioorg Med Chem Lett 22: 4990-3 (2012) Article DOI: 10.1016/j.bmcl.2012.06.032 BindingDB Entry DOI: 10.7270/Q29G5NWN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine N-methyltransferase (Homo sapiens (Human)) | BDBM50026331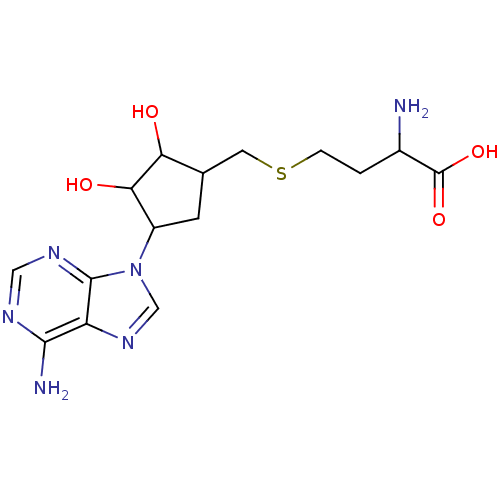 (2-Amino-4-[4-(6-amino-purin-9-yl)-2,3-dihydroxy-cy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated against Histamine N-methyl-transferase | J Med Chem 28: 478-82 (1985) BindingDB Entry DOI: 10.7270/Q2WM1DZS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Histamine N-methyltransferase (Homo sapiens (Human)) | BDBM50026331 (2-Amino-4-[4-(6-amino-purin-9-yl)-2,3-dihydroxy-cy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated against Histamine N-methyl-transferase | J Med Chem 28: 478-82 (1985) BindingDB Entry DOI: 10.7270/Q2WM1DZS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Histamine N-methyltransferase (Homo sapiens (Human)) | BDBM50009672 (AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated against Histamine N-methyl-transferase | J Med Chem 28: 478-82 (1985) BindingDB Entry DOI: 10.7270/Q2WM1DZS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine N-methyltransferase (Homo sapiens (Human)) | BDBM50009672 (AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant was evaluated against Histamine N-methyl-transferase | J Med Chem 28: 478-82 (1985) BindingDB Entry DOI: 10.7270/Q2WM1DZS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine N-methyltransferase (Cavia porcellus) | BDBM50016564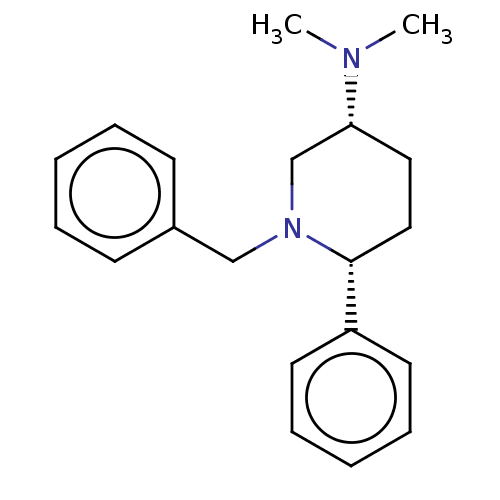 (CHEMBL3272448) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of histamine N-methyltransferase in guinea pig brain using S-adenosyl-L-methionine-14C as substrate by scintillation spectrophotometer | J Med Chem 19: 117-22 (1976) BindingDB Entry DOI: 10.7270/Q29W0H2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Cavia porcellus) | BDBM50016547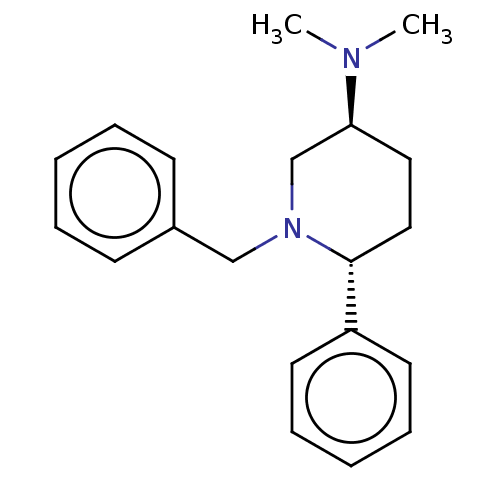 (CHEMBL3272447) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.47E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of histamine N-methyltransferase in guinea pig brain using S-adenosyl-L-methionine-14C as substrate by scintillation spectrophotometer | J Med Chem 19: 117-22 (1976) BindingDB Entry DOI: 10.7270/Q29W0H2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110309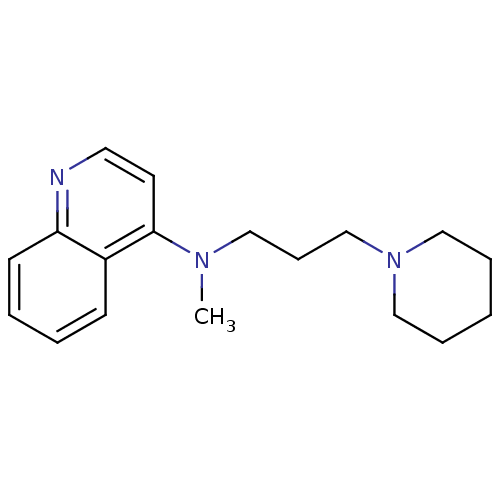 (CHEMBL14740 | Methyl-(3-piperidin-1-yl-propyl)-qui...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110306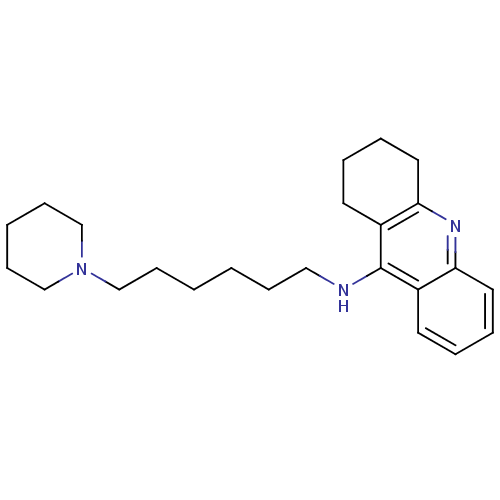 ((6-Piperidin-1-yl-hexyl)-(1,2,3,4-tetrahydro-acrid...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110285 (CHEMBL274041 | {3-[4-(3-Piperidin-1-yl-propoxy)-ph...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110303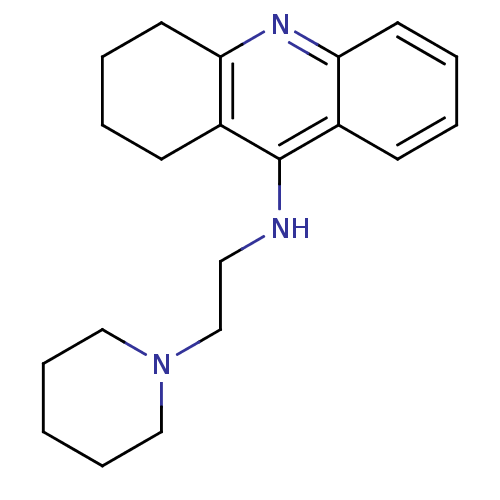 ((2-Piperidin-1-yl-ethyl)-(1,2,3,4-tetrahydro-acrid...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110286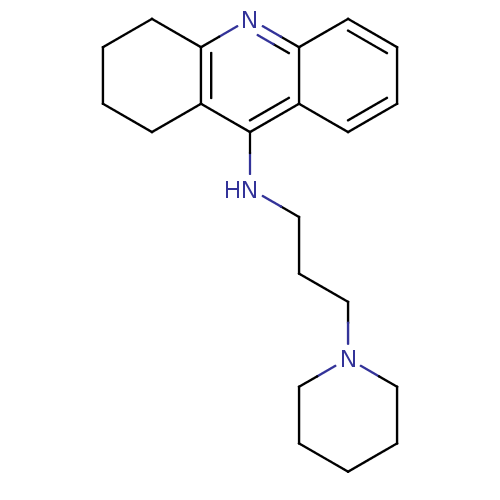 ((3-Piperidin-1-yl-propyl)-(1,2,3,4-tetrahydro-acri...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110296 (CHEMBL14868 | {4-[4-(3-Piperidin-1-yl-propoxy)-phe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110300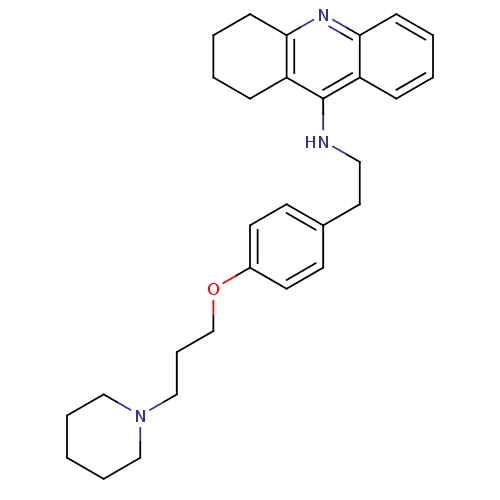 (CHEMBL15056 | N-(4-(3-(piperidin-1-yl)propoxy)phen...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110298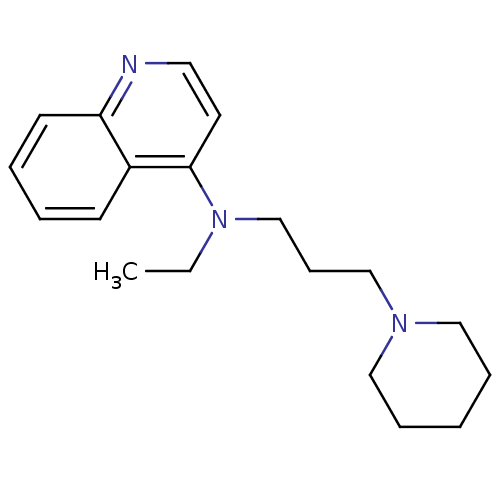 (CHEMBL277113 | Ethyl-(3-piperidin-1-yl-propyl)-qui...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110288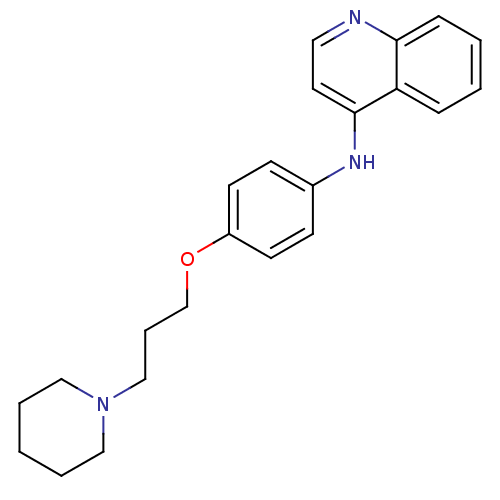 (CHEMBL15153 | N-(4-(3-(piperidin-1-yl)propoxy)phen...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110294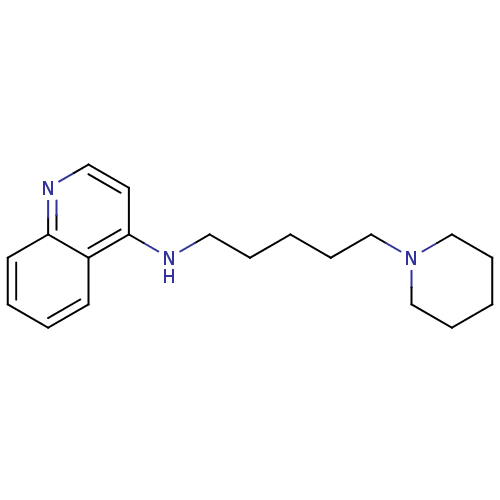 ((5-Piperidin-1-yl-pentyl)-quinolin-4-yl-amine; Oxa...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110284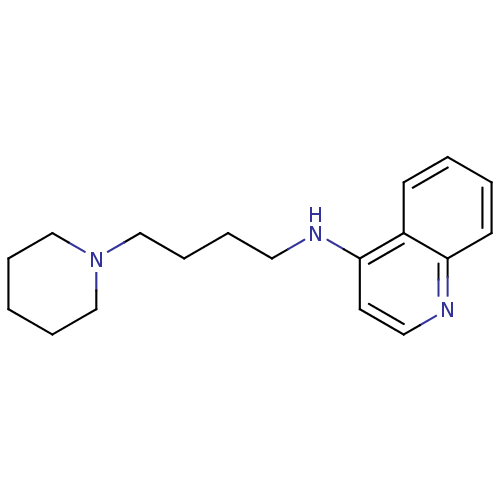 ((4-Piperidin-1-yl-butyl)-quinolin-4-yl-amine; Oxal...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110287 ((3-Piperidin-1-yl-propyl)-quinolin-4-yl-amine; Oxa...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110290 (CHEMBL14484 | {4-[4-(3-Piperidin-1-yl-propoxy)-phe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110297 ((6-Piperidin-1-yl-hexyl)-quinolin-4-yl-amine; Oxal...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110299 (CHEMBL14754 | {3-[4-(3-Piperidin-1-yl-propoxy)-phe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110302 (Acridin-9-yl-(3-piperidin-1-yl-propyl)-amine; Oxal...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110293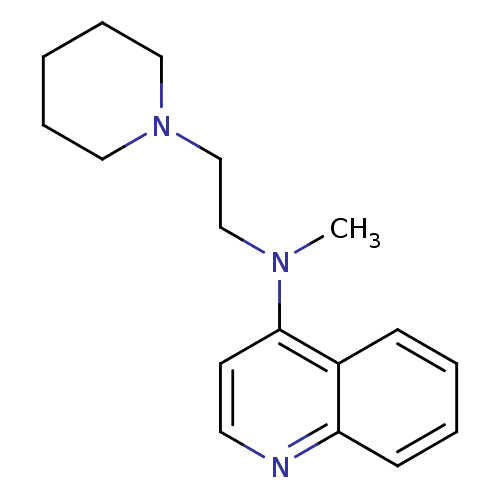 (CHEMBL14860 | Methyl-(2-piperidin-1-yl-ethyl)-quin...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110311 (CHEMBL276938 | {2-[4-(3-Piperidin-1-yl-propoxy)-ph...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110282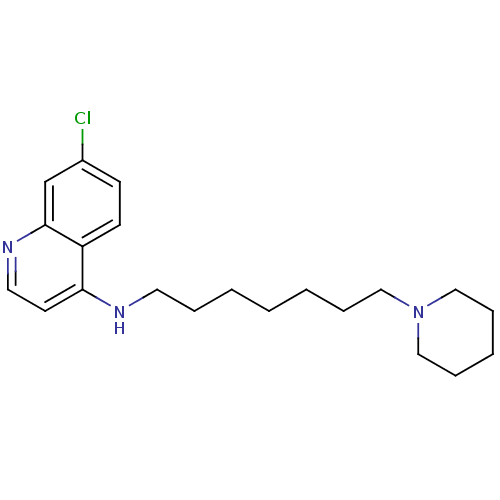 ((7-Chloro-quinolin-4-yl)-(7-piperidin-1-yl-heptyl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110304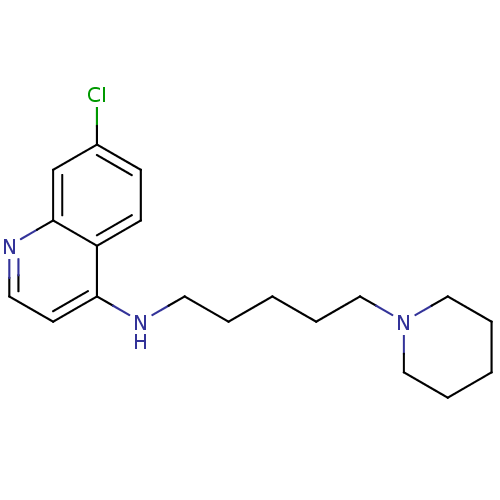 ((7-Chloro-quinolin-4-yl)-(5-piperidin-1-yl-pentyl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110283 ((7-Chloro-quinolin-4-yl)-(4-piperidin-1-yl-butyl)-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110295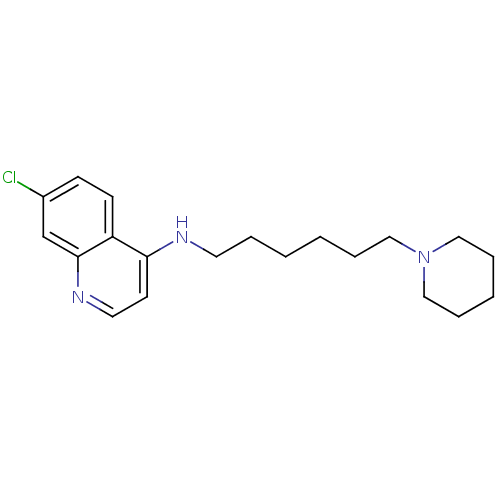 ((7-Chloro-quinolin-4-yl)-(6-piperidin-1-yl-hexyl)-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110305 ((7-Chloro-quinolin-4-yl)-(3-piperidin-1-yl-propyl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110313 ((2-Piperidin-1-yl-ethyl)-quinolin-4-yl-amine; Oxal...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110289 ((7-Chloro-quinolin-4-yl)-(10-piperidin-1-yl-decyl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110312 ((7-Chloro-quinolin-4-yl)-(8-piperidin-1-yl-octyl)-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110301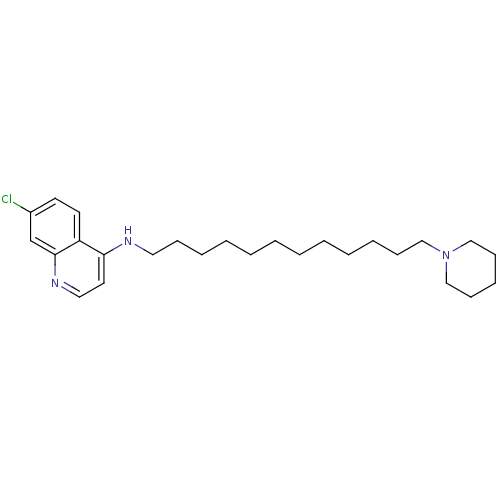 ((7-Chloro-quinolin-4-yl)-(12-piperidin-1-yl-dodecy...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110291 ((7-Chloro-quinolin-4-yl)-[4-(3-piperidin-1-yl-prop...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110310 ((3-Methyl-quinolin-4-yl)-(6-piperidin-1-yl-hexyl)-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110281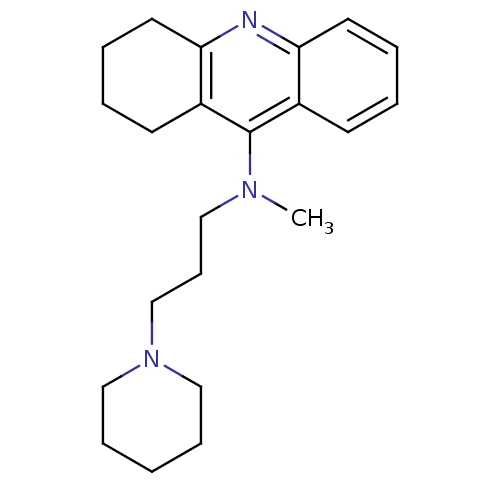 (CHEMBL14656 | Methyl-(3-piperidin-1-yl-propyl)-(1,...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110308 ((7-Chloro-quinolin-4-yl)-{2-[4-(3-piperidin-1-yl-p...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110307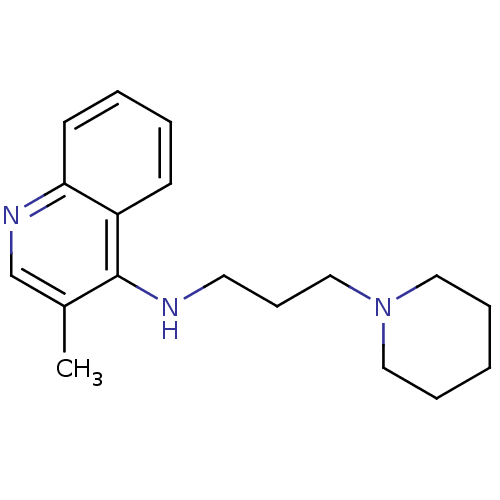 ((3-Methyl-quinolin-4-yl)-(3-piperidin-1-yl-propyl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50110292 (CHEMBL14781 | Methyl-(2-piperidin-1-yl-ethyl)-(1,2...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamine | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM27213 (4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition against histamine-metabolizing enzyme Histamine N-methyl-transferase | J Med Chem 43: 3987-94 (2000) BindingDB Entry DOI: 10.7270/Q2833SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine N-methyltransferase (Rattus norvegicus) | BDBM50126884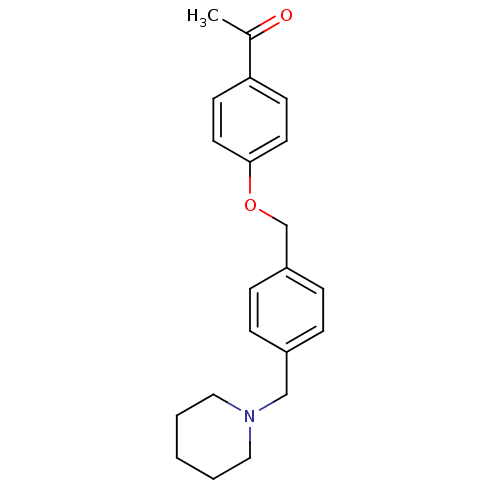 (1-[4-(4-Piperidin-1-ylmethyl-benzyloxy)-phenyl]-et...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Compound was tested against histamine N-methyl-transferase in rat brain [EC 2.1.1.8](relative weak affinity on rat enzyme) | J Med Chem 46: 1523-30 (2003) Article DOI: 10.1021/jm021084k BindingDB Entry DOI: 10.7270/Q2C24X5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||