

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nicotinic acetylcholine receptor alpha1 subunit (Heliothis virescens) | BDBM50376053 (PROTOSTEMODIOL) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at nAChR in Heliothis virescens neuronal cells by voltage clamp technique | J Nat Prod 71: 112-6 (2008) Article DOI: 10.1021/np070427k BindingDB Entry DOI: 10.7270/Q21Z4596 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinic acetylcholine receptor alpha1 subunit (Heliothis virescens) | BDBM50376055 (CHEMBL404139) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Agonist activity at nAChR in Heliothis virescens neuronal cells by voltage clamp technique | J Nat Prod 71: 112-6 (2008) Article DOI: 10.1021/np070427k BindingDB Entry DOI: 10.7270/Q21Z4596 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinic acetylcholine receptor alpha1 subunit (Heliothis virescens) | BDBM50376054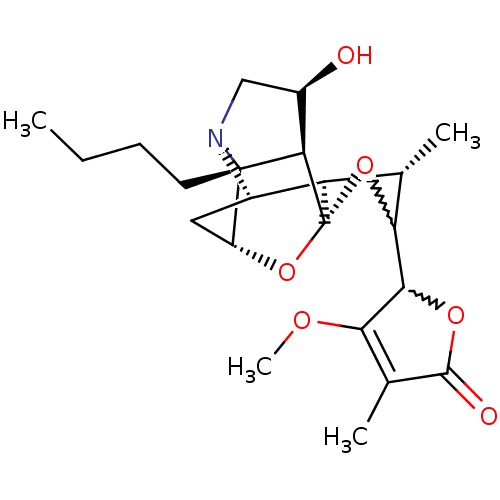 (CHEMBL255667) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 157 | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Agonist activity at nAChR in Heliothis virescens neuronal cells by voltage clamp technique | J Nat Prod 71: 112-6 (2008) Article DOI: 10.1021/np070427k BindingDB Entry DOI: 10.7270/Q21Z4596 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinic acetylcholine receptor alpha1 subunit (Heliothis virescens) | BDBM50376056 (STEMOFOLINE) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Agonist activity at nAChR in Heliothis virescens neuronal cells by voltage clamp technique | J Nat Prod 71: 112-6 (2008) Article DOI: 10.1021/np070427k BindingDB Entry DOI: 10.7270/Q21Z4596 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||