 Found 6477 hits Enz. Inhib. hit(s) with Target = 'Indoleamine 2,3-dioxygenase 1'
Found 6477 hits Enz. Inhib. hit(s) with Target = 'Indoleamine 2,3-dioxygenase 1' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604026

(CHEMBL5192384)Show SMILES CC(C)(O)c1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604023
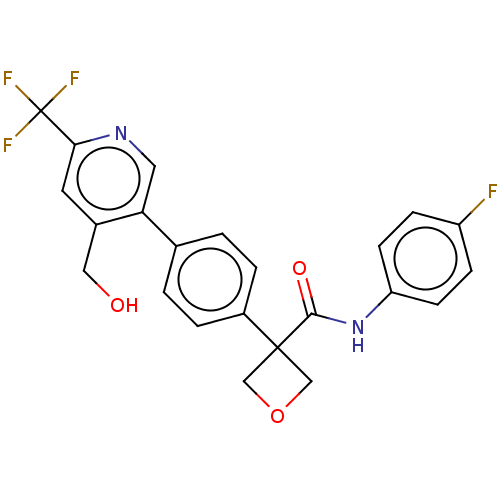
(CHEMBL5192977)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM370555
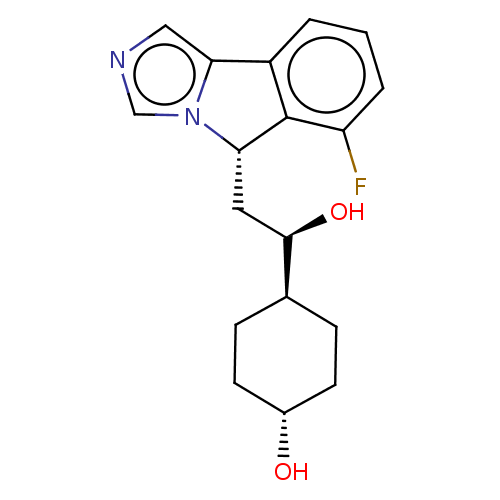
((1R,4r)-4-((R)-2-((S)-6-fluoro-5H-imidazo[5,1- a]i...)Show SMILES O[C@H](C[C@H]1c2c(cccc2F)-c2cncn12)[C@H]1CC[C@H](O)CC1 |r,wU:19.22,wD:3.2,1.0,16.19,(-.99,-3.03,;-.22,-1.7,;-.99,-.37,;-2.53,-.37,;-3.37,.92,;-4.85,.53,;-5.94,1.61,;-5.54,3.1,;-4.06,3.5,;-2.97,2.41,;-1.48,2.81,;-4.93,-1.01,;-5.9,-2.21,;-5.06,-3.5,;-3.58,-3.1,;-3.5,-1.56,;1.32,-1.7,;2.09,-3.03,;3.63,-3.03,;4.4,-1.7,;5.94,-1.7,;3.63,-.37,;2.09,-.37,)| Show InChI InChI=1S/C18H21FN2O2/c19-14-3-1-2-13-16-9-20-10-21(16)15(18(13)14)8-17(23)11-4-6-12(22)7-5-11/h1-3,9-12,15,17,22-23H,4-8H2/t11-,12-,15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation
Curated by ChEMBL
| Assay Description
Inhibition of purified human IDO1 using varying levels of L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measur... |
J Med Chem 62: 6705-6733 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00662
BindingDB Entry DOI: 10.7270/Q21G0QNZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126144

(CHEMBL3629569 | US10155972, Compound NewLink 1 | U...)Show InChI InChI=1S/C18H22N2O/c21-18(13-6-2-1-3-7-13)10-16-14-8-4-5-9-15(14)17-11-19-12-20(16)17/h4-5,8-9,11-13,16,18,21H,1-3,6-7,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) by in-vitro assay |
Eur J Med Chem 140: 293-304 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.025
BindingDB Entry DOI: 10.7270/Q2Q242VC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM370555

((1R,4r)-4-((R)-2-((S)-6-fluoro-5H-imidazo[5,1- a]i...)Show SMILES O[C@H](C[C@H]1c2c(cccc2F)-c2cncn12)[C@H]1CC[C@H](O)CC1 |r,wU:19.22,wD:3.2,1.0,16.19,(-.99,-3.03,;-.22,-1.7,;-.99,-.37,;-2.53,-.37,;-3.37,.92,;-4.85,.53,;-5.94,1.61,;-5.54,3.1,;-4.06,3.5,;-2.97,2.41,;-1.48,2.81,;-4.93,-1.01,;-5.9,-2.21,;-5.06,-3.5,;-3.58,-3.1,;-3.5,-1.56,;1.32,-1.7,;2.09,-3.03,;3.63,-3.03,;4.4,-1.7,;5.94,-1.7,;3.63,-.37,;2.09,-.37,)| Show InChI InChI=1S/C18H21FN2O2/c19-14-3-1-2-13-16-9-20-10-21(16)15(18(13)14)8-17(23)11-4-6-12(22)7-5-11/h1-3,9-12,15,17,22-23H,4-8H2/t11-,12-,15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50300305
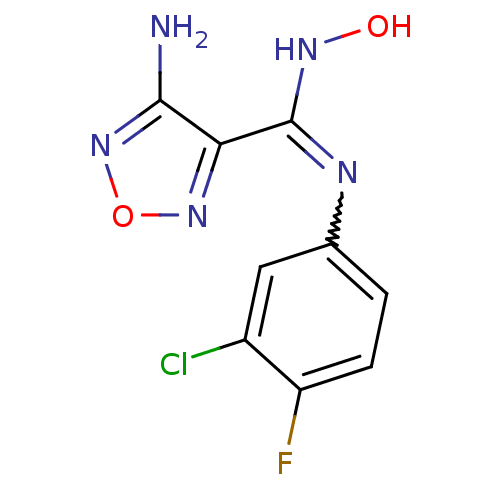
(4-Amino-N-(3-chloro-4-fluorophenyl)-N'-hydroxy-1,2...)Show InChI InChI=1S/C9H7ClFN5O2/c10-5-3-4(1-2-6(5)11)13-9(14-17)7-8(12)16-18-15-7/h1-3,17H,(H2,12,16)(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | CHEMBL5271653

| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM370555

((1R,4r)-4-((R)-2-((S)-6-fluoro-5H-imidazo[5,1- a]i...)Show SMILES O[C@H](C[C@H]1c2c(cccc2F)-c2cncn12)[C@H]1CC[C@H](O)CC1 |r,wU:19.22,wD:3.2,1.0,16.19,(-.99,-3.03,;-.22,-1.7,;-.99,-.37,;-2.53,-.37,;-3.37,.92,;-4.85,.53,;-5.94,1.61,;-5.54,3.1,;-4.06,3.5,;-2.97,2.41,;-1.48,2.81,;-4.93,-1.01,;-5.9,-2.21,;-5.06,-3.5,;-3.58,-3.1,;-3.5,-1.56,;1.32,-1.7,;2.09,-3.03,;3.63,-3.03,;4.4,-1.7,;5.94,-1.7,;3.63,-.37,;2.09,-.37,)| Show InChI InChI=1S/C18H21FN2O2/c19-14-3-1-2-13-16-9-20-10-21(16)15(18(13)14)8-17(23)11-4-6-12(22)7-5-11/h1-3,9-12,15,17,22-23H,4-8H2/t11-,12-,15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114524
BindingDB Entry DOI: 10.7270/Q2PZ5DW0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203289
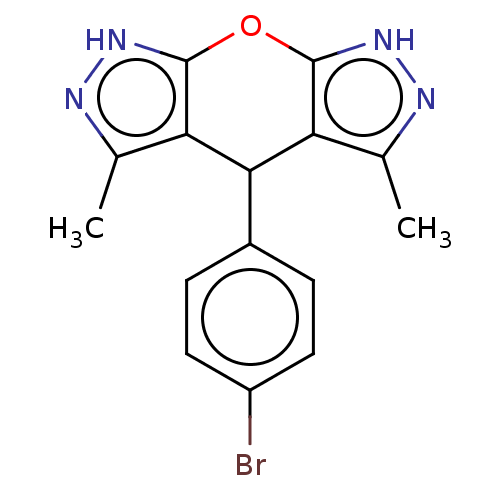
(CHEMBL3903361)Show InChI InChI=1S/C15H13BrN4O/c1-7-11-13(9-3-5-10(16)6-4-9)12-8(2)18-20-15(12)21-14(11)19-17-7/h3-6,13H,1-2H3,(H,17,19)(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203273
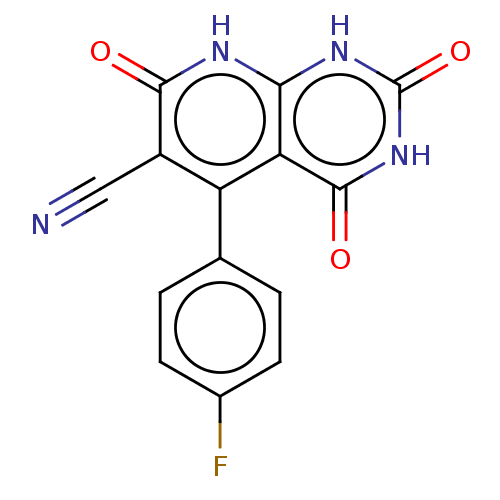
(CHEMBL3927664)Show SMILES Fc1ccc(cc1)-c1c(C#N)c(=O)[nH]c2[nH]c(=O)[nH]c(=O)c12 Show InChI InChI=1S/C14H7FN4O3/c15-7-3-1-6(2-4-7)9-8(5-16)12(20)17-11-10(9)13(21)19-14(22)18-11/h1-4H,(H3,17,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203278

(CHEMBL3986582)Show InChI InChI=1S/C15H13FN4O/c1-7-11-13(9-4-3-5-10(16)6-9)12-8(2)18-20-15(12)21-14(11)19-17-7/h3-6,13H,1-2H3,(H,17,19)(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203261

(CHEMBL3899644)Show SMILES Clc1ccc(cc1)-c1c(C#N)c(=O)[nH]c2[nH]c(=O)[nH]c(=O)c12 Show InChI InChI=1S/C14H7ClN4O3/c15-7-3-1-6(2-4-7)9-8(5-16)12(20)17-11-10(9)13(21)19-14(22)18-11/h1-4H,(H3,17,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203271

(CHEMBL3932970)Show SMILES NC1=C(C#N)C(c2ccc(Br)cc2)c2c(N1)[nH]c(=O)[nH]c2=O |c:1| Show InChI InChI=1S/C14H10BrN5O2/c15-7-3-1-6(2-4-7)9-8(5-16)11(17)18-12-10(9)13(21)20-14(22)19-12/h1-4,9H,17H2,(H3,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203265

(CHEMBL3917645)Show SMILES Fc1ccc(cc1Cl)-c1c(C#N)c(=O)[nH]c2[nH]c(=O)[nH]c(=O)c12 Show InChI InChI=1S/C14H6ClFN4O3/c15-7-3-5(1-2-8(7)16)9-6(4-17)12(21)18-11-10(9)13(22)20-14(23)19-11/h1-3H,(H3,18,19,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203277
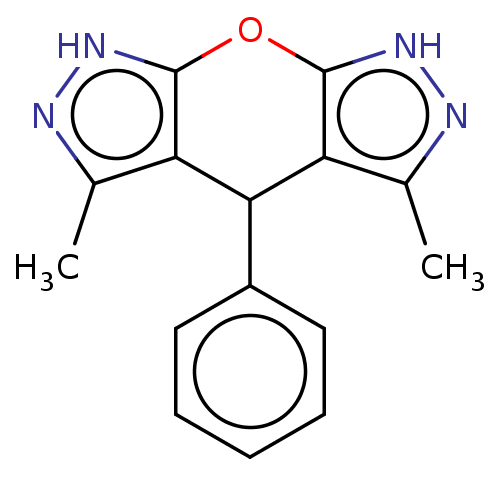
(CHEMBL3963472)Show InChI InChI=1S/C15H14N4O/c1-8-11-13(10-6-4-3-5-7-10)12-9(2)17-19-15(12)20-14(11)18-16-8/h3-7,13H,1-2H3,(H,16,18)(H,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203257

(CHEMBL3973927)Show SMILES NC1=C(C#N)C(c2ccccc2)c2c(N1)[nH]c(=O)[nH]c2=O |c:1| Show InChI InChI=1S/C14H11N5O2/c15-6-8-9(7-4-2-1-3-5-7)10-12(17-11(8)16)18-14(21)19-13(10)20/h1-5,9H,16H2,(H3,17,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203288

(CHEMBL3979528)Show SMILES Cc1n[nH]c2Oc3[nH]c(=O)[nH]c(=O)c3C(c12)c1ccccc1 Show InChI InChI=1S/C15H12N4O3/c1-7-9-10(8-5-3-2-4-6-8)11-12(20)16-15(21)17-13(11)22-14(9)19-18-7/h2-6,10H,1H3,(H,18,19)(H2,16,17,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203250

(CHEMBL3941951)Show SMILES NC1=C(C#N)C(c2ccc(F)cc2)c2c(N1)[nH]c(=O)[nH]c2=O |c:1| Show InChI InChI=1S/C14H10FN5O2/c15-7-3-1-6(2-4-7)9-8(5-16)11(17)18-12-10(9)13(21)20-14(22)19-12/h1-4,9H,17H2,(H3,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203251
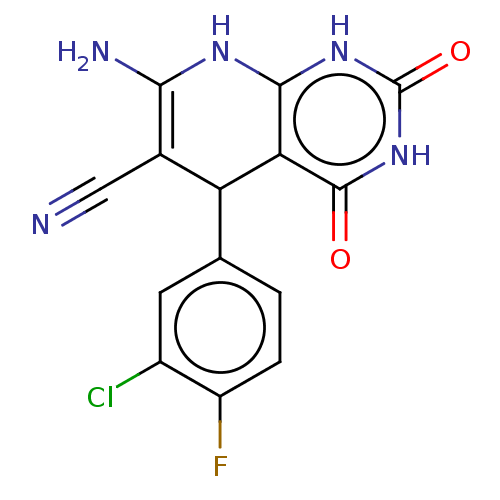
(CHEMBL3909806)Show SMILES NC1=C(C#N)C(c2ccc(F)c(Cl)c2)c2c(N1)[nH]c(=O)[nH]c2=O |c:1| Show InChI InChI=1S/C14H9ClFN5O2/c15-7-3-5(1-2-8(7)16)9-6(4-17)11(18)19-12-10(9)13(22)21-14(23)20-12/h1-3,9H,18H2,(H3,19,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203252

(CHEMBL3890673)Show SMILES Fc1ccc(cc1F)-c1c(C#N)c(=O)[nH]c2[nH]c(=O)[nH]c(=O)c12 Show InChI InChI=1S/C14H6F2N4O3/c15-7-2-1-5(3-8(7)16)9-6(4-17)12(21)18-11-10(9)13(22)20-14(23)19-11/h1-3H,(H3,18,19,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203253

(CHEMBL3960322)Show SMILES Cc1n[nH]c2Oc3[nH]c(=O)[nH]c(=O)c3C(c12)c1ccc(Cl)cc1 Show InChI InChI=1S/C15H11ClN4O3/c1-6-9-10(7-2-4-8(16)5-3-7)11-12(21)17-15(22)18-13(11)23-14(9)20-19-6/h2-5,10H,1H3,(H,19,20)(H2,17,18,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203254

(CHEMBL3949167)Show InChI InChI=1S/C15H13FN4O/c1-7-11-13(9-5-3-4-6-10(9)16)12-8(2)18-20-15(12)21-14(11)19-17-7/h3-6,13H,1-2H3,(H,17,19)(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203247
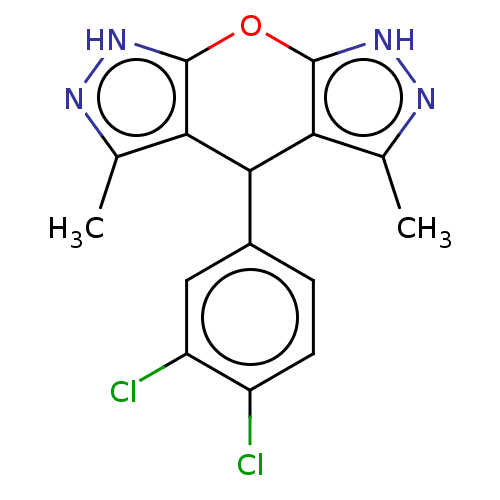
(CHEMBL3912388)Show SMILES Cc1n[nH]c2Oc3[nH]nc(C)c3C(c12)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C15H12Cl2N4O/c1-6-11-13(8-3-4-9(16)10(17)5-8)12-7(2)19-21-15(12)22-14(11)20-18-6/h3-5,13H,1-2H3,(H,18,20)(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203272

(CHEMBL3979980)Show SMILES O=c1[nH]c2[nH]c(=O)c(C#N)c(-c3ccccc3)c2c(=O)[nH]1 Show InChI InChI=1S/C14H8N4O3/c15-6-8-9(7-4-2-1-3-5-7)10-11(16-12(8)19)17-14(21)18-13(10)20/h1-5H,(H3,16,17,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203262

(CHEMBL3931346)Show InChI InChI=1S/C15H13ClN4O/c1-7-11-13(9-3-5-10(16)6-4-9)12-8(2)18-20-15(12)21-14(11)19-17-7/h3-6,13H,1-2H3,(H,17,19)(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203264

(CHEMBL3909309)Show SMILES Cc1n[nH]c2Oc3[nH]c(=O)[nH]c(=O)c3C(c12)c1cccc(F)c1 Show InChI InChI=1S/C15H11FN4O3/c1-6-9-10(7-3-2-4-8(16)5-7)11-12(21)17-15(22)18-13(11)23-14(9)20-19-6/h2-5,10H,1H3,(H,19,20)(H2,17,18,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203266

(CHEMBL3918239)Show SMILES Cc1n[nH]c2Oc3[nH]c(=O)[nH]c(=O)c3C(c12)c1ccc(F)cc1 Show InChI InChI=1S/C15H11FN4O3/c1-6-9-10(7-2-4-8(16)5-3-7)11-12(21)17-15(22)18-13(11)23-14(9)20-19-6/h2-5,10H,1H3,(H,19,20)(H2,17,18,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203249
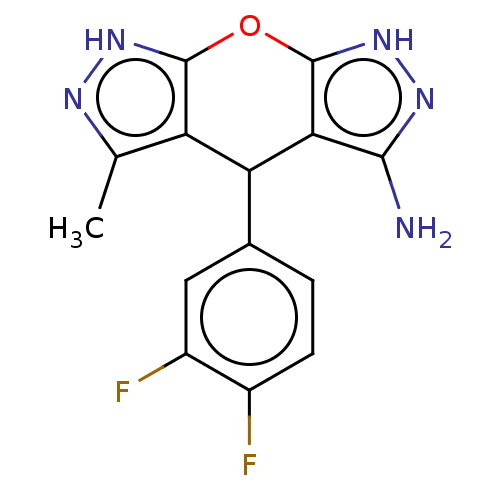
(CHEMBL3945146)Show SMILES Cc1n[nH]c2Oc3[nH]nc(N)c3C(c12)c1ccc(F)c(F)c1 Show InChI InChI=1S/C14H11F2N5O/c1-5-9-10(6-2-3-7(15)8(16)4-6)11-12(17)19-21-14(11)22-13(9)20-18-5/h2-4,10H,1H3,(H,18,20)(H3,17,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203259

(CHEMBL3900740)Show SMILES Fc1cccc(c1)-c1c(C#N)c(=O)[nH]c2[nH]c(=O)[nH]c(=O)c12 Show InChI InChI=1S/C14H7FN4O3/c15-7-3-1-2-6(4-7)9-8(5-16)12(20)17-11-10(9)13(21)19-14(22)18-11/h1-4H,(H3,17,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203267
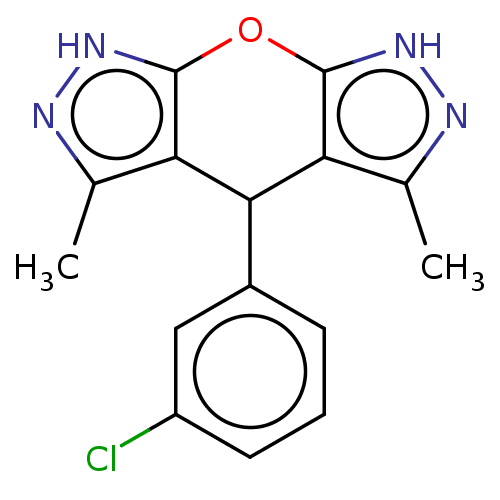
(CHEMBL3957792)Show InChI InChI=1S/C15H13ClN4O/c1-7-11-13(9-4-3-5-10(16)6-9)12-8(2)18-20-15(12)21-14(11)19-17-7/h3-6,13H,1-2H3,(H,17,19)(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
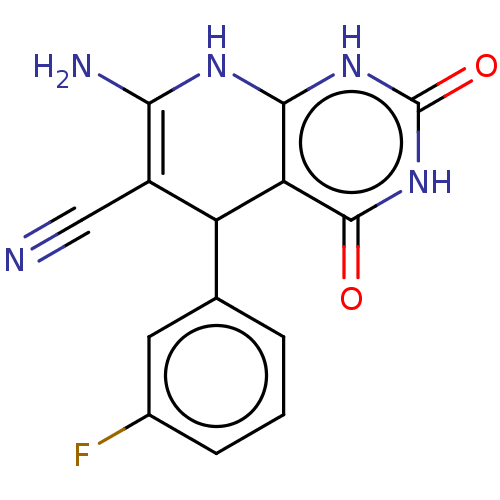
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203282
(CHEMBL3915008)Show SMILES NC1=C(C#N)C(c2cccc(F)c2)c2c(N1)[nH]c(=O)[nH]c2=O |c:1| Show InChI InChI=1S/C14H10FN5O2/c15-7-3-1-2-6(4-7)9-8(5-16)11(17)18-12-10(9)13(21)20-14(22)19-12/h1-4,9H,17H2,(H3,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
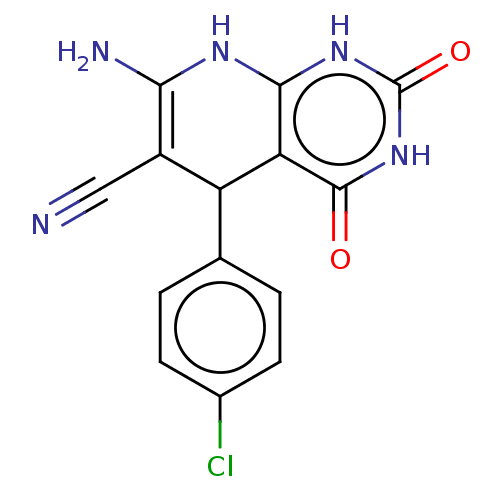
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203270
(CHEMBL3904973)Show SMILES NC1=C(C#N)C(c2ccc(Cl)cc2)c2c(N1)[nH]c(=O)[nH]c2=O |c:1| Show InChI InChI=1S/C14H10ClN5O2/c15-7-3-1-6(2-4-7)9-8(5-16)11(17)18-12-10(9)13(21)20-14(22)19-12/h1-4,9H,17H2,(H3,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203258

(CHEMBL3957215)Show SMILES NC1=C(C#N)C(c2ccc(F)c(F)c2)c2c(N1)[nH]c(=O)[nH]c2=O |c:1| Show InChI InChI=1S/C14H9F2N5O2/c15-7-2-1-5(3-8(7)16)9-6(4-17)11(18)19-12-10(9)13(22)21-14(23)20-12/h1-3,9H,18H2,(H3,19,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203283

(CHEMBL3985889)Show SMILES NC1=C(C#N)C(c2ccc(Cl)c(Cl)c2)c2c(N1)[nH]c(=O)[nH]c2=O |c:1| Show InChI InChI=1S/C14H9Cl2N5O2/c15-7-2-1-5(3-8(7)16)9-6(4-17)11(18)19-12-10(9)13(22)21-14(23)20-12/h1-3,9H,18H2,(H3,19,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203284

(CHEMBL3918741)Show SMILES Brc1ccc(cc1)-c1c(C#N)c(=O)[nH]c2[nH]c(=O)[nH]c(=O)c12 Show InChI InChI=1S/C14H7BrN4O3/c15-7-3-1-6(2-4-7)9-8(5-16)12(20)17-11-10(9)13(21)19-14(22)18-11/h1-4H,(H3,17,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203274
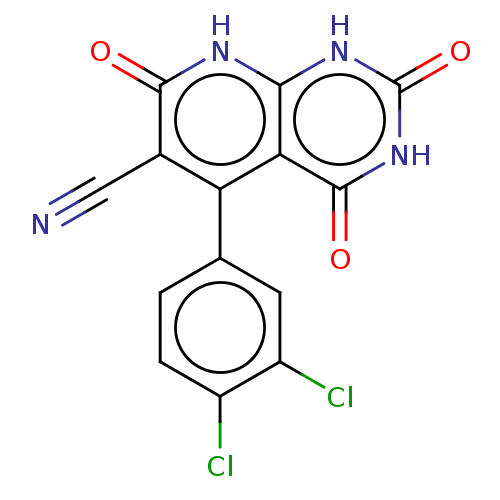
(CHEMBL3945545)Show SMILES Clc1ccc(cc1Cl)-c1c(C#N)c(=O)[nH]c2[nH]c(=O)[nH]c(=O)c12 Show InChI InChI=1S/C14H6Cl2N4O3/c15-7-2-1-5(3-8(7)16)9-6(4-17)12(21)18-11-10(9)13(22)20-14(23)19-11/h1-3H,(H3,18,19,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203285

(CHEMBL3890191)Show SMILES Cc1n[nH]c2Oc3[nH]c(=O)[nH]c(=O)c3C(c12)c1cccc(Cl)c1 Show InChI InChI=1S/C15H11ClN4O3/c1-6-9-10(7-3-2-4-8(16)5-7)11-12(21)17-15(22)18-13(11)23-14(9)20-19-6/h2-5,10H,1H3,(H,19,20)(H2,17,18,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203286

(CHEMBL3891251)Show SMILES Cc1n[nH]c2Oc3[nH]c(=O)[nH]c(=O)c3C(c12)c1ccc(Br)cc1 Show InChI InChI=1S/C15H11BrN4O3/c1-6-9-10(7-2-4-8(16)5-3-7)11-12(21)17-15(22)18-13(11)23-14(9)20-19-6/h2-5,10H,1H3,(H,19,20)(H2,17,18,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203276
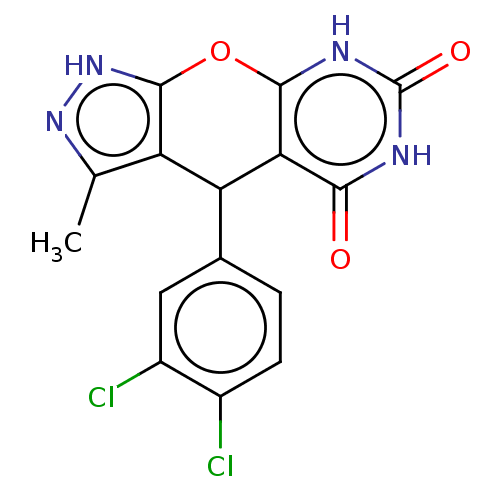
(CHEMBL3960467)Show SMILES Cc1n[nH]c2Oc3[nH]c(=O)[nH]c(=O)c3C(c12)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C15H10Cl2N4O3/c1-5-9-10(6-2-3-7(16)8(17)4-6)11-12(22)18-15(23)19-13(11)24-14(9)21-20-5/h2-4,10H,1H3,(H,20,21)(H2,18,19,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203287

(CHEMBL3983266)Show InChI InChI=1S/C14H12FN5O/c1-6-9-10(7-2-4-8(15)5-3-7)11-12(16)18-20-14(11)21-13(9)19-17-6/h2-5,10H,1H3,(H,17,19)(H3,16,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50324701
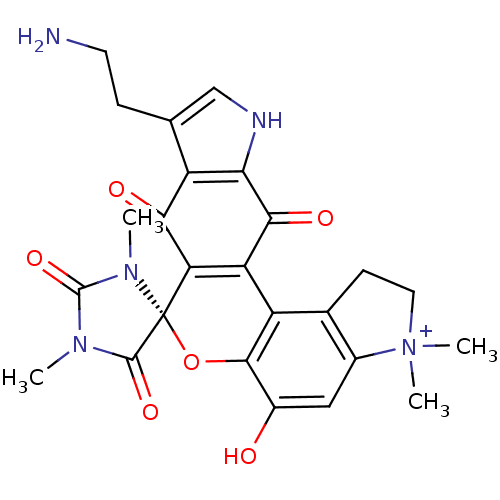
(CHEMBL1221472 | Exiguamine A)Show SMILES CN1C(=O)N(C)[C@]2(Oc3c(O)cc4c(CC[N+]4(C)C)c3C3=C2C(=O)c2c(CCN)c[nH]c2C3=O)C1=O |r,c:22| Show InChI InChI=1S/C25H25N5O6/c1-28-23(34)25(29(2)24(28)35)18-17(21(33)19-15(20(18)32)11(5-7-26)10-27-19)16-12-6-8-30(3,4)13(12)9-14(31)22(16)36-25/h9-10H,5-8,26H2,1-4H3,(H-,27,31,32,33)/p+1/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant indoleamine-2,3-dioxygenase |
Nat Chem Biol 4: 535-7 (2008)
Article DOI: 10.1038/nchembio.107
BindingDB Entry DOI: 10.7270/Q2P55NR5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM21975
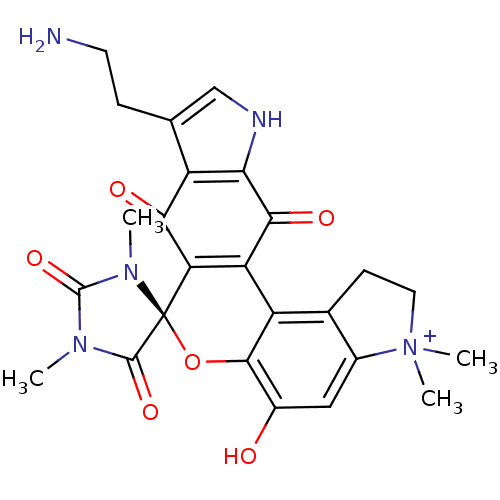
((4S)-16'-(2-aminoethyl)-9'-hydroxy-1,3,6',6'-tetra...)Show SMILES CN1C(=O)N(C)[C@@]2(Oc3c(O)cc4c(CC[N+]4(C)C)c3C3=C2C(=O)c2c(CCN)c[nH]c2C3=O)C1=O |r,c:22| Show InChI InChI=1S/C25H25N5O6/c1-28-23(34)25(29(2)24(28)35)18-17(21(33)19-15(20(18)32)11(5-7-26)10-27-19)16-12-6-8-30(3,4)13(12)9-14(31)22(16)36-25/h9-10H,5-8,26H2,1-4H3,(H-,27,31,32,33)/p+1/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | -43.9 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia
| Assay Description
The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... |
J Med Chem 51: 2634-7 (2008)
Article DOI: 10.1021/jm800143h
BindingDB Entry DOI: 10.7270/Q2MK6B65 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | CHEMBL5284391

| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM21975

((4S)-16'-(2-aminoethyl)-9'-hydroxy-1,3,6',6'-tetra...)Show SMILES CN1C(=O)N(C)[C@@]2(Oc3c(O)cc4c(CC[N+]4(C)C)c3C3=C2C(=O)c2c(CCN)c[nH]c2C3=O)C1=O |r,c:22| Show InChI InChI=1S/C25H25N5O6/c1-28-23(34)25(29(2)24(28)35)18-17(21(33)19-15(20(18)32)11(5-7-26)10-27-19)16-12-6-8-30(3,4)13(12)9-14(31)22(16)36-25/h9-10H,5-8,26H2,1-4H3,(H-,27,31,32,33)/p+1/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113631
BindingDB Entry DOI: 10.7270/Q2K35ZGP |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50442991

(CHEMBL432537 | GNF-Pf-3777 | US10669273, Compound ...)Show SMILES [O-][N+](=O)c1ccc-2c(c1)C(=O)c1nc3ccccc3c(=O)n-21 Show InChI InChI=1S/C15H7N3O4/c19-13-10-7-8(18(21)22)5-6-12(10)17-14(13)16-11-4-2-1-3-9(11)15(17)20/h1-7H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of human recombinant IDO1 using L-tryptophan as substrate |
J Med Chem 56: 8321-31 (2013)
Article DOI: 10.1021/jm401195n
BindingDB Entry DOI: 10.7270/Q2X34ZWS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203280

(CHEMBL3966057)Show SMILES Nc1n[nH]c2Oc3[nH]nc(N)c3C(c12)c1ccc(F)c(F)c1 Show InChI InChI=1S/C13H10F2N6O/c14-5-2-1-4(3-6(5)15)7-8-10(16)18-20-12(8)22-13-9(7)11(17)19-21-13/h1-3,7H,(H3,16,18,20)(H3,17,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50324700

(CHEMBL1221412 | Exiguamine B)Show SMILES CN1C(=O)N(C)[C@]2(Oc3c(O)cc4c([C@H](O)C[N+]4(C)C)c3C3=C2C(=O)c2c(CCN)c[nH]c2C3=O)C1=O |r,c:23| Show InChI InChI=1S/C25H25N5O7/c1-28-23(35)25(29(2)24(28)36)18-17(21(34)19-14(20(18)33)10(5-6-26)8-27-19)16-15-11(7-12(31)22(16)37-25)30(3,4)9-13(15)32/h7-8,13,32H,5-6,9,26H2,1-4H3,(H-,27,31,33,34)/p+1/t13-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant indoleamine-2,3-dioxygenase |
Nat Chem Biol 4: 535-7 (2008)
Article DOI: 10.1038/nchembio.107
BindingDB Entry DOI: 10.7270/Q2P55NR5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50324699

(CHEMBL1221473 | Seco-exiguamine)Show SMILES CN(C)CCc1ccc(O)c(O)c1C1=C(c2c(O)n(C)c(=O)n2C)C(=O)c2c(CCN)c[nH]c2C1=O |c:14| Show InChI InChI=1S/C25H29N5O6/c1-28(2)10-8-12-5-6-14(31)21(32)15(12)17-18(20-24(35)30(4)25(36)29(20)3)22(33)16-13(7-9-26)11-27-19(16)23(17)34/h5-6,11,27,31-32,35H,7-10,26H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant indoleamine-2,3-dioxygenase |
Nat Chem Biol 4: 535-7 (2008)
Article DOI: 10.1038/nchembio.107
BindingDB Entry DOI: 10.7270/Q2P55NR5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50203269
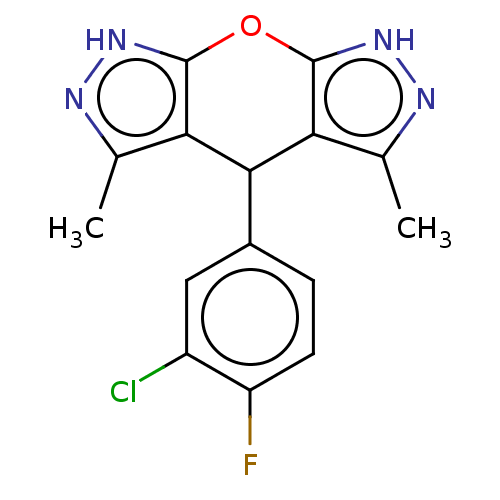
(CHEMBL3913434)Show SMILES Cc1n[nH]c2Oc3[nH]nc(C)c3C(c12)c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C15H12ClFN4O/c1-6-11-13(8-3-4-10(17)9(16)5-8)12-7(2)19-21-15(12)22-14(11)20-18-6/h3-5,13H,1-2H3,(H,18,20)(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft... |
ACS Med Chem Lett 7: 1167-1172 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00359
BindingDB Entry DOI: 10.7270/Q2TF009K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | CHEMBL5287590
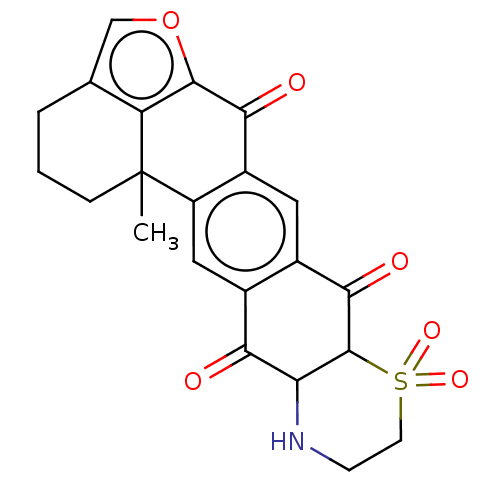
| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data