

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasmepsin II (Plasmodium falciparum) | BDBM50323472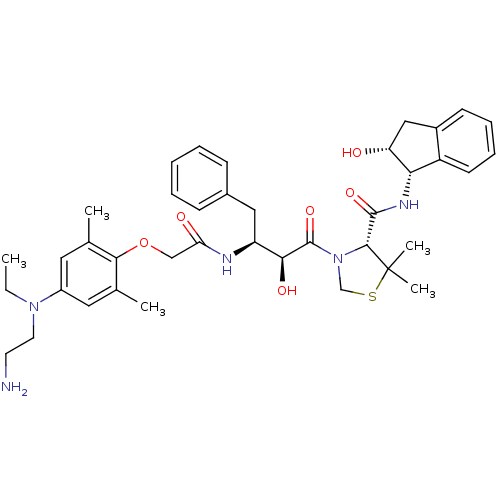 ((R)-3-((2S,3S)-3-(2-(4-((2-aminoethyl)(ethyl)amino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323469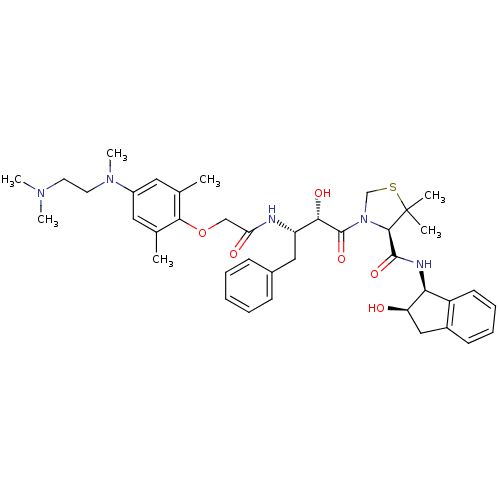 ((R)-3-((2S,3S)-3-(2-(4-((2-(dimethylamino)ethyl)(m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209553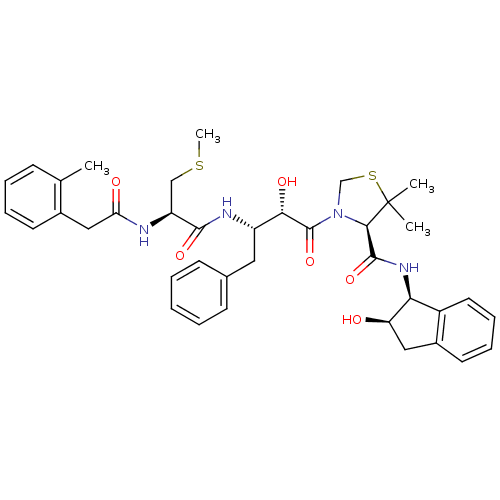 ((R)-N-((1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 17: 3048-52 (2007) Article DOI: 10.1016/j.bmcl.2007.03.052 BindingDB Entry DOI: 10.7270/Q2J102VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323737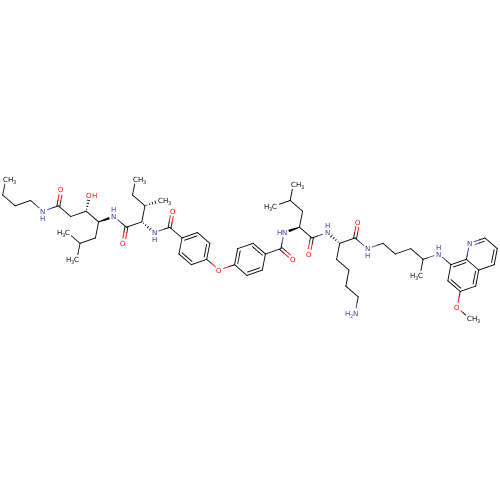 (CHEMBL1213687 | N-((2S)-1-((2S)-6-amino-1-(4-(6-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PLM2 using Lys-Glu-Phe-Val-Phe-NPhe-Ala-Leu-Lys as substrate by spectrophotometry | Bioorg Med Chem Lett 22: 5915-8 (2012) Article DOI: 10.1016/j.bmcl.2012.07.069 BindingDB Entry DOI: 10.7270/Q2M046HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM7977 ((3S,4S)-5-[(4-bromophenyl)methoxy]-N-[(1S)-1-{[(1S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | J Med Chem 53: 4234-47 (2010) Article DOI: 10.1021/jm100233b BindingDB Entry DOI: 10.7270/Q22807RR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209559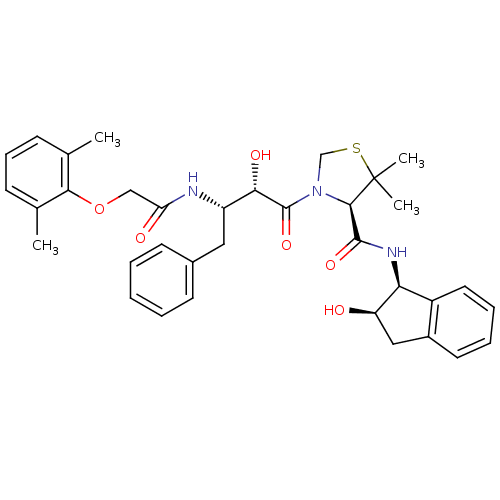 ((4R)-3-[(2S,3S)-3-{[(2,6-dimethylphenoxy)acetyl]am...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem 16: 10049-60 (2008) Article DOI: 10.1016/j.bmc.2008.10.011 BindingDB Entry DOI: 10.7270/Q2G160PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209559 ((4R)-3-[(2S,3S)-3-{[(2,6-dimethylphenoxy)acetyl]am...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 expressed in Escherichia coli BL21 (DE3) by fluorometric assay | Bioorg Med Chem 17: 5933-49 (2009) Article DOI: 10.1016/j.bmc.2009.06.065 BindingDB Entry DOI: 10.7270/Q2S46S0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209559 ((4R)-3-[(2S,3S)-3-{[(2,6-dimethylphenoxy)acetyl]am...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209559 ((4R)-3-[(2S,3S)-3-{[(2,6-dimethylphenoxy)acetyl]am...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 17: 3048-52 (2007) Article DOI: 10.1016/j.bmcl.2007.03.052 BindingDB Entry DOI: 10.7270/Q2J102VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM7974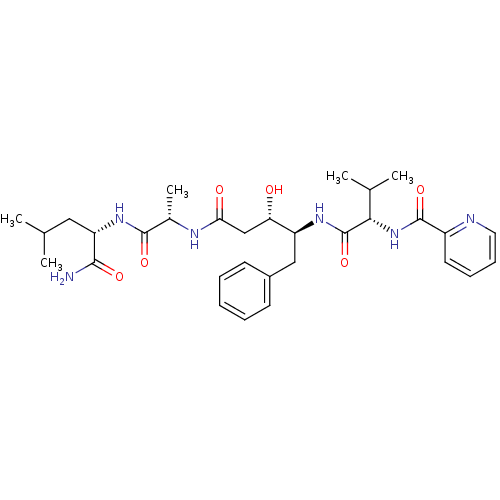 ((3S,4S)-N-[(1S)-1-{[(1S)-1-carbamoyl-3-methylbutyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin II of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM7974 ((3S,4S)-N-[(1S)-1-{[(1S)-1-carbamoyl-3-methylbutyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum recombinant plasmepsin 2 expressed in Escherichia coli BL21 (DE3) | J Med Chem 53: 4234-47 (2010) Article DOI: 10.1021/jm100233b BindingDB Entry DOI: 10.7270/Q22807RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM7974 ((3S,4S)-N-[(1S)-1-{[(1S)-1-carbamoyl-3-methylbutyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.560 | -52.3 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Linkoping University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 3353-66 (2004) Article DOI: 10.1021/jm031106i BindingDB Entry DOI: 10.7270/Q2WM1BMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323470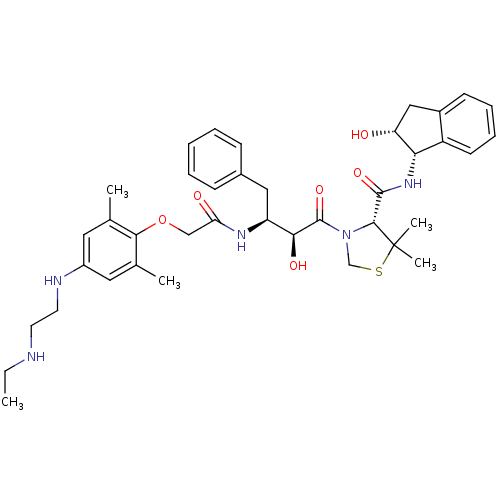 ((R)-3-((2S,3S)-3-(2-(4-(2-(ethylamino)ethylamino)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50169098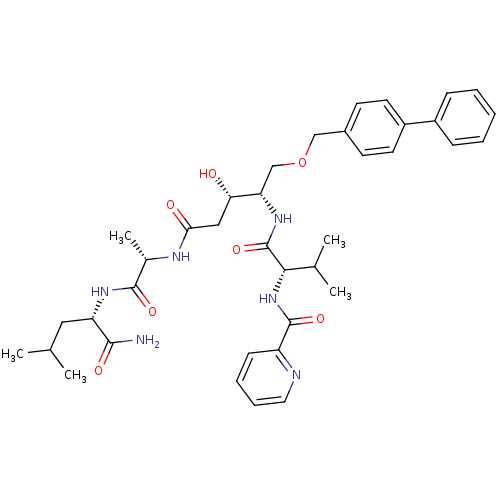 (CHEMBL264770 | Pyridine-2-carboxylic acid {(S)-1-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin II of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001073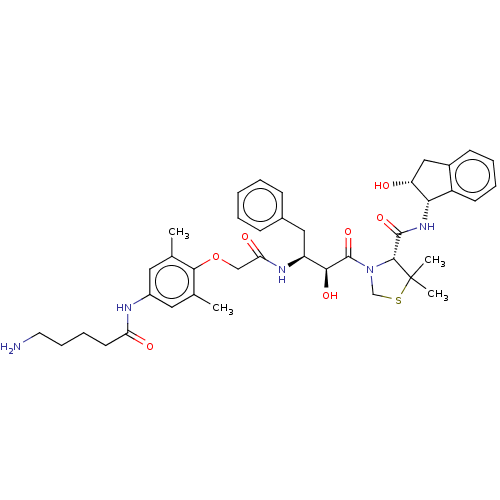 (CHEMBL3236067) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001072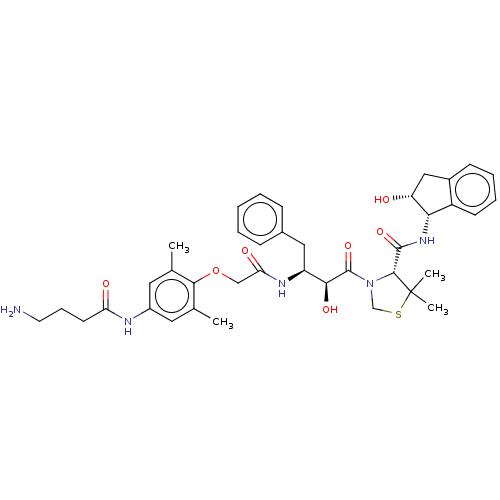 (CHEMBL3236066) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50169100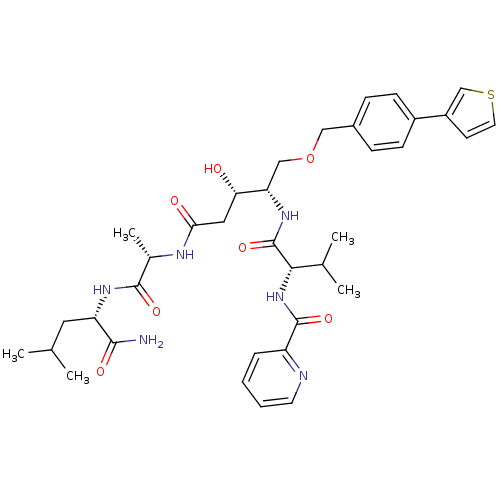 (CHEMBL191260 | Pyridine-2-carboxylic acid {(S)-1-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin II of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50169103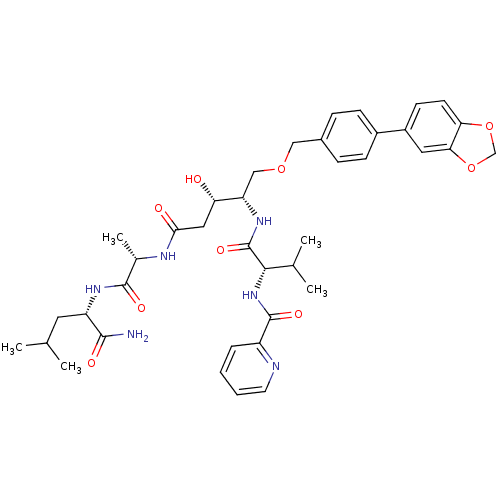 (CHEMBL191130 | Pyridine-2-carboxylic acid {(S)-1-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin II of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323468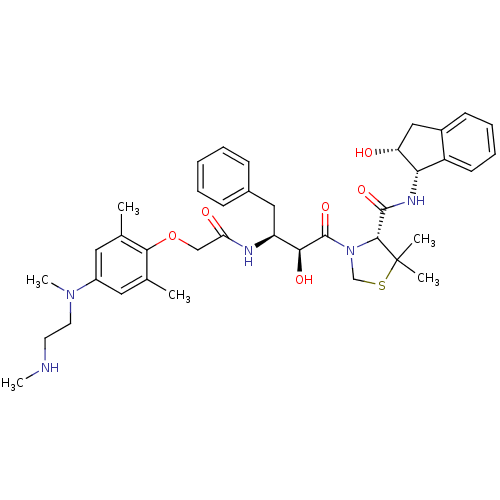 ((R)-3-((2S,3S)-3-(2-(2,6-dimethyl-4-(methyl(2-(met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001070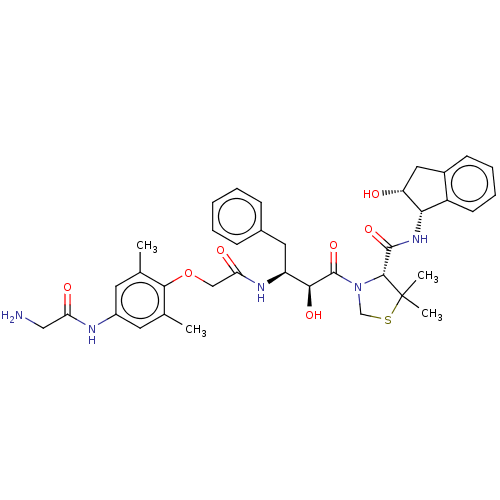 (CHEMBL3236064) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50390636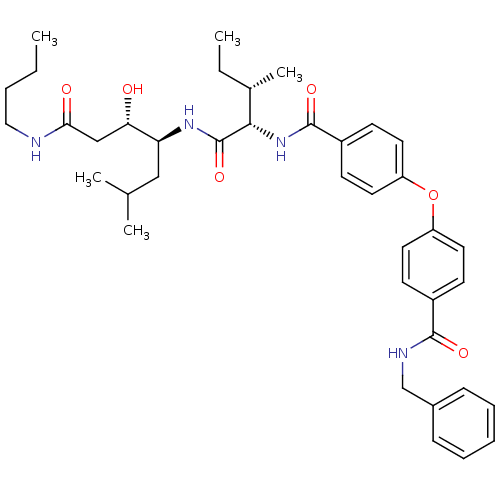 (CHEMBL2069615) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PLM2 using Lys-Glu-Phe-Val-Phe-NPhe-Ala-Leu-Lys as substrate by spectrophotometry | Bioorg Med Chem Lett 22: 5915-8 (2012) Article DOI: 10.1016/j.bmcl.2012.07.069 BindingDB Entry DOI: 10.7270/Q2M046HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001069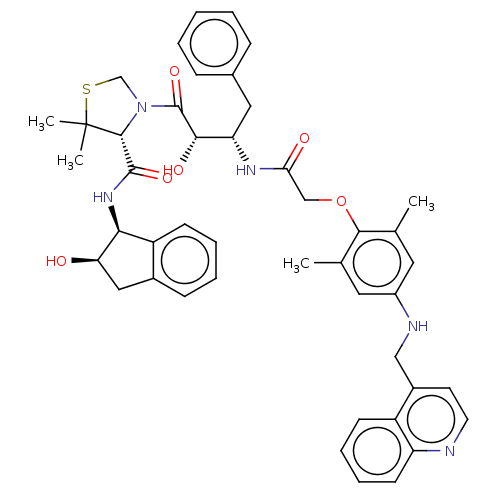 (CHEMBL3236063) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50390637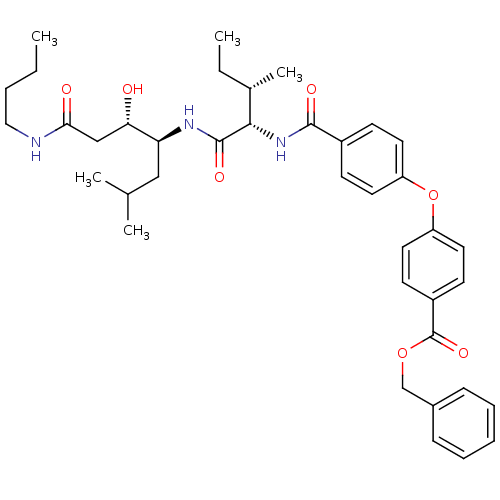 (CHEMBL2069616) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PLM2 using Lys-Glu-Phe-Val-Phe-NPhe-Ala-Leu-Lys as substrate by spectrophotometry | Bioorg Med Chem Lett 22: 5915-8 (2012) Article DOI: 10.1016/j.bmcl.2012.07.069 BindingDB Entry DOI: 10.7270/Q2M046HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50169104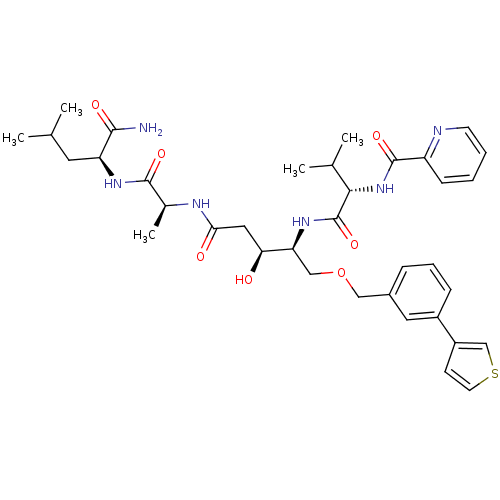 (CHEMBL371417 | Pyridine-2-carboxylic acid {(S)-1-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin II of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50076291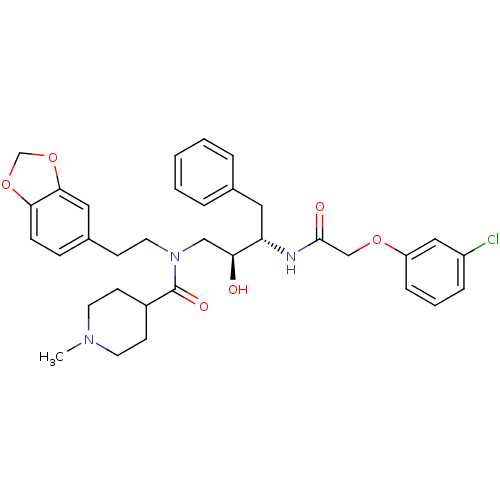 (1-Methyl-piperidine-4-carboxylic acid (2-benzo[1,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50076292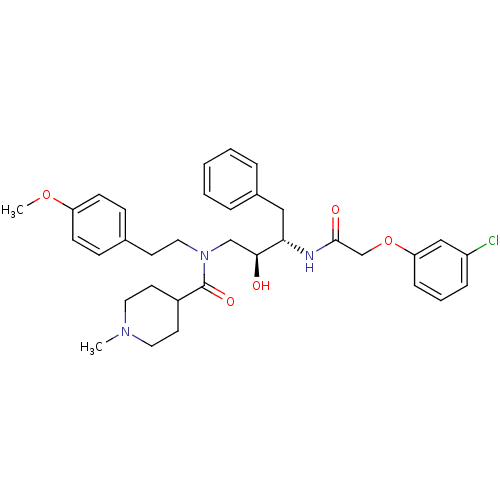 (1-Methyl-piperidine-4-carboxylic acid {(2S,3S)-3-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM7977 ((3S,4S)-5-[(4-bromophenyl)methoxy]-N-[(1S)-1-{[(1S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin II of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM7977 ((3S,4S)-5-[(4-bromophenyl)methoxy]-N-[(1S)-1-{[(1S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 2.20 | -48.9 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Linkoping University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 3353-66 (2004) Article DOI: 10.1021/jm031106i BindingDB Entry DOI: 10.7270/Q2WM1BMG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001071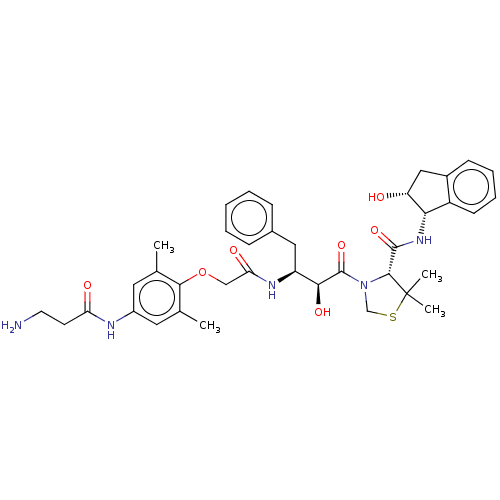 (CHEMBL3236065) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50390633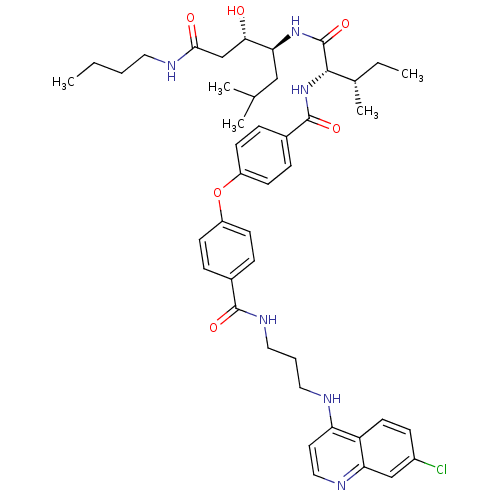 (CHEMBL2069612) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PLM2 using Lys-Glu-Phe-Val-Phe-NPhe-Ala-Leu-Lys as substrate by spectrophotometry | Bioorg Med Chem Lett 22: 5915-8 (2012) Article DOI: 10.1016/j.bmcl.2012.07.069 BindingDB Entry DOI: 10.7270/Q2M046HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50390635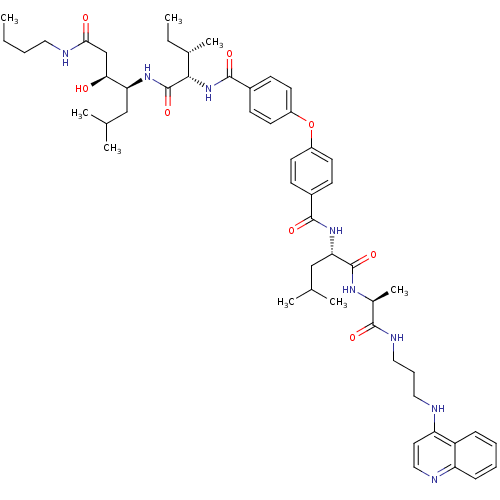 (CHEMBL2069614) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PLM2 using Lys-Glu-Phe-Val-Phe-NPhe-Ala-Leu-Lys as substrate by spectrophotometry | Bioorg Med Chem Lett 22: 5915-8 (2012) Article DOI: 10.1016/j.bmcl.2012.07.069 BindingDB Entry DOI: 10.7270/Q2M046HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50076285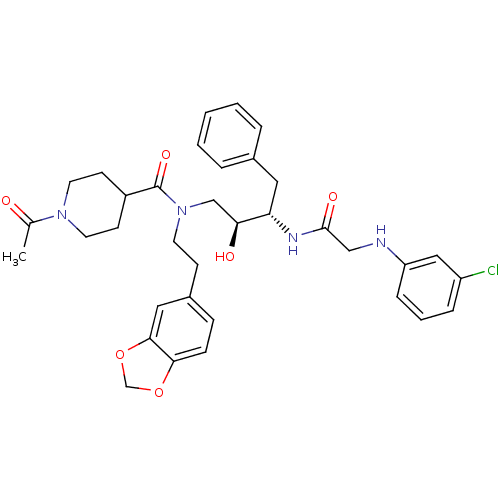 (1-Acetyl-piperidine-4-carboxylic acid (2-benzo[1,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209552 ((4R)-N-[(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 17: 3048-52 (2007) Article DOI: 10.1016/j.bmcl.2007.03.052 BindingDB Entry DOI: 10.7270/Q2J102VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50390634 (CHEMBL2069613) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PLM2 using Lys-Glu-Phe-Val-Phe-NPhe-Ala-Leu-Lys as substrate by spectrophotometry | Bioorg Med Chem Lett 22: 5915-8 (2012) Article DOI: 10.1016/j.bmcl.2012.07.069 BindingDB Entry DOI: 10.7270/Q2M046HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50200018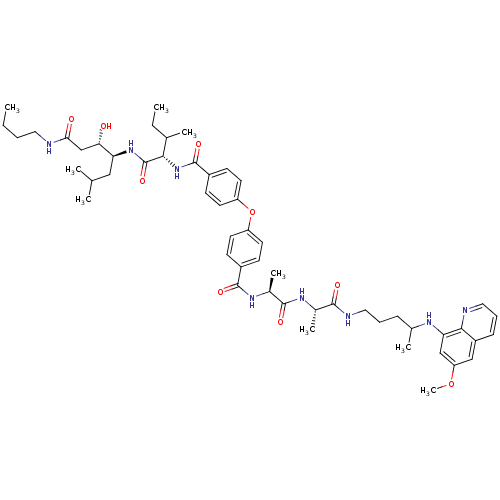 ((3S,4S)-4-((2S,3S)-2-(4-(4-((S)-((S)-1-(1-(4-(6-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum recombinant PLM2 | J Med Chem 49: 7440-9 (2006) Article DOI: 10.1021/jm061033d BindingDB Entry DOI: 10.7270/Q29P319W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209554 ((R)-N-((1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 17: 3048-52 (2007) Article DOI: 10.1016/j.bmcl.2007.03.052 BindingDB Entry DOI: 10.7270/Q2J102VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50076294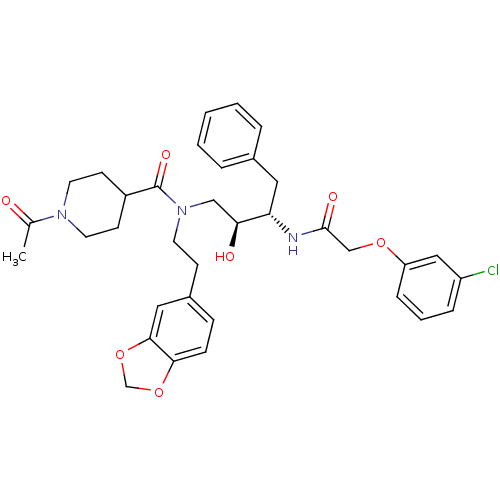 (1-Acetyl-piperidine-4-carboxylic acid (2-benzo[1,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50273723 ((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem 16: 10049-60 (2008) Article DOI: 10.1016/j.bmc.2008.10.011 BindingDB Entry DOI: 10.7270/Q2G160PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209563 ((R)-N-((1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 17: 3048-52 (2007) Article DOI: 10.1016/j.bmcl.2007.03.052 BindingDB Entry DOI: 10.7270/Q2J102VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209565 ((R)-3-((2S,3S)-3-((R)-2-(2-(2-aminophenyl)acetamid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 17: 3048-52 (2007) Article DOI: 10.1016/j.bmcl.2007.03.052 BindingDB Entry DOI: 10.7270/Q2J102VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50076287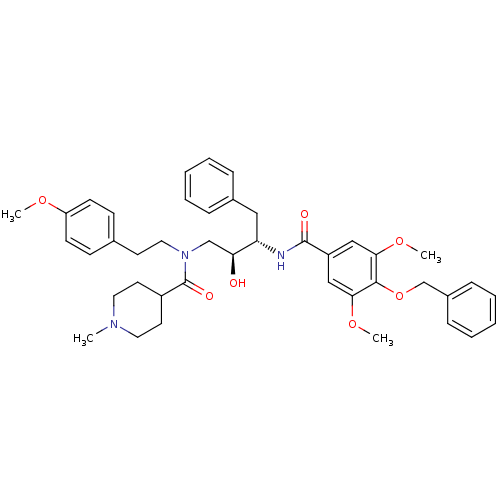 (1-Methyl-piperidine-4-carboxylic acid [(2S,3S)-3-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323473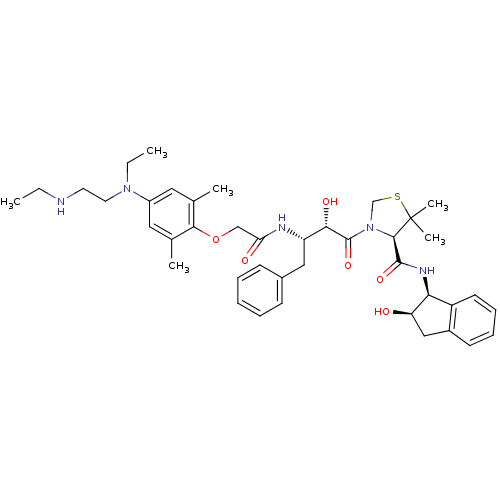 ((R)-3-((2S,3S)-3-(2-(4-(ethyl(2-(ethylamino)ethyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM8019 (2-(3-chlorophenoxy)-N-[(2S,3S)-3-hydroxy-4-{N-[2-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM8019 (2-(3-chlorophenoxy)-N-[(2S,3S)-3-hydroxy-4-{N-[2-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin II of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001075 (CHEMBL3236069) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM8019 (2-(3-chlorophenoxy)-N-[(2S,3S)-3-hydroxy-4-{N-[2-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001067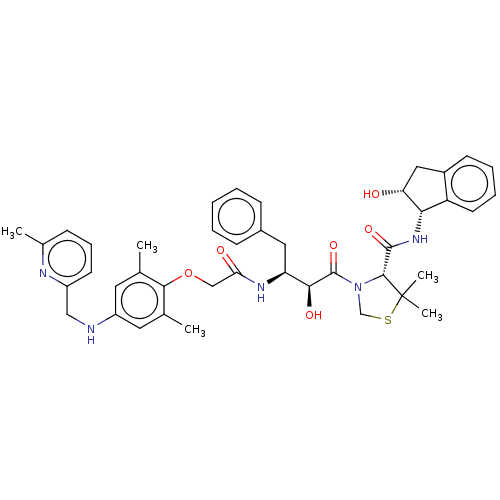 (CHEMBL3236061) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50169106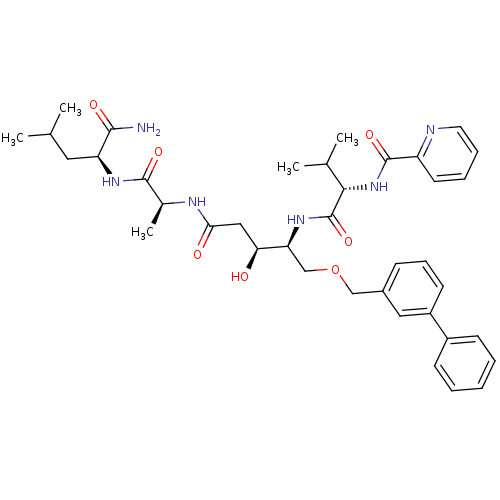 (CHEMBL363286 | Pyridine-2-carboxylic acid ((S)-1-{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin II of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001068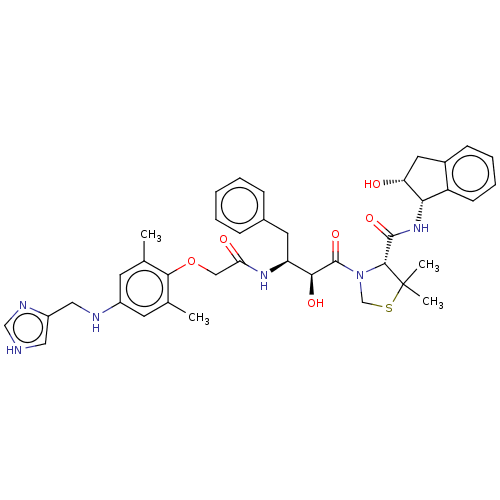 (CHEMBL3236062) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50076293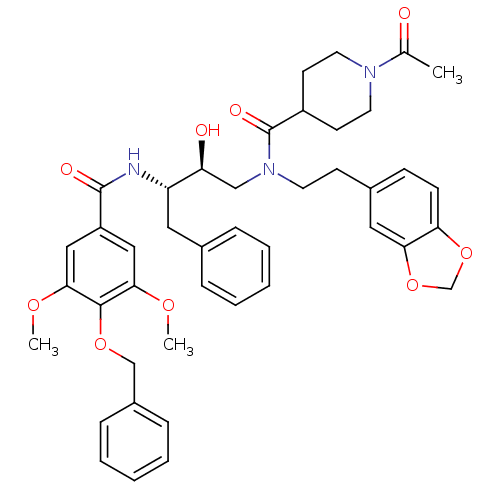 (1-Acetyl-piperidine-4-carboxylic acid (2-benzo[1,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Displayed 1 to 50 (of 729 total ) | Next | Last >> |