

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223288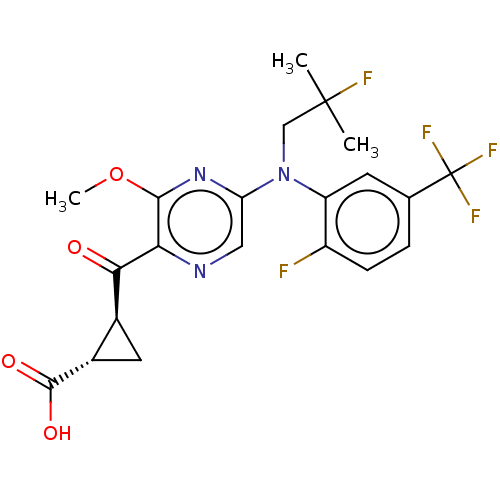 ((1S,2S)-2-[(5-{(2-Fluoro-2-methylpropyl)[2-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.248 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astrazeneca Ab US Patent | Assay Description In the assay, LTC4 synthase catalyses the reaction where the substrate LTA4 methyl ester is converted to LTC4 methyl ester. In order to obtain IC50-v... | US Patent US20160326143 (2016) BindingDB Entry DOI: 10.7270/Q2W66JN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223288 ((1S,2S)-2-[(5-{(2-Fluoro-2-methylpropyl)[2-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.248 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description In the assay, LTC4 synthase catalyses the reaction where the substrate LTA4 methyl ester is converted to LTC4 methyl ester.In order to obtain IC50-va... | US Patent US9657001 (2017) BindingDB Entry DOI: 10.7270/Q2SJ1NPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223288 ((1S,2S)-2-[(5-{(2-Fluoro-2-methylpropyl)[2-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519568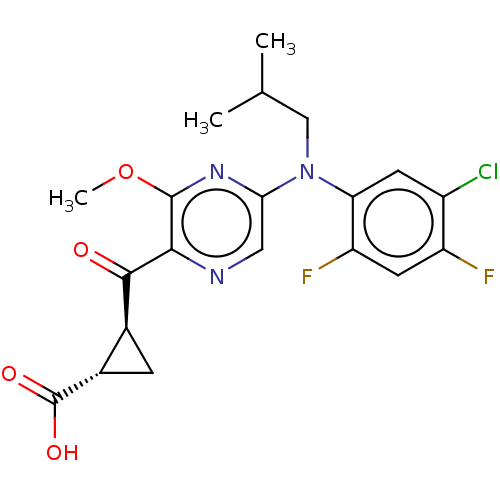 (CHEMBL4549822) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223287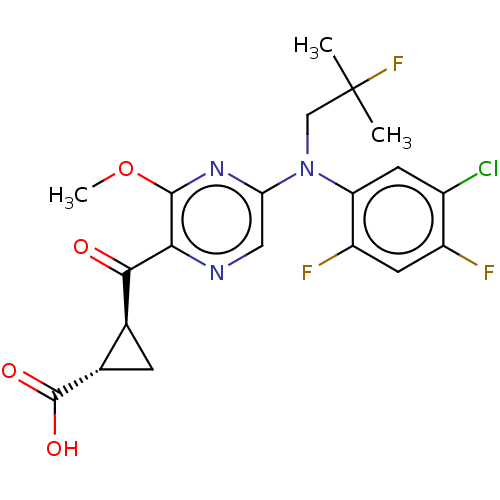 ((1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(2-flu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223287 ((1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(2-flu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.289 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astrazeneca Ab US Patent | Assay Description In the assay, LTC4 synthase catalyses the reaction where the substrate LTA4 methyl ester is converted to LTC4 methyl ester. In order to obtain IC50-v... | US Patent US20160326143 (2016) BindingDB Entry DOI: 10.7270/Q2W66JN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223287 ((1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(2-flu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.289 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description In the assay, LTC4 synthase catalyses the reaction where the substrate LTA4 methyl ester is converted to LTC4 methyl ester.In order to obtain IC50-va... | US Patent US9657001 (2017) BindingDB Entry DOI: 10.7270/Q2SJ1NPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519570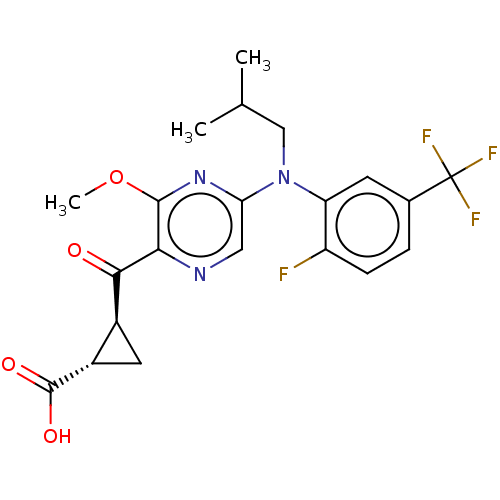 (CHEMBL4567083) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519585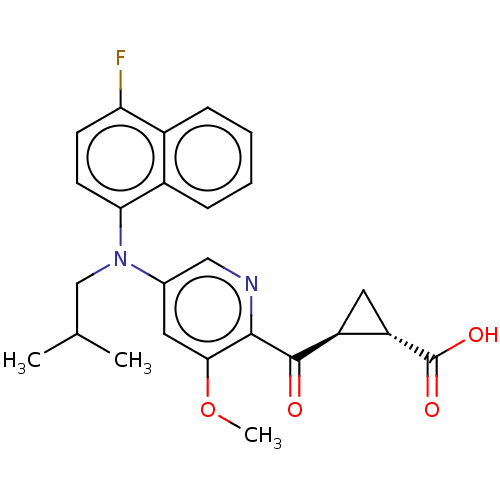 (CHEMBL4464773) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.381 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223289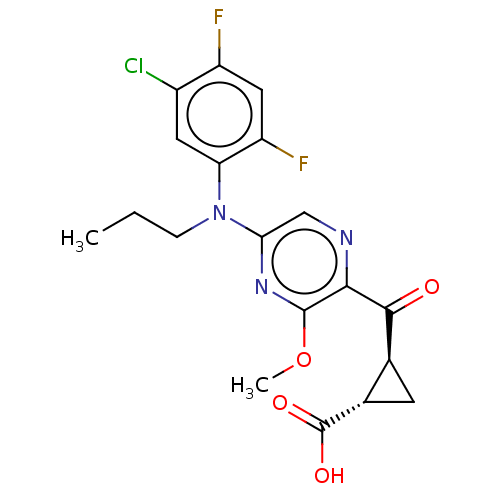 ((1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.483 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astrazeneca Ab US Patent | Assay Description In the assay, LTC4 synthase catalyses the reaction where the substrate LTA4 methyl ester is converted to LTC4 methyl ester. In order to obtain IC50-v... | US Patent US20160326143 (2016) BindingDB Entry DOI: 10.7270/Q2W66JN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM310035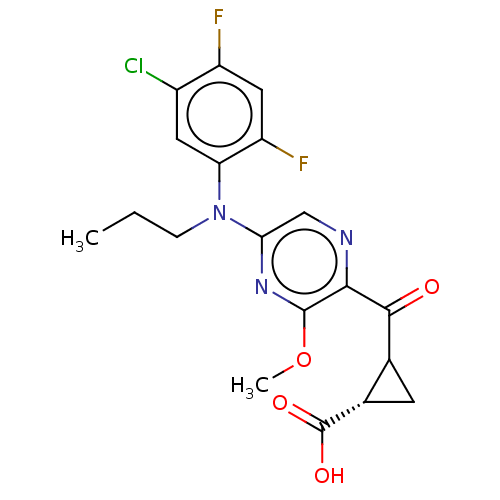 ((1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.483 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description In the assay, LTC4 synthase catalyses the reaction where the substrate LTA4 methyl ester is converted to LTC4 methyl ester.In order to obtain IC50-va... | US Patent US9657001 (2017) BindingDB Entry DOI: 10.7270/Q2SJ1NPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519591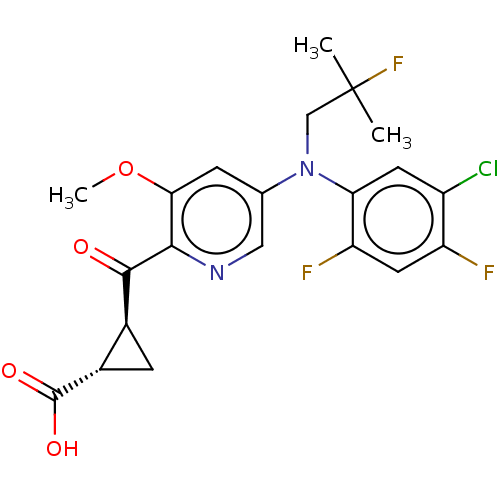 (CHEMBL4562583) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519574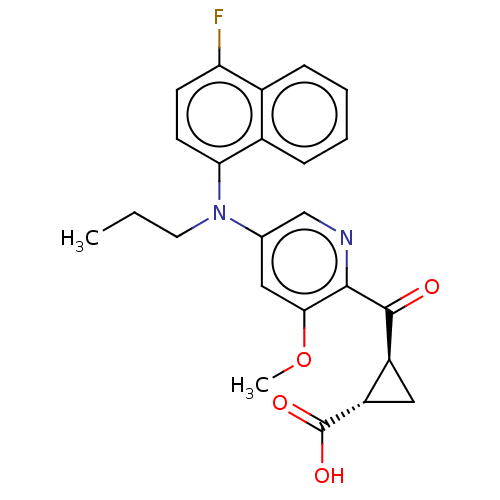 (CHEMBL4563433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.626 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519588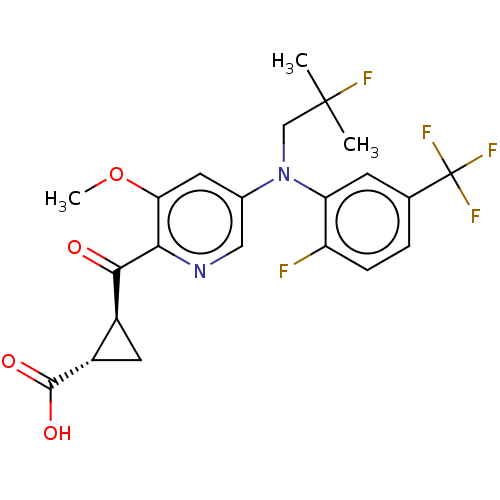 (CHEMBL4584584) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223290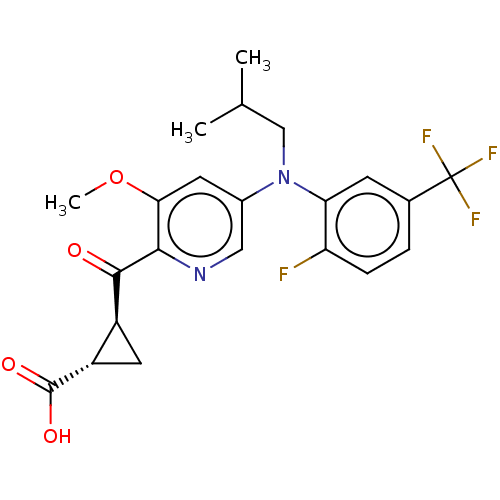 ((1S,2S)-2-[(5-{[2-Fluoro-5-(trifluoromethyl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.658 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description In the assay, LTC4 synthase catalyses the reaction where the substrate LTA4 methyl ester is converted to LTC4 methyl ester.In order to obtain IC50-va... | US Patent US9657001 (2017) BindingDB Entry DOI: 10.7270/Q2SJ1NPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223290 ((1S,2S)-2-[(5-{[2-Fluoro-5-(trifluoromethyl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.658 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astrazeneca Ab US Patent | Assay Description In the assay, LTC4 synthase catalyses the reaction where the substrate LTA4 methyl ester is converted to LTC4 methyl ester. In order to obtain IC50-v... | US Patent US20160326143 (2016) BindingDB Entry DOI: 10.7270/Q2W66JN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223290 ((1S,2S)-2-[(5-{[2-Fluoro-5-(trifluoromethyl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519580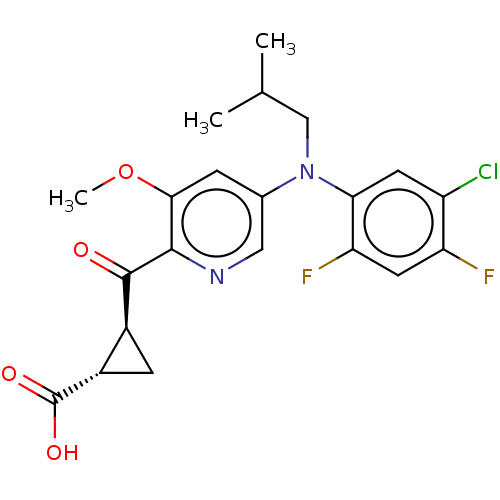 (CHEMBL4574439) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519565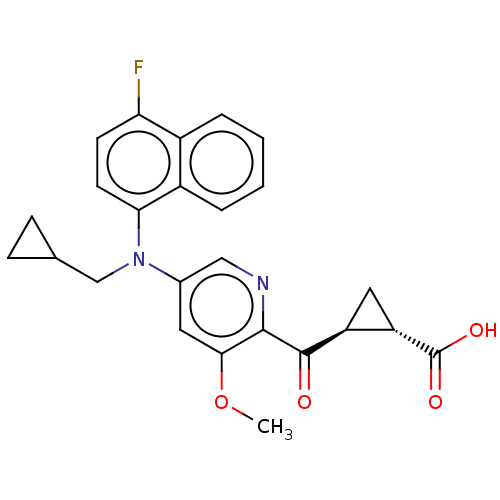 (CHEMBL4441362) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.758 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519567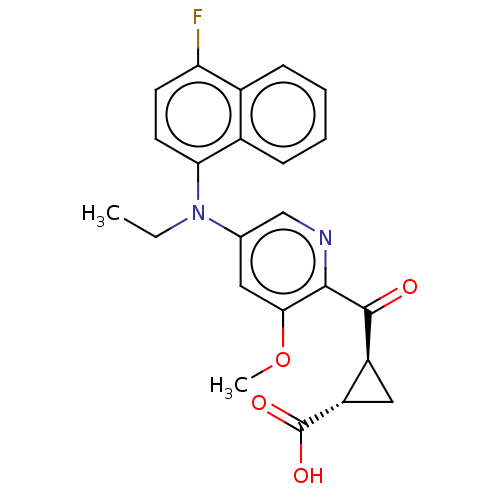 (CHEMBL4476024) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519571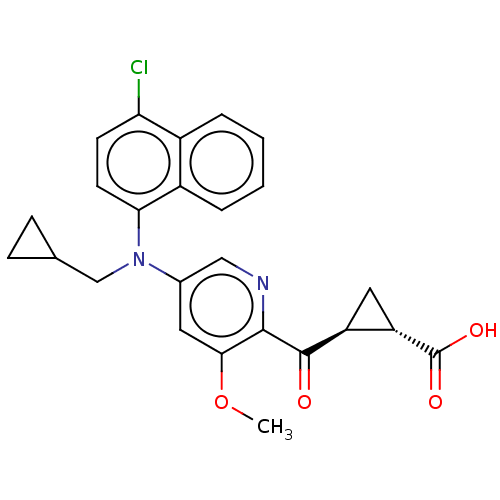 (CHEMBL4454609) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519595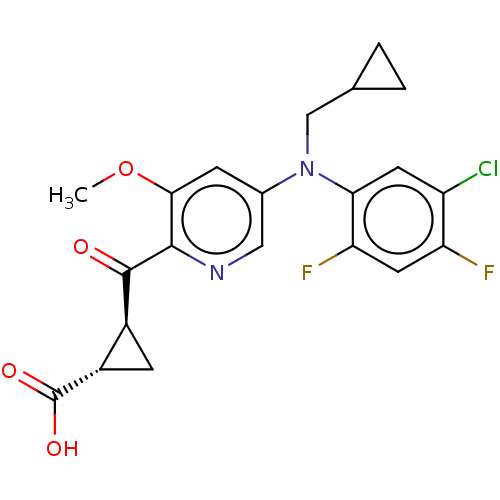 (CHEMBL4545014) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519569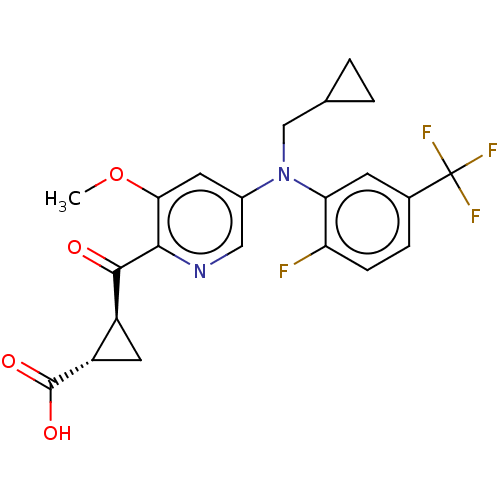 (CHEMBL4545950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519585 (CHEMBL4464773) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of LTC4S in zymosan-stimulated human PBMC assessed as inhibition of LTC4 production preincubated for 45 mins followed by zymosan stimulati... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519582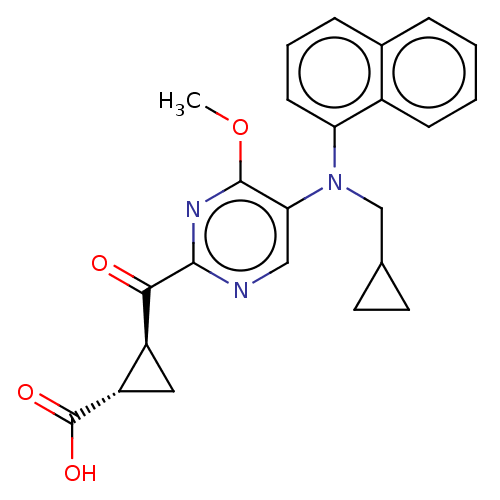 (CHEMBL4525972) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519570 (CHEMBL4567083) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of LTC4S in zymosan-stimulated human PBMC assessed as inhibition of LTC4 production preincubated for 45 mins followed by zymosan stimulati... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519587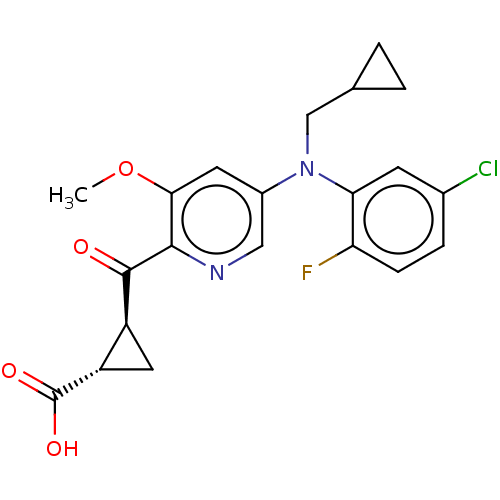 (CHEMBL4467942) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519568 (CHEMBL4549822) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of LTC4S in zymosan-stimulated human PBMC assessed as inhibition of LTC4 production preincubated for 45 mins followed by zymosan stimulati... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519590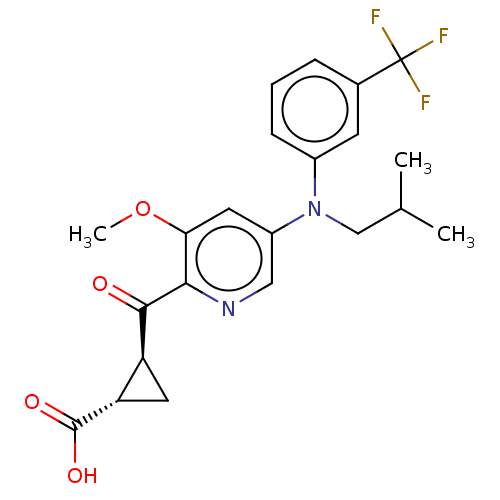 (CHEMBL4574370) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519592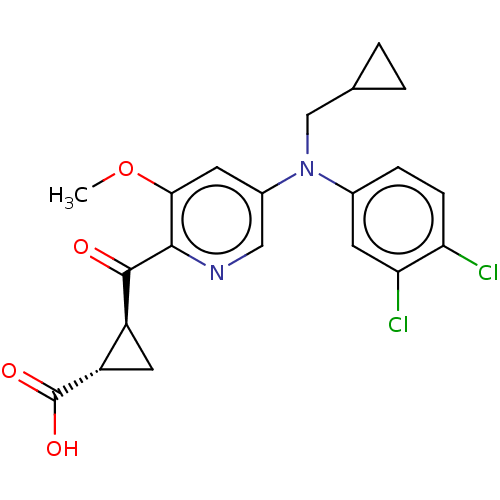 (CHEMBL4442047) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519579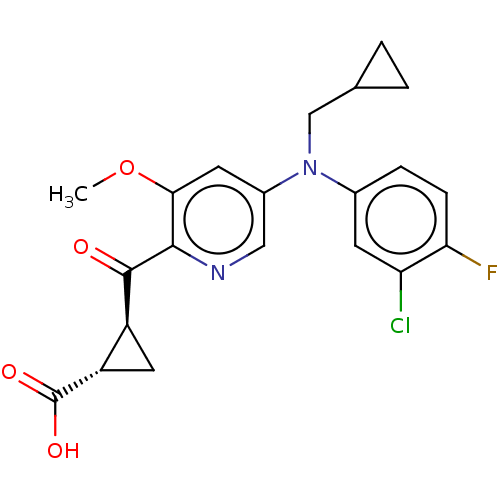 (CHEMBL4576647) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519575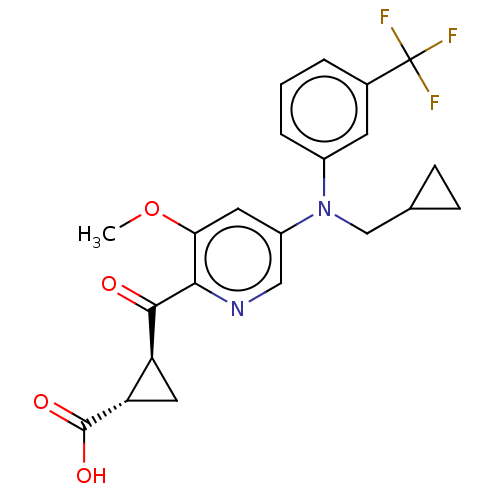 (CHEMBL4567339) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223290 ((1S,2S)-2-[(5-{[2-Fluoro-5-(trifluoromethyl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of LTC4S in zymosan-stimulated human PBMC assessed as inhibition of LTC4 production preincubated for 45 mins followed by zymosan stimulati... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519574 (CHEMBL4563433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of LTC4S in zymosan-stimulated human PBMC assessed as inhibition of LTC4 production preincubated for 45 mins followed by zymosan stimulati... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223288 ((1S,2S)-2-[(5-{(2-Fluoro-2-methylpropyl)[2-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of LTC4S in zymosan-stimulated human PBMC assessed as inhibition of LTC4 production preincubated for 45 mins followed by zymosan stimulati... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519586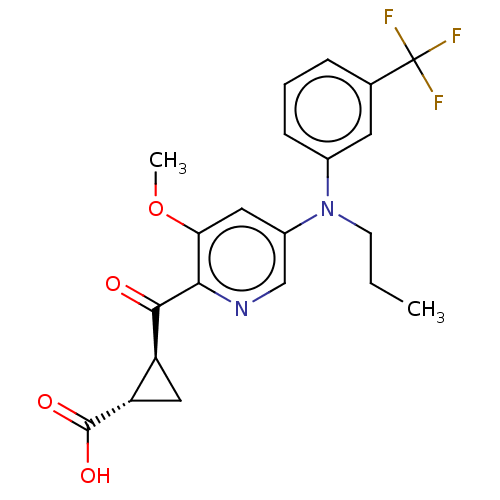 (CHEMBL4439499) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519580 (CHEMBL4574439) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of LTC4S in zymosan-stimulated human PBMC assessed as inhibition of LTC4 production preincubated for 45 mins followed by zymosan stimulati... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519577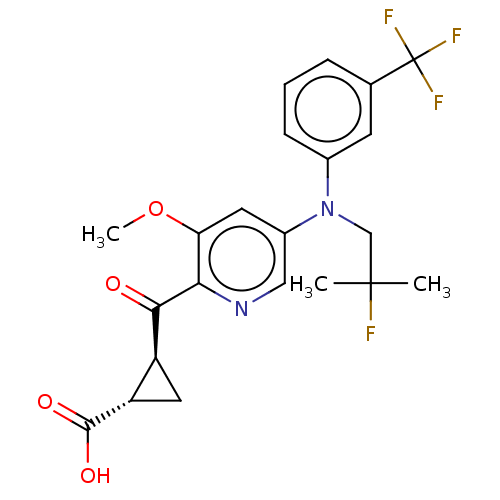 (CHEMBL4439148) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223261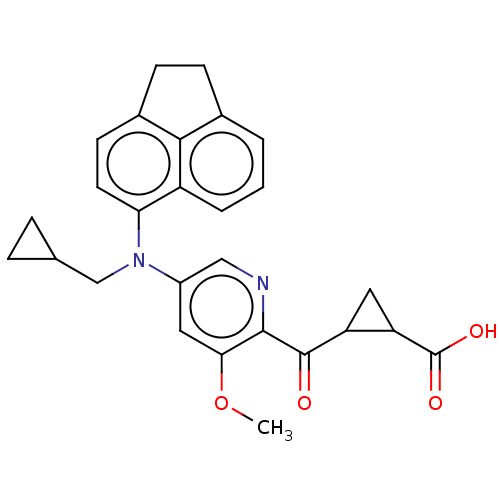 (2-(5-((Cyclopropylmethyl)(1,2-dihydroacenaphthylen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description In the assay, LTC4 synthase catalyses the reaction where the substrate LTA4 methyl ester is converted to LTC4 methyl ester.In order to obtain IC50-va... | US Patent US9657001 (2017) BindingDB Entry DOI: 10.7270/Q2SJ1NPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223261 (2-(5-((Cyclopropylmethyl)(1,2-dihydroacenaphthylen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Astrazeneca Ab US Patent | Assay Description In the assay, LTC4 synthase catalyses the reaction where the substrate LTA4 is converted to LTC4. Recombinant human LTC4 synthase is expressed in Pic... | US Patent US20160326143 (2016) BindingDB Entry DOI: 10.7270/Q2W66JN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519571 (CHEMBL4454609) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of LTC4S in zymosan-stimulated human PBMC assessed as inhibition of LTC4 production preincubated for 45 mins followed by zymosan stimulati... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223287 ((1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(2-flu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of LTC4S in zymosan-stimulated human PBMC assessed as inhibition of LTC4 production preincubated for 45 mins followed by zymosan stimulati... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519578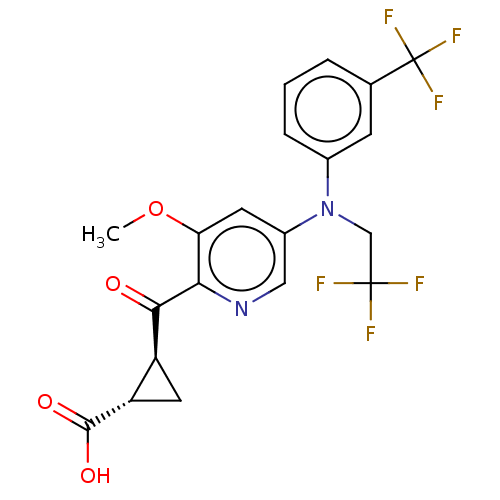 (CHEMBL4445815) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519589 (CHEMBL4531985) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519569 (CHEMBL4545950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of LTC4S in zymosan-stimulated human PBMC assessed as inhibition of LTC4 production preincubated for 45 mins followed by zymosan stimulati... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519566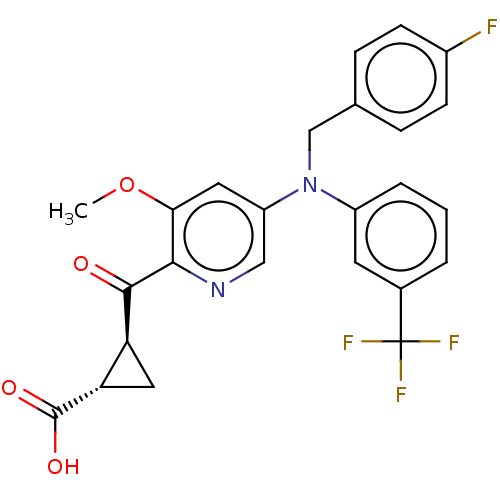 (CHEMBL4565208) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519573 (CHEMBL4456770) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519591 (CHEMBL4562583) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of LTC4S in zymosan-stimulated human PBMC assessed as inhibition of LTC4 production preincubated for 45 mins followed by zymosan stimulati... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223281 (2-(5-((Cyclopropylmethyl)(naphthalen-1-yl)amino)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Astrazeneca Ab US Patent | Assay Description In the assay, LTC4 synthase catalyses the reaction where the substrate LTA4 is converted to LTC4. Recombinant human LTC4 synthase is expressed in Pic... | US Patent US20160326143 (2016) BindingDB Entry DOI: 10.7270/Q2W66JN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223281 (2-(5-((Cyclopropylmethyl)(naphthalen-1-yl)amino)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description In the assay, LTC4 synthase catalyses the reaction where the substrate LTA4 methyl ester is converted to LTC4 methyl ester.In order to obtain IC50-va... | US Patent US9657001 (2017) BindingDB Entry DOI: 10.7270/Q2SJ1NPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 238 total ) | Next | Last >> |